Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids †
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Tryptamines in “Minor Psilocybin Genera” (Excl. Psilocybe)
2.2. Quantification of Tryptamines in the Genus Psilocybe
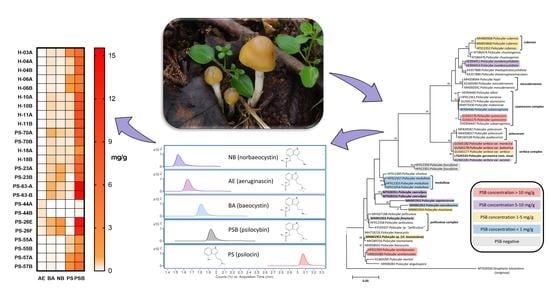
2.3. Quantification of Tryptamines in the Psilocybe serbica Complex
2.4. Remarks
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Collection of Mushroom Samples
3.3. Extraction
3.4. Instrumental Analysis
3.5. Molecular Methods and Phylogeny
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Jensen, N.; Gartz, J.; Laatsch, H. Aeruginascin, a trimethylammonium analogue of psilocybin from the hallucinogenic mushroom Inocybe aeruginascens. Planta Med. 2006, 72, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Wurst, M.; Kysilka, R.; Flieger, M. Psychoactive tryptamines from basidiomycetes. Folia Microbiol. 2002, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Sherwood, A.; Kargbo, R.; Hoffmeister, D. Taking different roads: L-tryptophan as the origin of Psilocybe natural products. ChemPlusChem 2021, 86, 28–35. [Google Scholar] [CrossRef]
- Gartz, J. Magic Mushrooms around the World; LIS Publs.: Los Angeles, CA, USA, 1996. [Google Scholar]
- Lenz, C.; Wick, J.; Hoffmeister, D. Identification of ω-N-Methyl-4-hydroxytryptamine (norpsilocin) as a Psilocybe natural product. J. Nat. Prod. 2017, 80, 2835–2838. [Google Scholar] [CrossRef]
- Blei, F.; Dörner, S.; Fricke, J.; Baldeweg, F.; Trottmann, F.; Komor, A.; Meyer, F.; Hertweck, C.; Hoffmeister, D. Simultaneous production of psilocybin and a cocktail of β-carboline monoamine oxidase inhibitors in “magic” mushrooms. Chem. Eur. J. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Schultes, R.E.; Hofmann, A. Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Froese, T.; Guzmán, G.; Guzmán-Dávalos, L. On the origin of the genus Psilocybe and its potential ritual use in ancient Africa and Europe. Econ. Bot. 2016, 70, 103–114. [Google Scholar] [CrossRef]
- Stijve, T. Psilocin, psilocybin, serotonin and urea in Panaeolus cyanescens from various origin. Persoonia 1992, 15, 117–121. [Google Scholar]
- Saupe, S.G. Occurrence of psilocybin/psilocin in Pluteus salicinus (Pluteaceae). Mycologia 1981, 73, 781–784. [Google Scholar] [CrossRef]
- Kreisel, H.; Lindequist, U. Gymnopilus purpuratus, ein psilocybinhaltiger Pilz adventiv in Berzirk Rostock. Z. Mykol. 1988, 54, 73–76. [Google Scholar]
- Halama, M.; Poliwoda, A.; Jasicka-Misiak, I.; Wieczorek, P.P.; Rutkowski, R. Pholiotina cyanopus, a rare fungus producing psychoactive tryptamines. Open Life Sci. 2015, 10, 40–51. [Google Scholar] [CrossRef]
- Besl, H. Galerina steglichii spec. nov., a hallucinogenic Galerina. Z. Mykol. 1993, 59, 215–218. [Google Scholar]
- Kosentka, P.; Sprague, S.L.; Ryberg, M.; Gartz, J.; May, A.L.; Campagna, S.R.; Matheny, P.B. Evolution of the toxins muscarine and psilocybin in a family of mushroom-forming fungi. PLoS ONE 2013, 8, e64646. [Google Scholar] [CrossRef]
- Boyce, G.R.; Gluck-Thaler, E.; Slot, J.C.; Stajich, J.E.; Davis, W.J.; James, T.Y.; Cooley, J.R.; Panaccione, D.G.; Eilenberg, J.; Henrik, H. Psychoactive plant-and mushroom-associated alkaloids from two behavior modifying cicada pathogens. Fungal Ecol. 2019, 41, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Letcher, A. Shroom: A Cultural History of the Magic Mushroom; Ecco Harper Collins: New York, NY, USA, 2007. [Google Scholar]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wark, C.; Galliher, J.F. Timothy Leary, Richard Alpert (Ram Dass) and the changing definition of psilocybin. Int. J. Drug Policy 2010, 21, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P.; Csémy, L. Self-experimentations with psychedelics among mental health professionals: LSD in the former Czechoslovakia. J. Psychoact. Drugs 2014, 46, 11–19. [Google Scholar] [CrossRef]
