Dipeptidyl Peptidase-4 Inhibitor-Related Bullous Pemphigoid: Clinical, Laboratory, and Histological Features, and Possible Pathogenesis
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients with DBP and IBP
2.2. Pathological Assessment of Patients with DBP and IBP
2.3. Heatmap of Expressed Genes Related to the Dermal–Epidermal Junction in DPP4i-Treated vs. Vehicle-Treated Primary Keratinocytes
2.4. Vildagliptin Decreased the Expression of DYS and Collagen 17A1 in a Time-Dependent Manner
2.5. Vildagliptin Decreased the Expression of LAMA3, LAMB3, and LAMC2 in a Time-Dependent Manner
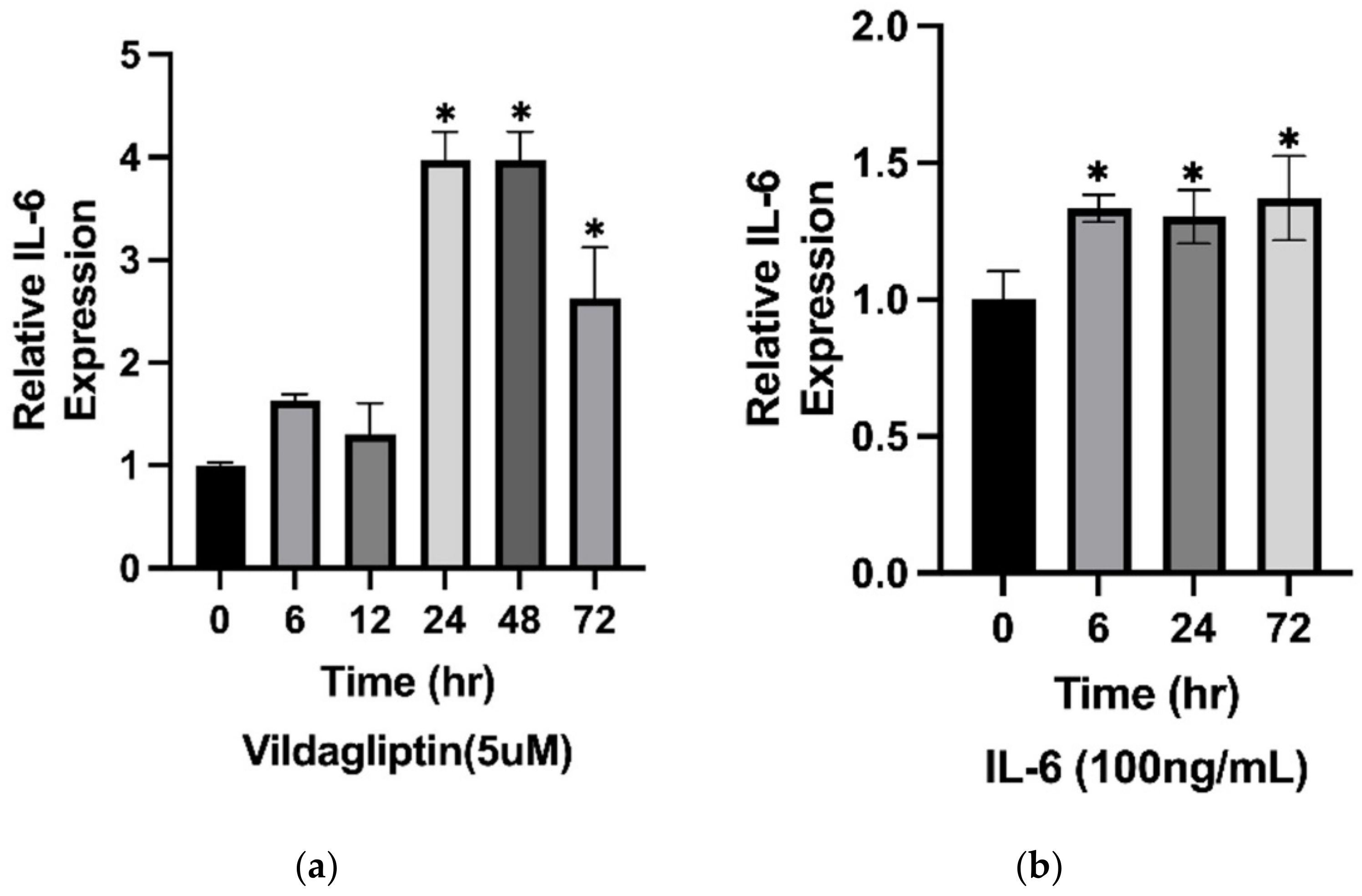
2.6. The Expression of IL-6 Was Stimulated by Vildagliptin-Treated HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Evaluation of Clinical Characteristics of BP
4.3. Laboratory Findings
4.4. Histology and Immunopathology
4.5. Cell Culture
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genovese, G.; Di Zenzo, G.; Cozzani, E.; Berti, E.; Cugno, M.; Marzano, A.V. New Insights Into the Pathogenesis of Bullous Pemphigoid: 2019 Update. Front. Immunol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Stavropoulos, P.G.; Soura, E.; Antoniou, C. Drug-induced pemphigoid: A review of the literature. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.L.; Chen, Y.L.; Lin, K.T.; Chiang, C.P.; Chung, C.H.; Hung, C.T.; Lin, F.H.; Tsao, C.H.; Chien, W.C.; Wang, W.M. Risk of radiotherapy-associated autoimmune bullous disease among Taiwanese patients with breast cancer: A case-control study. Arch Dermatol. Res. 2020, 312, 69–75. [Google Scholar] [CrossRef]
- Tan, C.W.; Pang, Y.; Sim, B.; Thirumoorthy, T.; Pang, S.M.; Lee, H.Y. The association between drugs and bullous pemphigoid. Br. J. Dermatol. 2017, 176, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.D.; Chen, W.T.; Chi, C.C. Association Between Medication Use and Bullous Pemphigoid: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Bastuji-Garin, S.; Joly, P.; Picard-Dahan, C.; Bernard, P.; Vaillant, L.; Pauwels, C.; Salagnac, V.; Lok, C.; Roujeau, J.C. Drugs associated with bullous pemphigoid. A case-control study. Arch. Dermatol. 1996, 132, 272–276. [Google Scholar] [CrossRef]
- Bastuji-Garin, S.; Joly, P.; Lemordant, P.; Sparsa, A.; Bedane, C.; Delaporte, E.; Roujeau, J.C.; Bernard, P.; Guillaume, J.C.; Ingen-Housz-Oro, S.; et al. Risk factors for bullous pemphigoid in the elderly: A prospective case-control study. J. Investig. Dermatol. 2011, 131, 637–643. [Google Scholar] [CrossRef]
- Verheyden, M.J.; Bilgic, A.; Murrell, D.F. A Systematic Review of Drug-Induced Pemphigoid. Acta Derm.-Venereol. 2020, 100, adv00224. [Google Scholar] [CrossRef]
