Abstract
In this experimental research, different types of essential oils (EOs) were blended with polyhydroxybutyrate (PHB) to study the influence of these additives on PHB degradation. The blends were developed by incorporating three terpenoids at two concentrations (1 and 3%). The mineralization rate obtained from CO2 released from each sample was the factor that defined biodegradation. Furthermore, scanning electron microscope (SEM), differential scanning calorimetry (DSC), and dynamic mechanical analysis (DMA) were used in this research. The biodegradation percentages of PHB blended with 3% of eucalyptol, limonene, and thymol after 226 days were reached 66.4%, 73.3%, and 76.9%, respectively, while the rate for pure PHB was 100% after 198 days, and SEM images proved these results. Mechanical analysis of the samples showed that eucalyptol had the highest resistance level, even before the burial test. The other additives showed excellent mechanical properties although they had less mechanical strength than pure PHB after extrusion. The samples’ mechanical properties improved due to their crystallinity and decreased glass transition temperature (Tg). DSC results showed that blending terpenoids caused a reduction in Tg, which is evident in the DMA results, and a negligible reduction in melting point (Tm).
1. Introduction
Plastics have been used indiscriminately for decades, but they have eventually raised strong ecological concerns. In this regard, the negative influence of petroleum-based plastics on the environment has led to the development of biobased and biodegradable plastics [1,2]. Plastics derived from renewable resources have received significant attention as an environmentally friendlier alternative to petroleum-based polymers due to their biodegradability, which can occur in aerobic, and sometimes even anaerobic, conditions [3,4,5,6]. One of the most studied materials in this class is polyhydroxybutyrate (PHB), which can be used in many applications. It is a thermoplastic polymer that is renewable as it is produced by many Gram-negative and Gram-positive bacteria [7] as carbon- and energy-storage material [8,9].
The chemical structures of polymers, as well as the environment to which they are subjected, have a major impact on how quickly they degrade. Other factors that have a significant impact on biodegradability include temperature, oxygen levels, humidity, the predominance of particular microbial communities, and the availability of nutrients. PHB is known as a completely degradable polymer in a variety of controlled and natural biologically active conditions [10]. PHB is a thoroughly studied biodegradable polymer with biocompatibility and biodegradability, and its properties are suitable in food-packaging and agricultural applications; yet, due to characteristics, such as its melting temperature, lack of flexibility, and low extension to break, its commercial applicability has been limited so far [11]. Moreover, it has a high degree of crystallinity, as well as low impact strength, as compared to other biodegradable polymers [12].
Despite having some favorable properties, PHB has some limitations. First, this polymer’s price and availability are the critical factors restricting its broader use so far. Second, the material has less favorable mechanical and thermal properties [13,14]. To eliminate these problems, many studies have been performed [9,15], using various types of copolymers (which could provide materials with more favorable morphology) [16,17], crosslinking agents [18], natural compounds [19,20], antimicrobial agents [21], and chain extenders [22,23]. Third, the rapid biodegradation of PHB is a drawback that restricts its use in some agricultural applications, especially those which require direct contact with soil.
The latter issue could be controlled by adding compounds with mild antimicrobial properties that can slow down, but not completely prevent, the biodegradation of the material in the soil. Natural, simple terpenoids appear to be attractive candidates for such compounds. Previously, terpenoids were studied in PHB materials mainly to prevent the adsorption of pathogenic bacteria on its surface. Narayanan and his colleagues [24] studied the antimicrobial effect of the addition of eugenol to PHB against Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli. The results showed that eugenol–PHB films, in conjunction with another antimicrobial, pediocin, could effectively control the growth of food spoilage microorganisms, food-contaminating molds, and foodborne pathogens. Oliveira et al. [25] studied the antibacterial effect of eucalyptol and carvacrol against Pseudomonas fluorescens, Aeromonas hydrophila, and Listeria monocytogenes, as model organisms in a mixed culture. They reported that carvacrol’s minimum inhibition concentration (MIC), which shows the minimum required concentration of an antimicrobial [26], was about 30 times lower than eucalyptol, but both had sufficient potential as antimicrobial agents. Porfirio et al. [27] and Huang et al. [28] studied the antibacterial and antifungal effects of carvone and citral. The first study reported that the MIC of carvone was 4 times higher than that of citral; however, Huang and his research group found that, as an antifungal, the MIC of citral was about 67% higher than that of carvone.
The compounds in the discussed group can also act as plasticizers in polymeric materials in somewhat higher concentrations [29,30]. Mangeon et al. [4] studied the influence of three different terpenoids as plasticizers on PHB. Their results revealed that these additives enhanced the elongation at break by up to 650%. Moreover, a reduction in Tg and the storage modulus was observed after adding these compounds.
As explained, previous studies did not investigate the influence of antibacterial terpenoid addition on the biodegradation of PHB-based materials in soil. Here, we explore this phenomenon and show a way to control the biodegradation of such materials. These findings can be further exploited to develop more functionally advanced materials to be used in agriculture, e.g., mulching films or other products that should be completely biodegradable in the soil, but which must retain their properties during their limited service lives.
2. Results and Discussion
2.1. Preliminary Biodegradation Experiment
As a preliminary study, 10 PHB materials with terpenoids were prepared to find the most effective ones. The majority of the compounds were selected based on their MICs. The materials were evaluated after 65 days of mineralization and a simple burial test in which the disintegration of the samples was observed. The results indicated that thymol, eucalyptol, and limonene were the most promising additives for slowing the biodegradation of the materials in the soil, while the effect of the other tested compounds seemed marginal.