- Nutt, D.J.; King, L.A.; Phillips, L.D. Drug harms in the UK: A multicriteria decision analysis. Lancet 2010, 376, 1558–1565. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Ross, S. Therapeutic applications of classic hallucinogens. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 361–391. [Google Scholar]
- Dos Santos, R.G.; Hallak, J.E.C. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci. Biobehav. Rev. 2020, 108, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Froese, T.; Leenen, I.; Páleníček, T. A role for enhanced functions of sleep in psychedelic therapy? Adapt. Behav. 2018, 26, 129–135. [Google Scholar] [CrossRef]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin–summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Psilocybin Mushrooms of the World; Ten Speed Press: Berkeley, CA, USA, 1996. [Google Scholar]
- Lenz, C.; Wick, J.; Braga, D.; García-Altares, M.; Lackner, G.; Hertweck, C.; Gressler, M.; Hoffmeister, D. Injury-triggered blueing reactions of Psilocybe “magic” mushrooms. Angew. Chem. Int. Ed. 2020, 132, 1466–1470. [Google Scholar] [CrossRef]
- Borovička, J. The wood-rotting bluing Psilocybe species in Central Europe—an identification key. Czech Mycol. 2008, 60, 173–192. [Google Scholar] [CrossRef]
- Menolli, N.; Justo, A.; Arrillaga, P.; Pradeep, C.; Minnis, A.M.; Capelari, M. Taxonomy and phylogeny of Pluteus glaucotinctus sensu lato (Agaricales, Basidiomycota), a multicontinental species complex. Phytotaxa 2014, 188, 78–90. [Google Scholar] [CrossRef]
- Benedict, R.; Tyler, V.; Watling, R. Blueing in Conocybe, Psilocybe and a Stropharia species and detection if psilocybin. Lloydia 1967, 30, 150. [Google Scholar]
- Hatfield, G.; Valdes, L. The occurrence of psilocybin in Gymnopilus species. Lloydia 1978, 41, 140–144. [Google Scholar]
- Moser, M.; Horak, E. Psilocybe serbica spec. nov: Eine neue Psilocybin und Psilocin bildende Art aus Serbien. Z. Pilzkd. 1968, 34, 137–144. [Google Scholar]
- Ott, J.; Guzmán, G. Detection of psilocybin in species of Psilocybe, Panaeolus and Psathyrella. Lloydia 1976, 39, 258–260. [Google Scholar] [PubMed]
- Semerdžieva, M. Halluzinogene Pilze in der Tschechoslowakei. Česká Mykol. 1973, 27, 42–47. [Google Scholar]
- Weeks, R.A.; Singer, R.; Hearn, W.L. A new psilocybian species of Copelandia. J. Nat. Prod. 1979, 42, 469–474. [Google Scholar] [CrossRef]
- Michaelis, H. Psilocybe semilanceata (Fr.) Quél. (Spitzkegliger Kahlkopf): Nachweis von Psilocybin in deutschen Funden. Z. Pilzkd. 1977, 43, 305–310. [Google Scholar]
- Allen, J.W.; Gartz, J.; Molter, D. The occurrence, cultivation, and chemistry of Psilocybe ovoideocystidiata, a new bluing species (Agaricales) from Ohio, Pennsylvania and West Virginia. Ethnomycol. J. Sacred Mushroom Stud. 2009, 8, 70–81. [Google Scholar]
- Gartz, J.; Allen, J.W.; Merlin, M.D. Ethnomycology, biochemistry, and cultivation of Psilocybe samuiensis Guzmán, Bandala and Allen, a new psychoactive fungus from Koh Samui, Thailand. J. Ethnopharmacol. 1994, 43, 73–80. [Google Scholar] [CrossRef]
- Christiansen, A.; Rasmussen, K. Analysis of indole alkaloids in Norwegian Psilocybe semilanceata using high-performance liquid chromatography and mass spectrometry. J. Chromatogr. A 1982, 244, 357–364. [Google Scholar] [CrossRef]