- American Diabetes, A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S90–S102. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Bergman, R. Association of Bullous Pemphigoid With Dipeptidyl-Peptidase 4 Inhibitors in Patients With Diabetes: Estimating the Risk of the New Agents and Characterizing the Patients. JAMA Dermatol. 2018, 154, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Varpuluoma, O.; Forsti, A.K.; Jokelainen, J.; Turpeinen, M.; Timonen, M.; Huilaja, L.; Tasanen, K. Vildagliptin Significantly Increases the Risk of Bullous Pemphigoid: A Finnish Nationwide Registry Study. J. Investig. Dermatol. 2018, 138, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Shimauchi, R.; Nishibori, N.; Kawashima, K.; Oshitani, S.; Fujiya, A.; Shibata, T.; Ohashi, N.; Izumi, K.; Nishie, W.; et al. Dipeptidyl peptidase-4 inhibitors-associated bullous pemphigoid: A retrospective study of 168 pemphigoid and 9304 diabetes mellitus patients. J. Diabetes Investig. 2019, 10, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Charlton, O.; Smith, S.D. Dipeptidyl peptidase-4 inhibitors and bullous pemphigoid: A systematic review and adjusted meta-analysis. Australas J. Dermatol. 2020, 61, e15–e21. [Google Scholar] [CrossRef]
- Skandalis, K.; Spirova, M.; Gaitanis, G.; Tsartsarakis, A.; Bassukas, I.D. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 249–253. [Google Scholar] [CrossRef]
- Hung, C.T.; Liu, J.S.; Cheng, C.Y.; Chung, C.H.; Chiang, C.P.; Chien, W.C.; Wang, W.M. Increased risk of bullous pemphigoid in dipeptidyl peptidase 4 inhibitors: A nationwide, population-based, cohort study in Taiwan. J. Dermatol. 2020, 47, 245–250. [Google Scholar] [CrossRef]
- Sugiyama, S.; Yamamoto, T.; Aoyama, Y. Clinical features of dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid in Japan: A nationwide retrospective observational study. J. Dermatol. 2022, 49, 697–702. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Wu, C.; Zhou, Y.; Wang, C. Analysis of the Clinical Characteristics of Dipeptidyl Peptidase-4 Inhibitor-Induced Bullous Pemphigoid. Ann. Pharmacother. 2022, 56, 205–212. [Google Scholar] [CrossRef]
- Izumi, K.; Nishie, W.; Mai, Y.; Wada, M.; Natsuga, K.; Ujiie, H.; Iwata, H.; Yamagami, J.; Shimizu, H. Autoantibody Profile Differentiates between Inflammatory and Noninflammatory Bullous Pemphigoid. J. Investig. Dermatol. 2016, 136, 2201–2210. [Google Scholar] [CrossRef]