2.2. Mineralization of Materials in Soil
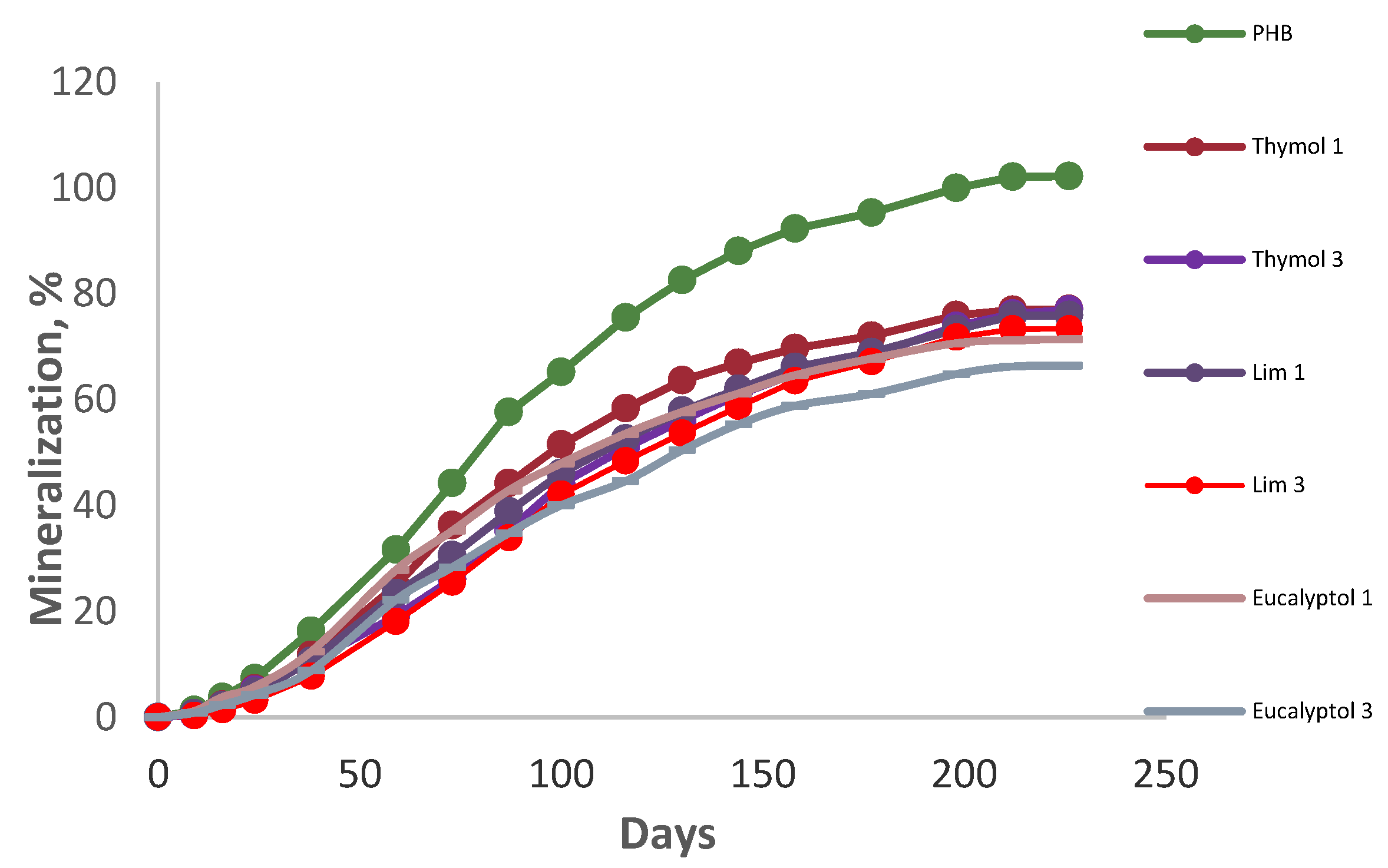
The CO2 evolution measurement monitored the mineralization of the materials. The data proved a substantial retardation of biodegradation by all of the additives (Figure 1). As can be seen, the blend with 3% eucalyptol showed the best resistance against degradation. Although pure PHB had been degraded completely prior to 200 days, PHB blended with 3% limonene was the second-best sample in retarding biodegradation, and 3% thymol showed moderate retardation of biodegradation. In this regard, after 226 days of incubation, 3% eucalyptol, 3% thymol, and 3% limonene reached 66.4, 77, and 73.3% of mineralization, respectively, due to their antimicrobial activities [19,31]. The influence of limonene in retarding biodegradation was more substantial than that of thymol due to its higher antifungal activity [32]. The film degradation in the preliminary study proved the more substantial influence of eucalyptol in PHB degradation, which was also due to its high antimicrobial and antioxidant activities [33]. Furthermore, blending essential oils with PHB is a technique for enhancing the polymer’s crystallinity, which reduces biodegradation [19].
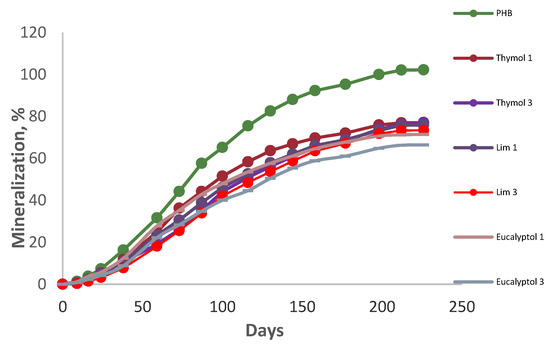
Figure 1.
Mineralization of the additives at room temperature.
2.3. Visual and Microscopic Analysis
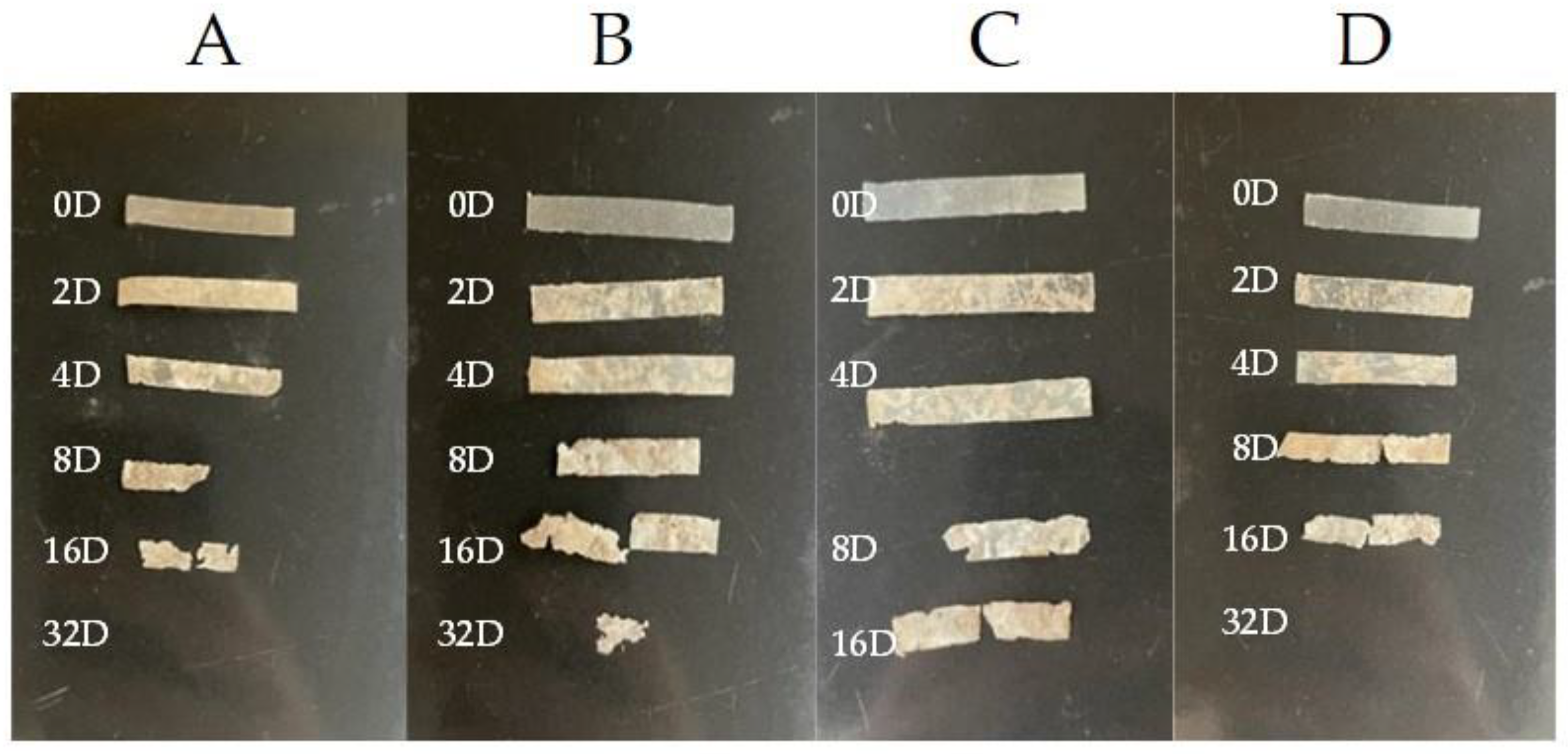
The physical shapes of the samples are shown in Figure 2. As can be seen, the blend with 3% of eucalyptol was the only sample that could be identified after 32 days; the other samples had disintegrated prior to that. The images also depict that samples with thymol and limonene were more resistant than pure PHB.