- Ohenoja, E.; Jokiranta, J.; Mäkinen, T.; Kaikkonen, A.; Airaksinen, M. The occurrence of psilocybin and psilocin in Finnish Fungi. J. Nat. Prod. 1987, 50, 741–744. [Google Scholar] [CrossRef]
- Semerdžieva, M.; Wurst, M. Psychotrope Inhaltsstoffe zweier Psilocybe Arten-Kahlkopfe aus der CSSR. Mykol. Mitteilungsblatt 1986, 29, 65–70. [Google Scholar]
- Stříbrný, J.; Borovička, J.; Sokol, M. Psilocibin content of several macrofungal species. Soud. Lék. 2003, 48, 45–49. (In Czech) [Google Scholar]
- Vanhaelen-Fastré, R.; Vanhaelen, M. Qualitative and quantitative determinations of hallucinogenic components of Psilocybe mushrooms by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1984, 312, 467–472. [Google Scholar] [CrossRef]
- Wurst, M.; Kysilka, R.; Koza, T. Analysis and isolation of indole alkaloids of fungi by high-performance liquid chromatography. J. Chromatogr. A 1992, 593, 201–208. [Google Scholar] [CrossRef]
- Wurst, M.; Semerdžieva, M.; Vokoun, J. Analysis of psychotropic compounds in fungi of the genus Psilocybe by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1984, 286, 229–235. [Google Scholar] [CrossRef]
- Gartz, J. Detection of tryptamine derivatives in fungi of the genera Gerronema, Hygrocybe, Psathyrella and Inocybe. Biochem. Physiol. Pflanzen 1986, 181, 275–278. [Google Scholar] [CrossRef]
- Gartz, J. Analysis of aeruginascin in fruit bodies of the mushroom Inocybe aeruginascens. Int. J. Crude Drug Res. 1989, 27, 141–144. [Google Scholar] [CrossRef]
- Repke, D.B.; Leslie, D.T.; Guzmán, G. Baeocystin in Psilocybe, Conocybe and Panaeolus. Lloydia 1977, 40, 566–578. [Google Scholar]
- Stijve, T.; Kuyper, T.W. Occurrence of psilocybin in various higher fungi from several European countries. Planta Med. 1985, 51, 385–387. [Google Scholar] [CrossRef]
- Gartz, J. New aspects of the occurrence, chemistry and cultivation of European hallucinogenic mushrooms. Ann. Musei Civ. Rovereto 1992, 8, 107–124. [Google Scholar]
- Thomas, B. Boletus manicus Heim. J. Psychoact. Drugs 2003, 35, 393–394. [Google Scholar] [CrossRef]
- Gotvaldová, K.; Hájková, K.; Borovička, J.; Jurok, R.; Cihlářová, P.; Kuchař, M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Drug Test. Anal. 2021, 13, 439–446. [Google Scholar] [CrossRef]
- Stijve, T.; Klán, J.; Kuyper, T.W. Occurrence of psilocybin and baeocystin in the genus Inocybe (Fr.) Fr. Persoonia 1985, 12, 469–473. [Google Scholar]
- Læssøe, T.; Petersen, J.H. Fungi of Temperate Europe; Princeton University Press: Princeton, NJ, USA, 2019; p. 813. [Google Scholar]
- Besl, H.; Mack, P.; Schmid-Heckel, H. Giftpilze in den Gattungen Galerina und Leipiota. Z. Mykol. 1984, 50, 183–192. [Google Scholar]
- Stijve, T.; Kuyper, T.W. Absence of psilocybin in species of fungi previously reported to contain psilocybin and related tryptamine derivatives. Persoonia 1988, 13, 463–465. [Google Scholar]
- Gerhardt, E. Taxonomische Revision der Gattungen Panaeolus and Panaeolina (Fungi, Agaricales, Coprinaceae); Bibliotheca Botanica: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Stamets, P.; Gartz, J. A new caerulescent Psilocybe from the Pacific Coast of Northwestern America. Integration 1995, 6, 21–28. [Google Scholar]
- Stijve, T.; De Meijer, A. Macromycetes from the State of Paraná, Brazil. The psychoactive species. Arq. Biol. Tecnol. 1993, 36, 313–329. [Google Scholar]