- Chijiwa, C.; Takeoka, S.; Kamata, M.; Tateishi, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; Ohnishi, T.; et al. Decrease in eosinophils infiltrating into the skin of patients with dipeptidyl peptidase-4 inhibitor-related bullous pemphigoid. J. Dermatol. 2018, 45, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Tuusa, J.; Kokkonen, N.; Mattila, A.; Huilaja, L.; Varpuluoma, O.; Rannikko, S.; Glumoff, V.; Miettunen, J.; Tasanen, K. Dipeptidyl peptidase 4 InhibitorAssociated Bullous Pemphigoid Is Characterized by an Altered Expression of Cytokines in the Skin. J. Investig. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nishie, W.; Tasanen, K. Gliptin-Associated Bullous Pemphigoid: A Valuable Model of the Mechanism of Breakdown of Immune Tolerance against BP180. J. Investig. Dermatol. 2019, 139, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Perez White, B.E.; Patel, P.M.; Amber, K.T. Inhibition of dipeptidyl-peptidase 4 induces upregulation of the late cornified envelope cluster in keratinocytes. Arch. Dermatol. Res. 2022, 314, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Giusti, D.; Le Jan, S.; Gatouillat, G.; Bernard, P.; Pham, B.N.; Antonicelli, F. Biomarkers related to bullous pemphigoid activity and outcome. Exp. Dermatol. 2017, 26, 1240–1247. [Google Scholar] [CrossRef]
- Ou, X.; O’Leary, H.A.; Broxmeyer, H.E. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood 2013, 122, 161–169. [Google Scholar] [CrossRef]
- Chanprapaph, K.; Pratumchart, N.; Limtong, P.; Rutnin, S.; Sukasem, C.; Kungvalpivat, P.; Triamchaisri, S.; Suchonwanit, P. Dipeptidyl peptidase-4 inhibitor-related bullous pemphigoid: A comparative study of 100 patients with bullous pemphigoid and diabetes mellitus. J. Dermatol. 2021, 48, 486–496. [Google Scholar] [CrossRef]
- Tasanen, K.; Varpuluoma, O.; Nishie, W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Front. Immunol. 2019, 10, 1238. [Google Scholar] [CrossRef]
- Plaquevent, M.; Tetart, F.; Fardet, L.; Ingen-Housz-Oro, S.; Valeyrie-Allanore, L.; Bernard, P.; Hebert, V.; Roussel, A.; Avenel-Audran, M.; Chaby, G.; et al. Higher Frequency of Dipeptidyl Peptidase-4 Inhibitor Intake in Bullous Pemphigoid Patients than in the French General Population. J. Investig. Dermatol. 2019, 139, 835–841. [Google Scholar] [CrossRef]
- Ujiie, H.; Muramatsu, K.; Mushiroda, T.; Ozeki, T.; Miyoshi, H.; Iwata, H.; Nakamura, A.; Nomoto, H.; Cho, K.Y.; Sato, N.; et al. HLA-DQB1*03:01 as a Biomarker for Genetic Susceptibility to Bullous Pemphigoid Induced by DPP-4 Inhibitors. J. Investig. Dermatol. 2018, 138, 1201–1204. [Google Scholar] [CrossRef]
- Horikawa, H.; Kurihara, Y.; Funakoshi, T.; Umegaki-Arao, N.; Takahashi, H.; Kubo, A.; Tanikawa, A.; Kodani, N.; Minami, Y.; Meguro, S.; et al. Unique clinical and serological features of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors. Br. J. Dermatol. 2018, 178, 1462–1463. [Google Scholar] [CrossRef] [PubMed]