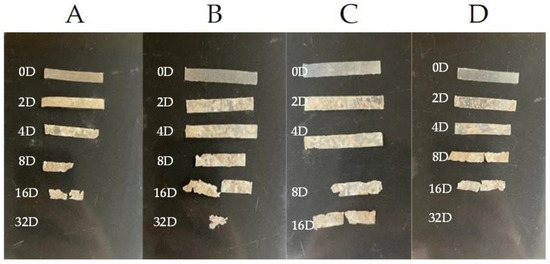
Figure 2.
Physical shapes of the samples during incubation, observed at different times: 0, 2, 4, 8, 16, and 32 days: (A) pure PHB, (B) PHB + 3% eucalyptol, (C) PHB + 3% limonene, and (D) PHB + 3% thymol.
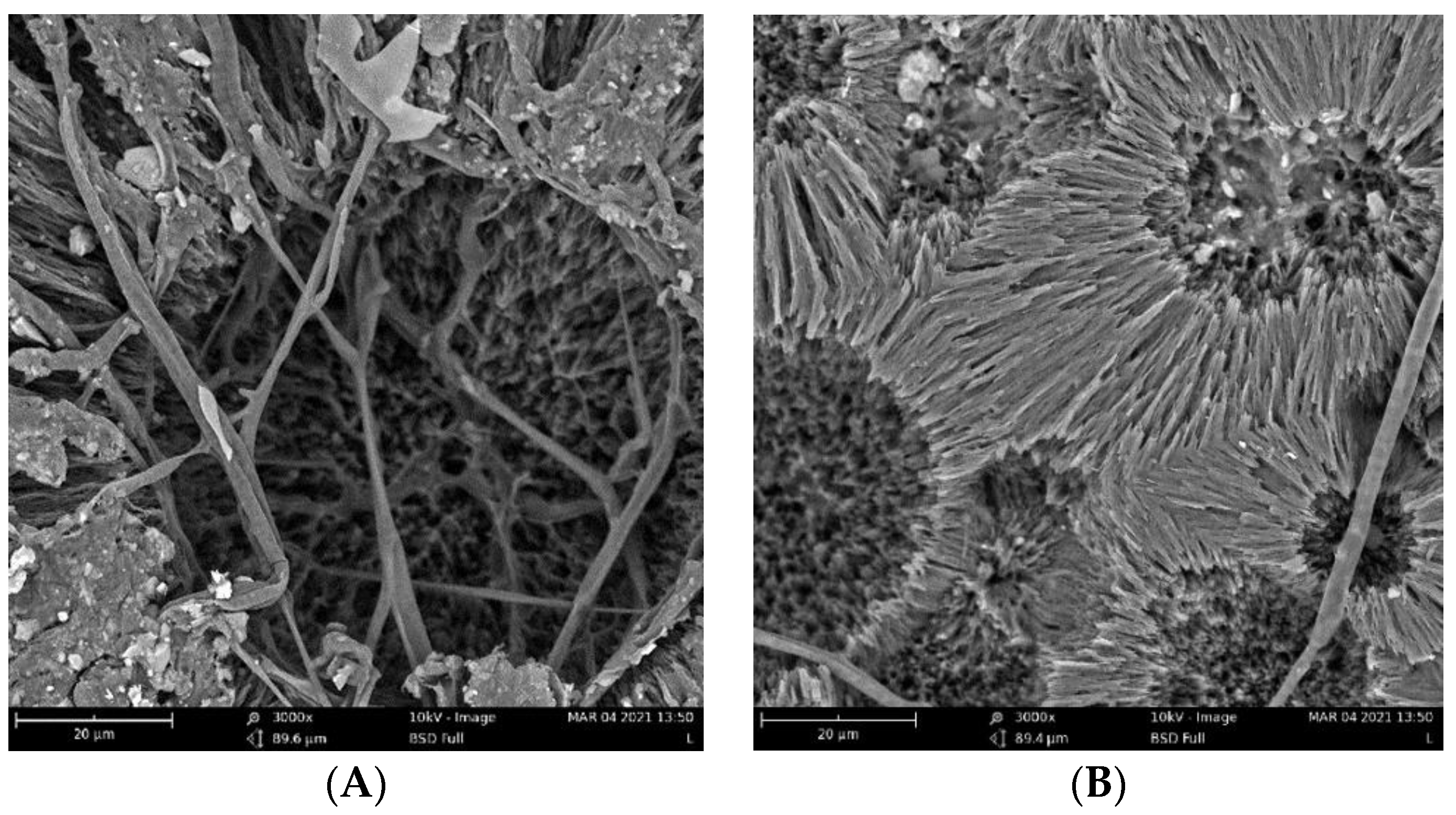
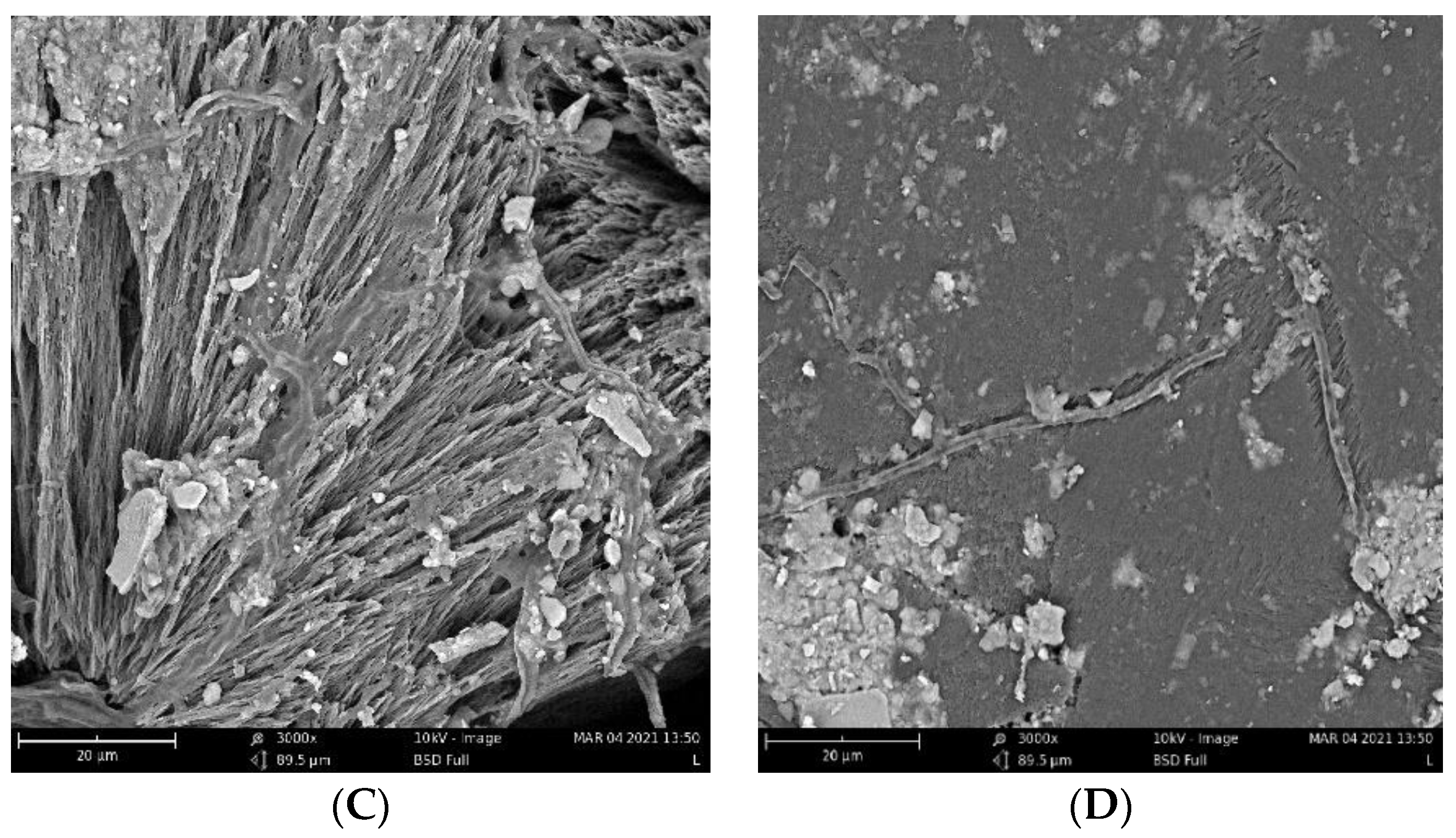
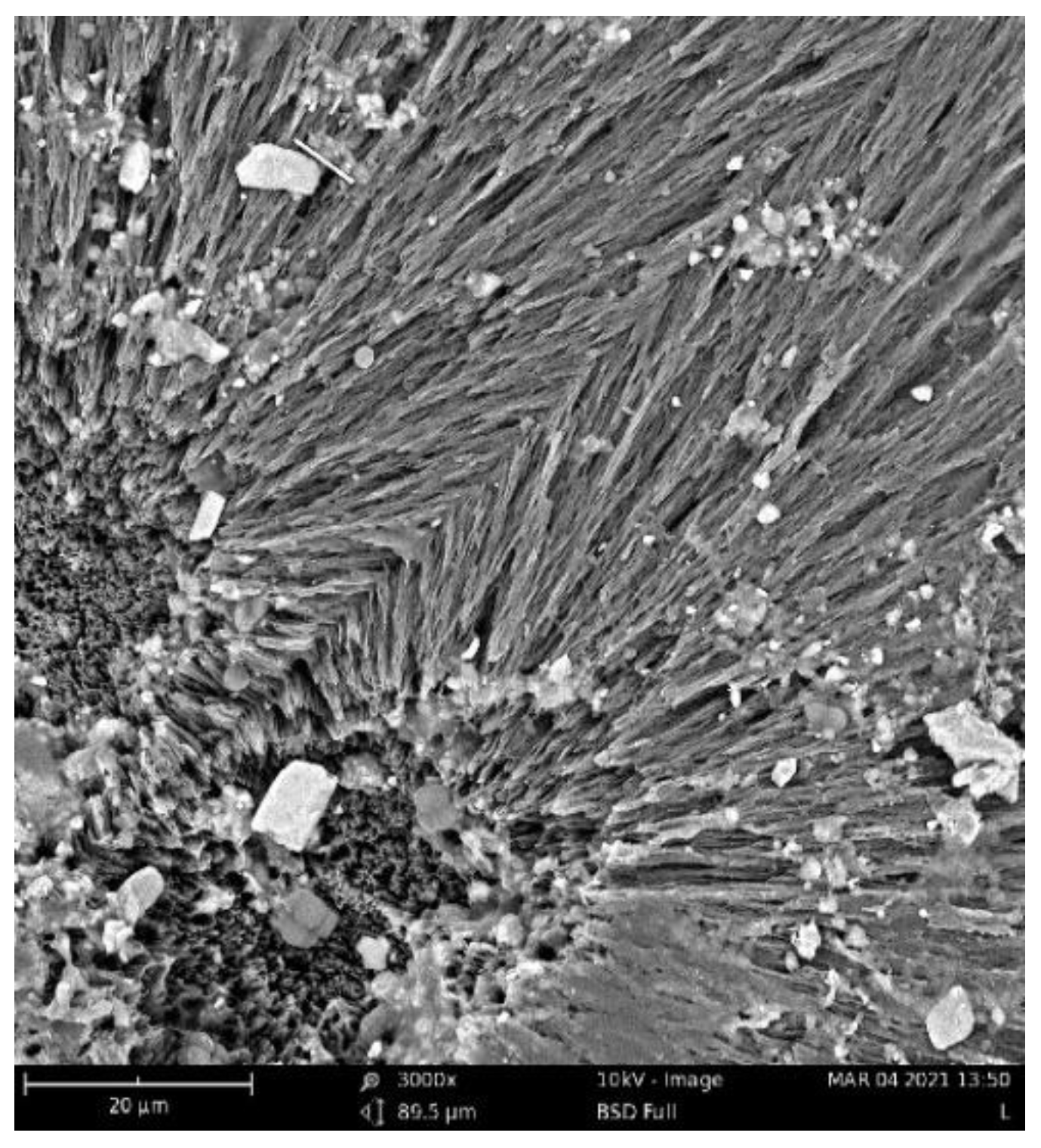
The biodegradation results were supported qualitatively by SEM images (Figure 3). These images show that pure PHB has a higher potential to degrade than that of the other samples, as is evident from its substantial biodeterioration. The material with 3% eucalyptol showed a relatively smooth surface, which could mean a higher level of degradation resistance than that of the other samples. Moreover, the sample with 3% thymol showed better resistance to degradation than did the sample with limonene and pure PHB, as depicted in Figure 2.