- Allen, J.W.; Merlin, M.D. Observations regarding the suspected psychoactive properties of Panaeolina foenisecii Maire. In Yearbook for Ethnomedicine and the Study of Consciousness; Rätsch, C., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 99–115. [Google Scholar]
- Perkal, M.; Blackman, G.L.; Ottrey, A.; Turner, L. Determination of hallucinogenic components of Psilocybe mushrooms using high-performance liquid chromatography. J. Chromatogr. A 1980, 196, 180–184. [Google Scholar] [CrossRef]
- Anastos, N.; Barnett, N.W.; Lewis, S.W.; Gathergood, N.; Scammells, P.J.; Sims, D.N. Determination of psilocin and psilocybin using flow injection analysis with acidic potassium permanganate and tris (2, 2′-bipyridyl) ruthenium (II) chemiluminescence detection respectively. Talanta 2005, 67, 354–359. [Google Scholar] [CrossRef]
- Leung, A.Y.; Smith, A.; Paul, A. Production of psilocybin in Psilocybe baeocystis saprophytic culture. J. Pharm. Sci. 1965, 54, 1576–1579. [Google Scholar] [CrossRef]
- Borovička, J.; Oborník, M.; Stříbrný, J.; Noordeloos, M.; Parra Sánchez, L.A.; Gryndler, M. Phylogenetic and chemical studies in the potential psychotropic species complex of Psilocybe atrobrunnea with taxonomic and nomenclatural notes. Persoonia 2015, 34, 1–9. [Google Scholar] [CrossRef]
- Reynolds, H.T.; Vijayakumar, V.; Gluck-Thaler, E.; Korotkin, H.B.; Matheny, P.B.; Slot, J.C. Horizontal gene cluster transfer increased hallucinogenic mushroom diversity. Evol. Lett. 2018, 2, 88–101. [Google Scholar] [CrossRef]
- Redhead, S.A.; Moncalvo, J.-M.; Vilgalys, R.; Matheny, P.B.; Guzmán-Dávalos, L.; Guzmán, G. (1757) Proposal to conserve the name Psilocybe (Basidiomycota) with a conserved type. Taxon 2007, 56, 255–257. [Google Scholar]
- Guzmán, G. The Genus Psilocybe; Beihefte Nova Hedwigia 74; Cramer, J., Ed.; Lubrecht & Cramer Ltd.: Vaduz, Liechtenstein, 1983. [Google Scholar]
- Noordeloos, M.E. Fungi Europaei, Volume 13: Strophariaceae s.l.; Edizioni Candusso: Caronno Pertusella, Italy, 2011. [Google Scholar]
- Cortés-Pérez, A.; Ramírez-Guillén, F.; Guzmán, G.; Guzmán-Dávalos, L.; Rockefeller, A.; Ramírez-Cruz, V. Type studies in five species of Psilocybe (Agaricales, Basidiomycota). Nova Hedwig. 2021, 112, 197–221. [Google Scholar] [CrossRef]
- Hofmann, A.; Heim, R.; Brack, A.; Kobel, H. Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim. Experientia 1958, 14, 107–109. [Google Scholar] [CrossRef]
- Hofmann, A.; Heim, R.; Brack, A.; Kobel, H.; Frey, A.; Ott, H.; Petrzilka, T.; Troxler, F. Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helv. Chim. Acta 1959, 42, 1557–1572. [Google Scholar] [CrossRef]
- Borovička, J.; Noordeloos, M.E.; Gryndler, M.; Oborník, M. Molecular phylogeny of Psilocybe cyanescens complex in Europe, with reference to the position of the secotioid Weraroa novae-zelandiae. Mycol. Prog. 2011, 10, 149–155. [Google Scholar] [CrossRef]
- Fricke, J.; Blei, F.; Hoffmeister, D. Enzymatic synthesis of psilocybin. Angew. Chem. Int. Ed. 2017, 56, 12352–12355. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, K.; Kanamori, T.; Iwata, Y.; Ohmae, Y.; Sugita, R.; Inoue, H.; Kishi, T. Morphological and chemical analysis of magic mushrooms in Japan. Forensic Sci. Int. 2003, 138, 85–90. [Google Scholar] [CrossRef]
- Saito, K.; Toyo’oka, T.; Kato, M.; Fukushima, T.; Shirota, O.; Goda, Y. Determination of psilocybin in hallucinogenic mushrooms by reversed-phase liquid chromatography with fluorescence detection. Talanta 2005, 66, 562–568. [Google Scholar] [CrossRef]
- Keller, T.; Schneider, A.; Regenscheit, P.; Dirnhofer, R.; Rücker, T.; Jaspers, J.; Kisser, W. Analysis of psilocybin and psilocin in Psilocybe subcubensis Guzmán by ion mobility spectrometry and gas chromatography–mass spectrometry. Forensic Sci. Int. 1999, 99, 93–105. [Google Scholar] [CrossRef]