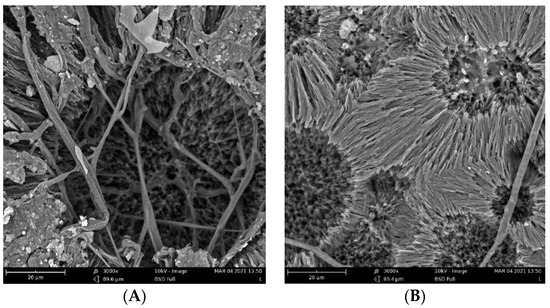
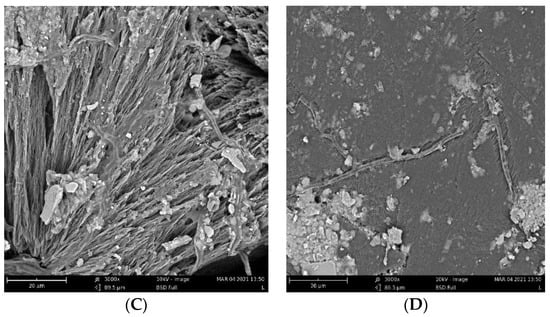
Figure 3.
SEM images of the samples after 16 days of incubation: (A) pure PHB, (B) limonene 3%, (C) thymol 3%, and (D) eucalyptol 3%.
Fungi hyphae are easily discernible in the pictures, supporting the assumption that fungi play an essential role in PHB biodegradation in soil [34,35,36]. The materials, however, were not covered by a dense biofilm of cells. The very distinct structure of the crystalline lamellae revealed during the biodegradation is also clearly visible and suggests that the biodegradation of highly crystalline PHB starts in the more amorphous matter in the center of the crystallites and between the lamellae.
2.4. DSC Analysis
Thermal parameters, such as melting point, glass transition temperature, and they crystallinity of the PHB samples were studied using differential scanning calorimetry (DSC). The obtained data are summarized in Table 1 and Table 2. As can be seen, the addition of the selected compounds caused a Tg reduction (Table 3). The decrease in Tg values showed that eucalyptol, thymol, and limonene were miscible with the amorphous phase of PHB and that they increased the molecular mobility of the polymer matrix [3]. The biggest Tg changes were observed in materials with limonene (29%). Furthermore, the Tm was evaluated. After the addition of the selected compounds, two melting transitions (Tm1 and Tm2) were observed in the melting region of the PHB blends, while pure PHB showed only one sharp maximum at 172 °C (Tm2). After partial biodegradation, two melting transition temperatures were observed in all of the tested compositions. This indicates that the prepared mixtures underwent the melting and recrystallization of their subphases. During biodegradation, only minor changes in the Tm were marked in the PHB modified by the terpenoids. Furthermore, all mixtures displayed a very weak degree of cold crystallization Tcc (Table 2). It was found that the Tcc moves slightly towards higher values (max. change of 3%) with increased biodegradation time. It seems that Tcc is affected significantly by neither terpenoid presence nor degradation time.

Table 1.
DSC: Crystallinity (second heating).

Table 2.
DMA: Complex modulus E* for PHB mixtures at 30 °C.

Table 3.
DSC results of PHB/terpenoid blends (second heating).
The crystallinity of the materials during their biodegradation was also studied with the help of DSC (Table 3). As shown in Figure 4, microorganisms started to degrade the materials from the amorphous part, so the crystalline parts were exposed. The DSC data indeed support this assumption (Table 1 and Table 2). As can be seen, pure PHB was substantially degraded after 16 days (Figure 3), which was faster than that of other samples. The drop in the crystallinity of the PHB at different degradation times was faster than in the PHB/terpenoid blends (Table 3), except for the composition with eucalyptol. At the beginning of the experiment and after 4 days of degradation, the crystallinity of the tested samples was similar (a max. change of 5% compared to pure PHB), but after 8 days, the samples blended with terpenoids exhibited higher values of crystallinity compared to the pure PHB samples (Table 3, Figure 5). In the case of the PHB/eucalyptol blend, a rapid decrease in crystallinity had already been observed after 4 days, but a renewed increase was also detected in the sample after 32 days. This phenomenon was asserted by Beltrami and her colleagues [36], who proposed that enzymatic degradation started first in the amorphous phase, thus increasing the crystallinity of the samples. The PHB/limonene mixture showed the most stable level of crystallinity over time. The explanation of this behavior may also be connected to the antimicrobial properties of terpenoids.
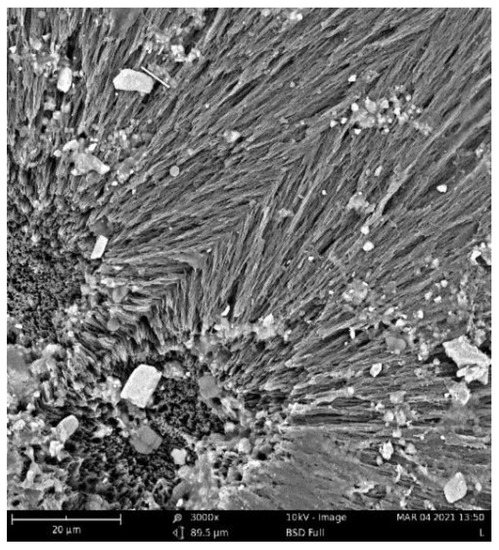
Figure 4.
SEM image of PHB/eucalyptol 3% after 32 days of incubation.
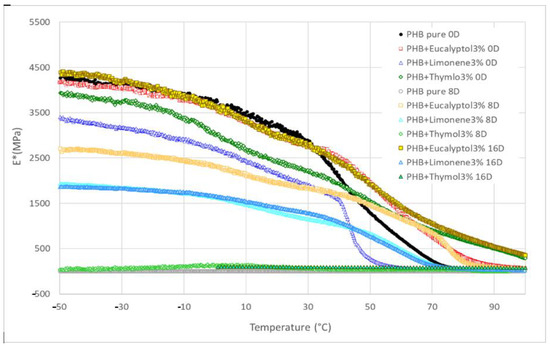
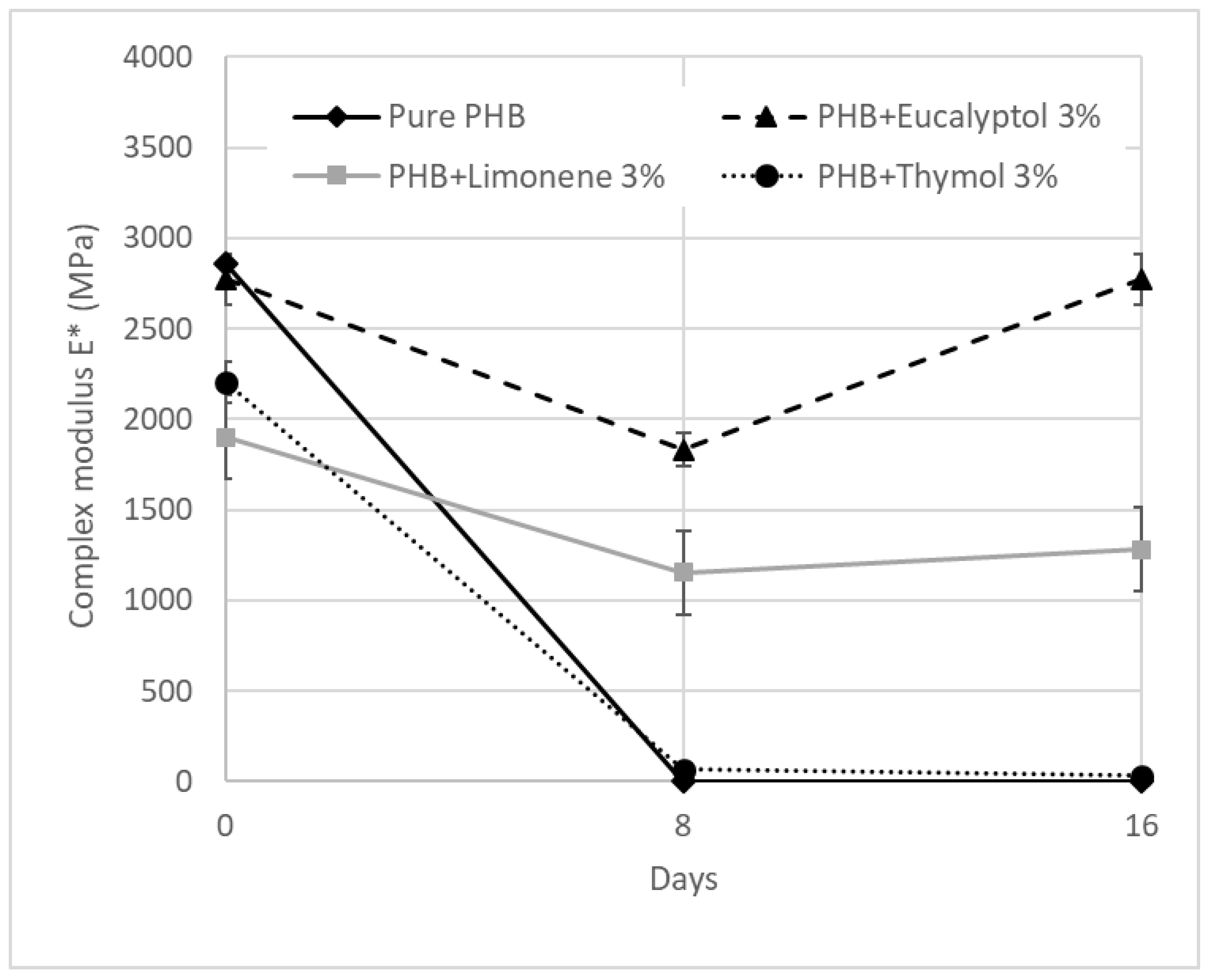
Figure 5.