- Arkhipchenko, I.; Shaposhnikov, A.; Kravchenko, L.V. Tryptophan concentration of animal wastes and organic fertilizers. Appl. Soil Ecol. 2006, 34, 62–64. [Google Scholar] [CrossRef]
- Guzmán-Dávalos, L.; Mueller, G.M.; Cifuentes, J.; Miller, A.N.; Santerre, A. Traditional infrageneric classification of Gymnopilus is not supported by ribosomal DNA sequence data. Mycologia 2003, 95, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cruz, V.; Guzmán, G.; Villalobos-Arámbula, A.R.; Rodríguez, A.; Matheny, P.B.; Sánchez-García, M.; Guzmán-Dávalos, L. Phylogenetic inference and trait evolution of the psychedelic mushroom genus Psilocybe sensu lato (Agaricales). Botany 2013, 91, 573–591. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Eyre, C.A.; Hayden, K.M.; Dhillon, J.; Garbelotto, M.M. Back to basics: An evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and Oomycete samples. Mol. Ecol. Resour. 2013, 13, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Borovička, J.; Rockefeller, A.; Werner, P.G. Psilocybe allenii—A new bluing species from the Pacific Coast, USA. Czech Mycol. 2012, 64, 181–195. [Google Scholar] [CrossRef]
- Ma, T.; Ling, X.-F.; Hyde, K.D. Species of Psilocybe (Hymenogastraceae) from Yunnan, southwest China. Phytotaxa 2016, 284, 181–193. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; De Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.; Cardinali, G.; Houbraken, J. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for Fungal species and higher taxon delimitation. Stud. Mycol. 2018, 91, 23–36. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Tzean, S.-S. Dung-associated, potentially hallucinogenic mushrooms from Taiwan. Taiwania 2015, 60, 160–168. [Google Scholar]
- Zhang, X.; Yu, H.; Yang, Q.; Wang, Z.; Xia, R.; Chen, C.; Qu, Y.; Tan, R.; Shi, Y. A forensic detection method for hallucinogenic mushrooms via High-Resolution Melting (HRM) analysis. Genes 2021, 12, 199. [Google Scholar] [CrossRef]
- Ma, T.; Feng, Y.; Lin, X.-F.; Karunarathna, S.C. Psilocybe chuxiongensis, a new bluing species from subtropical China. Phytotaxa 2014, 156, 211–220. [Google Scholar] [CrossRef][Green Version]
- Katoh, K.; Toh, H. Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinform. 2008, 9, 1–13. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions throughcomparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Borovička, J.; Braeuer, S.; Walenta, M.; Hršelová, H.; Leonhardt, T.; Sácký, J.; Kaňa, A.; Goessler, W. A new mushroom hyperaccumulator: Cadmium and arsenic in the ectomycorrhizal basidiomycete Thelephora penicillata. Sci. Total Environ. 2022, 826, 154227. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, T.; Borovička, J.; Sácký, J.; Šantrůček, J.; Kameník, J.; Kotrba, P. Zn overaccumulating Russula species clade together and use the same mechanism for the detoxification of excess Zn. Chemosphere 2019, 225, 618–626. [Google Scholar] [CrossRef] [PubMed]





| Mushroom Species | fb | n | AE | BA | NB | PS | PSB |
|---|---|---|---|---|---|---|---|
| Gymnopilus dilepis | 2 | 1 | <LOD | 0.002–0.005 | <LOD | 0.024–0.063 | 0.031–0.131 |
| Inocybe aeruginascens | 2 | 1 | 0.280–0.283 | 0.038–0.039 | <LOD | 0.005 | 0.124–0.128 |
| Inocybe calamistrata | 9 | 5 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Inocybe corydalina | 4 | 2 | 0.016–0.365 | 0.499–0.975 | 0.036–0.097 | 0.000–0.006 | 0.076–0.282 |