DMA: Complex modulus E* for PHB mixtures before and after degradation.
2.5. Mechanical Properties
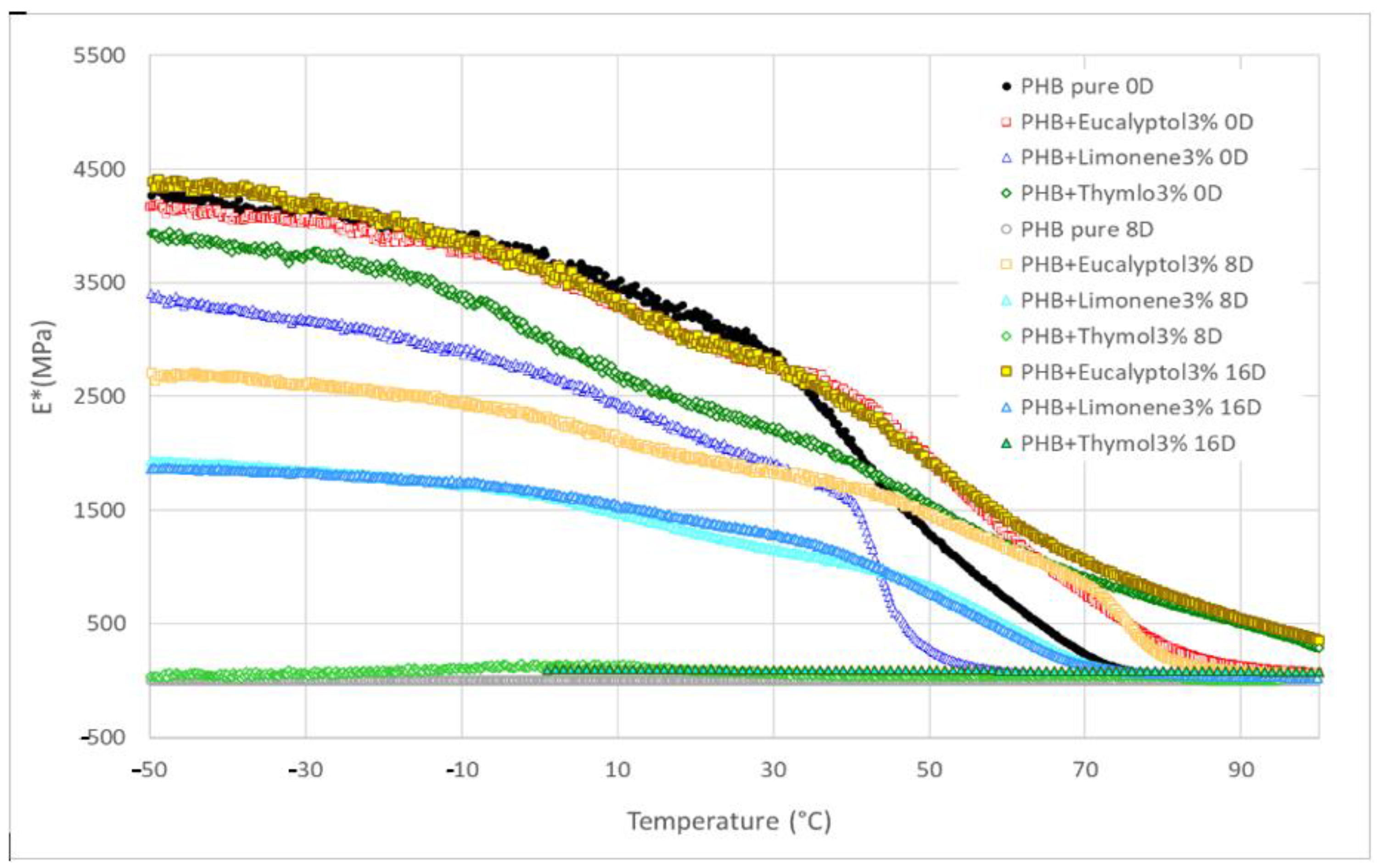
We used DMA to investigate the dynamic mechanical properties of the materials during biodegradation. Figure 6 presents the complex storage modulus E* trend for PHB compositions with terpenoids. In general, the E* remained constant and high in the glassy state and then dropped dramatically following the Tg before stabilizing in the rubbery state. The pure PHB and PHB with 3% limonene exhibited the fastest drop from a glassy to a rubbery state at the beginning of the experiment (0 days). Other compositions showed a more gradual decline during the thermal-mechanical exposition on day 0.
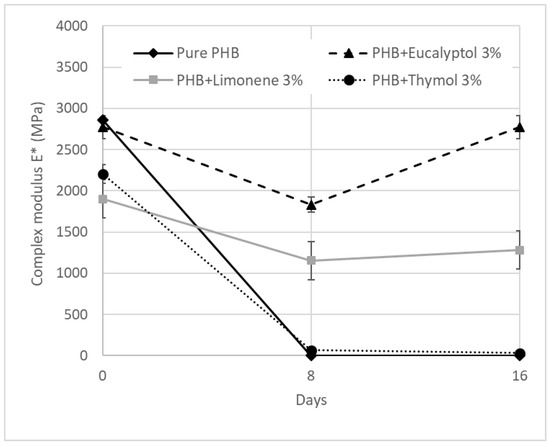
Figure 6.
DMA: Complex modulus E* for PHB mixtures at 30 °C.
The E* values for 30 °C are summarized in Table 3 and Figure 5. The complex modulus (Figure 6) decreased after adding terpenoids. The most significant drop was observed in the samples containing limonene. These data are in correlation with the DSC measurements. Terpenoids act like plasticizers in the PHB matrix and could decrease intermolecular stresses throughout the polymer chain, enhancing chain mobility and flexibility [30]. During incubation in the soil, another decrease occurred. After 8 days, the pure PHB showed shallow values for E*, close to 0 MPa. The composition with thymol also exhibited very low values for E* modulus. For this material, a further decline was recorded between 8 and 16 days of degradation. The material lost 99% of the E* modulus when compared to the same material before degradation.
On the other hand, stable E* values were exhibited by the material containing eucalyptol. This effect could relate to the antimicrobial properties of eucalyptol and the higher stability of these materials in the soil environment. During biodegradation, PHB with 3% limonene showed a decrease in the E* modulus of about 30%. The value was then stable after 8 and 16 days of incubation. Furthermore, a very small increase after 16 days was observed in this mixture. This effect could be related to the increasing degree of crystallinity, which could have enhanced the rigidity of the samples because the growing level of crystallinity led to a restriction of movement in the polymer chains [37]. Another reason for this behavior could be connected to terpenoid loss during incubation in soil.
3. Materials and Methods
3.1. Materials
The PHB used in this research was in powder form, and it was obtained from Tiatan Biologic Materials Co, Ltd. (Beilun, Ningbo, China) with a molecular weight of 66,500 g mol−1. The natural compounds used as additives to the PHB in this study were purchased from Sigma Aldrich and are summarized in Table 4.

Table 4.
The properties of essential oils blended with PHB.
3.2. Sample Preparation
The additives were mixed well in a bowl with pure PHB powder to reach the given concentrations before being loaded into the micro-extruder. A twin-screw micro-extruder (HAAKE Minilab, Thermo Fisher Scientific, Waltham, MA, USA) was used in this study. The processing temperature was set at 185 °C, with a speed of 50 rpm. A period of 2 min of mixing at the given conditions was evaluated as being sufficient and did not induce unwanted degradation of the material. Before the extrusion, the PHB was dried for 10 h at 100 °C. Then, compression molding was performed at the same temperature as that of the micro-extruder (185 °C) to make the films. The heating time was about 2 min, and after that, the films cooled down to room temperature for about 10 min. The specimens’ thickness was 120–130 m, and the dimensions were 50mm × 300 mm. The same process was also used to prepare the pure PHB films used as controls.
3.3. Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) analysis of the samples was performed on a DSC1/700 analyzer (Mettler Toledo, Columbus, OH, USA) containing a mechanical cooling system. The measurement was performed in a nitrogen atmosphere and calibrated by an indium standard. DSC was used to measure melting point, crystallinity, and Tg. The samples were placed in a DSC aluminum pan and scanned between −40 and 200 °C. First, the samples were heated from −40 to 200 °C with a heating rate of 10 °C min−1. This process was followed by cooling from 200 to −40 °C with a cooling rate of −10 °C min−1. In this method, two heating processes were introduced to the material. The initial heating scan was performed to erase the sample’s previous thermal history, and the second heating cycle of the DSC curves yielded the thermal characteristics.
3.4. Dynamic Mechanical Analysis
Dynamic mechanical analysis (DMA) was performed on a DMA Analyzer (Mettler Toledo, Columbus, OH, USA). The temperature ranged from −40 to 100 °C; the film-tension mode had a heating rate of 2 °C min−1 with a frequency of 1 Hz and a load of 0.5 N. The DMA analysis of the samples was carried out before the burial test, as well as 8 days after the burial test.
3.5. Biodegradation in Soil
When organic materials degrade aerobically, oxygen is consumed, and carbon is transformed to gaseous carbon dioxide (CO2). The proportion of solid organic carbon in the test sample, transforming into CO2, quantifies the mineralization. Tests were carried out in 500 mL biometric flasks. Polymer film samples were cut into 2 mm pieces (50 mg); 15 g of dry soil, a mineral medium (11 mL) [32], and 5 g of perlite were all placed in flasks (ISO 17556).
Mineralization percentage (Dt) was calculated as:
where (CO2)t is the accumulated CO2 released by each sample, (CO2)b is the accumulated CO2 released by the blank flasks, and ThCO2 is the test material’s theoretical carbon dioxide in the test flasks. A mass spectrometer HPR-40 DSA (HIDEN Analytical, 2020, Warrington, UK) was used to assess the released carbon dioxide. For each sample, three parallel flasks were used, along with four blank flasks.
3.6. Scanning Electron Microscopy
Scanning electron microscopy (Phenom Pro Desktop SEM, Thermo Fisher Scientific, Waltham, MA, USA) was implemented to observe surface changes on films in high vacuum mode at an acceleration voltage of 10 kV. The samples were coated with a thin layer of Au/Pd to reduce sample damage and prevent charging.
4. Conclusions
This research studied the effect of blending monoterpenes into PHB. These compounds were selected from 10 terpenoids with previously proven antimicrobial properties. The preliminary study identified eucalyptol, limonene, and thymol as being the most effective in retarding the biodegradation of the PHB-based materials in the soil. The thermal and mechanical properties of the materials and their evolution during biodegradation were also studied. Eucalyptol was evaluated as being the most effective in retarding biodegradation during the biodegradation test. Limonene and thymol also provided some resistance against biodegradation (more than a 35% reduction under some conditions). The most probable reason for this was the antimicrobial activity of these compounds. Thus, it is possible to use eucalyptol, an available compound, to increase the necessary resistance of biodegradable materials based on PHB that are in contact with soil, e.g., in agricultural applications. Under the laboratory conditions supporting the maximal biodegradation rate, this material was stable for about 32 days. One might assume that under real field conditions, an even longer time could be expected. Furthermore, the combination of PHB with eucalyptol seems to offer comparable values of complex modulus with pure PHB, but for a longer time during biodegradation. In the case of the PHB blended with limonene, a decrease in the mechanical properties of about 30% was marked at the beginning of the biodegradation. After this decrease, the material seemed to be relatively stable during the tested period. These additives are fully biobased and do not prohibit the ultimate biodegradation of the material at the end of its service life.
Author Contributions
Conceptualization, A.F.; Data curation, A.K.; Investigation, A.F. and A.K.; Methodology, A.F., M.J., D.Š., M.K. (Marek Koutný), A.K. and M.K. (Markéta Kadlečková); Project administration, M.K. (Marek Koutný); Supervision, M.K. (Marek Koutný). All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under European Union’s Horizon 2020 Research and Innovation Program under grant agreement No 862910(SEALIVE), and under the Internal Grant Agency of Tomas Bata University in Zlín (IGA/FT/2020/005, IGA/FT/2020/009, and IGA/FT/2022/003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Emmerich, L.; Wu, J.; Vana, P.; Zhang, K. Hydroplastic polymers as eco-friendly hydrosetting plastics. Nat. Sustain. 2021, 4, 877–883. [Google Scholar] [CrossRef]
- Fogašová, M.; Figalla, S.; Danišová, L.; Medlenová, E.; Hlaváčiková, S.; Bočkaj, J.; Plavec, R.; Alexy, P.; Repiská, M.; Přikryl, R.; et al. PLA/PHB-Based Materials Fully Biodegradable under Both Industrial and Home-Composting Conditions. Polymers 2022, 14, 4113. [Google Scholar] [CrossRef] [PubMed]
- Mangeon, C.; Michely, L.; Rios De Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural Terpenes Used as Plasticizers for Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015, 370164. [Google Scholar] [CrossRef]
- Rech, C.R.; da Silva Brabes, K.C.; Bagnara e Silva, B.E.; Bittencourt, P.R.S.; Koschevic, M.T.; da Silveira, T.F.S.; Martines, M.A.U.; Caon, T.; Martelli, S.M. Biodegradation of eugenol-loaded polyhydroxybutyrate films in different soil types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Šerá, J.; Kadlečková, M.; Fayyazbakhsh, A.; Kučabová, V.; Koutný, M. Occurrence and analysis of thermophilic poly(Butylene adipate-co-terephthalate)-degrading microorganisms in temperate zone soils. Int. J. Mol. Sci. 2020, 21, 7857. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Yan, X.; Yu, L.P.; Liu, X.Y.; Chen, G.Q. Hyperproduction of 3-hydroxypropionate by Halomonas bluephagenesis. Nat. Commun. 2021, 12, 1513. [Google Scholar] [CrossRef]