| Panaeolus cinctulus | 12 | 3 | ― | 0.118–1.525 | 0.045–0.477 | 0.007–0.257 | 0.114–1.578 |
| Panaeolus foenisecii | 8 | 2 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Panaeolus olivaceus | 2 | 1 | ― | <LOD | <LOD | <LOD | <LOD |
| Panaeolus papilionaceus | 2 | 1 | ― | <LOD | <LOD | <LOD | <LOD |
| Pholiotina cyanopus | 3 | 1 | ― | 0.821–1.360 | 0.247–0.565 | 0.000–0.062 | 0.000–0.859 |
| Pluteus americanus | 6 | 3 | 0.008–0.014 | 0.154–0.410 | 0.067–0.162 | 0.123–0.347 | 1.172–2.428 |
| Pluteus glaucotinctus | 1 | 1 | 0.012 | 0.224 | 0.484 | 0.013 | 1.939 |
| Pluteus salicinus | 3 | 2 | 0.014–0.024 | 0.032–0.157 | 0.018–0.044 | 0.037–0.070 | 0.306–1.353 |
| Psilocybe caerulescens | 2 | 1 | <LOQ | 0.009–0.013 | <LOD | 0.341–0.413 | 0.225–0.310 |
| Psilocybe caerulipes | 4 | 2 | 0.018–0.028 | 0.063–0.141 | 0.019–0.052 | 0.501–2.770 | 2.234–5.674 |
| Psilocybe cubensis | 9 | 4 | 0.026–0.053 | 0.139–0.881 | 0.044–0.161 | 0.208–5.344 | 0.651–3.509 |
| Psilocybe cyanescens | 13 | 4 | 0.011–0.039 | 0.216–2.852 | 0.102–0.978 | 0.409–10.018 | 2.340–13.808 |
| Psilocybe fimetaria | 4 | 2 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Psilocybe fuscofulva | 6 | 3 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Psilocybe medullosa | 11 | 3 | <LOD | 0.007–0.406 | 0.000–0.215 | 0.000–0.051 | 0.143–1.003 |
| Psilocybe mexicana | 2 | 1 | 0.006–0.007 | 0.254–0.328 | 0.159–0.203 | 1.944–1.974 | 3.286–3.934 |
| Psilocybe ovoideocystidiata | 8 | 3 | 0.004–0.013 | 0.164–0.699 | 0.031–0.568 | 0.026–5.464 | 0.914–7.172 |
| Psilocybe semilanceata | 18 | 5 | 0.010–0.033 | 0.725–4.467 | 0.121–0.510 | 0.033–0.619 | 1.280–11.421 |
| Psilocybe serbica | 5 | 1 | ― | 1.748–3.385 | 1.010–1.946 | 0.215–3.810 | 1.562–3.957 |
| Psilocybe serbica var. arcana | 54 | 18 | 0.000–0.024 | 0.000–2.237 | 0.000–0.794 | 0.412–7.922 | 0.002–8.878 |
| Psilocybe serbica var. bohemica | 20 | 8 | 0.008–0.093 | 0.234–2.473 | 0.173–1.282 | 0.027–2.485 | 1.553–15.543 |
| Psilocybe serbica var. moravica | 6 | 1 | 0.053–0.249 | 0.293–0.822 | 0.453–2.012 | 0.061–0.386 | 5.655–14.158 |
| Psilocybe sp. | 6 | 1 | ― | 0.282–0.965 | 0.031–0.144 | 1.489–2.051 | 0.512–1.892 |
| Psilocybe subaeruginosa | 2 | 1 | ― | 0.009–0.011 | <LOQ | 0.081–0.326 | 0.102–0.195 |
| Psilocybe zapotecorum | 2 | 1 | 0.021 | 0.430–0.481 | 0.208–0.211 | 0.293–0.369 | 9.022–9.655 |
| Stropharia aeruginosa * | 3 | 1 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Agaricus bisporus * | 20 | 1 | <LOD | <LOD | <LOD | <LOD | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotvaldová, K.; Borovička, J.; Hájková, K.; Cihlářová, P.; Rockefeller, A.; Kuchař, M. Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids. Int. J. Mol. Sci. 2022, 23, 14068. https://doi.org/10.3390/ijms232214068
Gotvaldová K, Borovička J, Hájková K, Cihlářová P, Rockefeller A, Kuchař M. Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids. International Journal of Molecular Sciences. 2022; 23(22):14068. https://doi.org/10.3390/ijms232214068
Chicago/Turabian StyleGotvaldová, Klára, Jan Borovička, Kateřina Hájková, Petra Cihlářová, Alan Rockefeller, and Martin Kuchař. 2022. "Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids" International Journal of Molecular Sciences 23, no. 22: 14068. https://doi.org/10.3390/ijms232214068
APA StyleGotvaldová, K., Borovička, J., Hájková, K., Cihlářová, P., Rockefeller, A., & Kuchař, M. (2022). Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids. International Journal of Molecular Sciences, 23(22), 14068. https://doi.org/10.3390/ijms232214068