- Gao, M.; Du, D.; Bo, Z.; Sui, L. Poly-β-hydroxybutyrate (PHB)-accumulating Halomonas improves the survival, growth, robustness and modifies the gut microbial composition of Litopenaeus vannamei postlarvae. Aquaculture 2019, 500, 607–612. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.-G. Alternative optimization routes for improving the performance of poly(3-hydroxybutyrate) (PHB) based plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.J.; Moon, M.; Lee, J.-S.; Min, K. Recent progress and challenges in microbial polyhydroxybutyrate (PHB) production from CO2 as a sustainable feedstock: A state-of-the-art review. Bioresour. Technol. 2021, 339, 125616. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yang, Y.H.; Choi, K.Y. One-pot production of thermostable PHB biodegradable polymer by co-producing bio-melanin pigment in engineered Escherichia coli. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Agnelli, J.A.M.; Belem, L.P. Thermal, mechanical and morphological properties of poly (hydroxybutyrate) and polypropylene blends after processing. Mater. Res. 2009, 12, 159–164. [Google Scholar] [CrossRef]
- Aydemir, D.; Gardner, D.J. Biopolymer blends of polyhydroxybutyrate and polylactic acid reinforced with cellulose nanofibrils. Carbohydr. Polym. 2020, 250, 116867. [Google Scholar] [CrossRef] [PubMed]
- Mohanrasu, K.; Premnath, N.; Siva Prakash, G.; Sudhakar, M.; Boobalan, T.; Arun, A. Exploring multi potential uses of marine bacteria; an integrated approach for PHB production, PAHs and polyethylene biodegradation. J. Photochem. Photobiol. B Biol. 2018, 185, 55–65. [Google Scholar] [CrossRef]
- Robledo-Ortíz, J.R.; González-López, M.E.; Martín del Campo, A.S.; Pérez-Fonseca, A.A. Lignocellulosic Materials as Reinforcement of Polyhydroxybutyrate and its Copolymer with Hydroxyvalerate: A Review. J. Polym. Environ. 2021, 29, 1350–1364. [Google Scholar] [CrossRef]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Nigmatullin, R.; Paxinou, A.; Ahmad, M.H.; Gurumayum, B.; Roy, I. Antimicrobial materials with lime oil and a poly(3-hydroxyalkanoate) produced via valorisation of sugar cane molasses. J. Funct. Biomater. 2020, 11, 24. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Ogura, T.; Ito, Y.; Nishida, M. Multi-scale instrumental analyses of plasticized polyhydroxyalkanoates (PHA) blended with polycaprolactone (PCL) and the effects of crosslinkers and graft copolymers. RSC Adv. 2019, 9, 1551–1561. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2020, 29, 1202–1211. [Google Scholar] [CrossRef]
- Miao, L.; Walton, W.C.; Wang, L.; Li, L.; Wang, Y. Characterization of polylactic acids-polyhydroxybutyrate based packaging film with fennel oil, and its application on oysters. Food Packag. Shelf Life 2019, 22, 100388. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Yang, Y.; Xu, S.; Wong, E.H.H.; Boyer, C. Antibiofilm Platform based on the Combination of Antimicrobial Polymers and Essential Oils. Biomacromolecules 2020, 21, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ventura, H.; Laguna-Gutiérrez, E.; Rodriguez-Perez, M.A.; Ardanuy, M. Effect of chain extender and water-quenching on the properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) foams for its production by extrusion foaming. Eur. Polym. J. 2016, 85, 14–25. [Google Scholar] [CrossRef]
- Duangphet, S.; Szegda, D.; Song, J.; Tarverdi, K. The Effect of Chain Extender on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Thermal Degradation, Crystallization, and Rheological Behaviours. J. Polym. Environ. 2014, 22, 1–8. [Google Scholar] [CrossRef]
- Narayanan, A.; Neera; Mallesha; Ramana, K.V. Synergized antimicrobial activity of eugenol incorporated polyhydroxybutyrate films against food spoilage microorganisms in conjunction with pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.Á.R.; de Sousa, J.P.; da Costa Medeiros, J.A.; de Figueiredo, R.C.B.Q.; Magnani, M.; de Siqueira, J.P.; de Souza, E.L. Synergistic inhibition of bacteria associated with minimally processed vegetables in mixed culture by carvacrol and 1,8-cineole. Food Control. 2015, 47, 334–339. [Google Scholar] [CrossRef]
- Monistero, V.; Barberio, A.; Biscarini, F.; Cremonesi, P.; Castiglioni, B.; Graber, H.U.; Bottini, E.; Ceballos-Marquez, A.; Kroemker, V.; Petzer, I.M.; et al. Different distribution of antimicrobial resistance genes and virulence profiles of Staphylococcus aureus strains isolated from clinical mastitis in six countries. J. Dairy Sci. 2020, 103, 3431–3446. [Google Scholar] [CrossRef]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; De Carvalho, M.G.; Costa, R.A.; Catunda, F.E.A. In vitro antibacterial and antibiofilm activity of lippia alba essential oil, citral, and carvone against staphylococcus aureus. Sci. World J. 2017, 2017, 4962707. [Google Scholar] [CrossRef]
- Huang, F.; Kong, J.; Ju, J.; Zhang, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Xie, Y.; Yao, W. Membrane damage mechanism contributes to inhibition of trans-cinnamaldehyde on Penicillium italicum using Surface-Enhanced Raman Spectroscopy (SERS). Sci. Rep. 2019, 9, 490. [Google Scholar] [CrossRef]
- Beltrami, L.V.R.; Bandeira, J.A.V.; Scienza, L.C.; Zattera, A.J. Biodegradable composites: Morphological, chemical, thermal, and mechanical properties of composites of poly(hydroxybutyrate-co-hydroxyvalerate) with curaua fibers after exposure to simulated soil. J. Appl. Polym. Sci. 2014, 131, 8769–8776. [Google Scholar] [CrossRef]
- da Costa, R.C.; Daitx, T.S.; Mauler, R.S.; da Silva, N.M.; Miotto, M.; Crespo, J.S.; Carli, L.N. Poly(hydroxybutyrate-co-hydroxyvalerate)-based nanocomposites for antimicrobial active food packaging containing oregano essential oil. Food Packag. Shelf Life 2020, 26, 100602. [Google Scholar] [CrossRef]
- Marcet, I.; Weng, S.; Sáez-Orviz, S.; Rendueles, M.; Díaz, M. Production and characterisation of biodegradable PLA nanoparticles loaded with thymol to improve its antimicrobial effect. J. Food Eng. 2018, 239, 26–32. [Google Scholar] [CrossRef]
- Šašinková, D.; Serbruyns, L.; Julinová, M.; FayyazBakhsh, A.; De Wilde, B.; Koutný, M. Evaluation of the biodegradation of polymeric materials in the freshwater environment—An attempt to prolong and accelerate the biodegradation experiment. Polym. Degrad. Stab. 2022, 203, 110085. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Swiontek Brzezinka, M.; Richert, A.; Kalwasińska, A.; Świątczak, J.; Deja-Sikora, E.; Walczak, M.; Michalska-Sionkowska, M.; Piekarska, K.; Kaczmarek-Szczepańska, B. Microbial degradation of polyhydroxybutyrate with embedded polyhexamethylene guanidine derivatives. Int. J. Biol. Macromol. 2021, 187, 309–318. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.; Alves, M.M.; Vicente, A.A. Factors affecting polyhydroxyalkanoates biodegradation in soil. Polym. Degrad. Stab. 2020, 182, 109408. [Google Scholar] [CrossRef]
- Sharma, B.; Jain, P. Deciphering the advances in bioaugmentation of plastic wastes. J. Clean. Prod. 2020, 275, 123241. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.c.; Huang, S.; Mei, C.; Wu, Q. Enhancing mechanical properties of poly(lactic acid) through its in-situ crosslinking with maleic anhydride-modified cellulose nanocrystals from cottonseed hulls. Ind. Crops Prod. 2018, 112, 449–459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).