- Salemme, A.; Fania, L.; Scarabello, A.; Caproni, M.; Marzano, A.V.; Cozzani, E.; Feliciani, C.; De Simone, C.; Papini, M.; Satta, R.R.; et al. Gliptin-associated bullous pemphigoid shows peculiar features of anti-BP180 and -BP230 humoral response: Results of a multicenter study. J. Am. Acad. Dermatol. 2022, 87, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kinyo, A.; Hanyecz, A.; Lengyel, Z.; Varszegi, D.; Olah, P.; Gyomorei, C.; Kalman, E.; Berki, T.; Gyulai, R. Clinical, Laboratory and Histological Features of Dipeptidyl Peptidase-4 Inhibitor Related Noninflammatory Bullous Pemphigoid. J. Clin. Med. 2021, 10, 1916. [Google Scholar] [CrossRef]
- Patsatsi, A.; Kyriakou, A.; Meltzanidou, P.; Trigoni, A.; Lamprou, F.; Kokolios, M.; Giannakou, A. Betaullous pemphigoid in patients with DPP-4 inhibitors at the onset of disease: Does this differ from common bullous pemphigoid? Eur. J. Dermatol. 2018, 28, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Salemme, A.; Provini, A.; Pagnanelli, G.; Collina, M.C.; Abeni, D.; Didona, B.; Di Zenzo, G.; Mazzanti, C. Detection and characterization of IgG, IgE, and IgA autoantibodies in patients with bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors. J. Am. Acad. Dermatol. 2018, 78, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diez, I.; Ivars-Lleo, M.; Lopez-Aventin, D.; Ishii, N.; Hashimoto, T.; Iranzo, P.; Pujol, R.M.; Espana, A.; Herrero-Gonzalez, J.E. Bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. Eight cases with clinical and immunological characterization. Int. J. Dermatol. 2018, 57, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, T.; Merlo, S.; Spampinato, S.F.; Pasquale, R.D.; Sortino, M.A. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front. Pharmacol. 2015, 6, 126. [Google Scholar] [CrossRef]
- Shiobara, T.; Chibana, K.; Watanabe, T.; Arai, R.; Horigane, Y.; Nakamura, Y.; Hayashi, Y.; Shimizu, Y.; Takemasa, A.; Ishii, Y. Dipeptidyl peptidase-4 is highly expressed in bronchial epithelial cells of untreated asthma and it increases cell proliferation along with fibronectin production in airway constitutive cells. Respir. Res. 2016, 17, 28. [Google Scholar] [CrossRef]
- Patel, P.M.; Jones, V.A.; Kridin, K.; Amber, K.T. The role of Dipeptidyl Peptidase-4 in cutaneous disease. Exp. Dermatol. 2021, 30, 304–318. [Google Scholar] [CrossRef]
- Gines, S.; Marino, M.; Mallol, J.; Canela, E.I.; Morimoto, C.; Callebaut, C.; Hovanessian, A.; Casado, V.; Lluis, C.; Franco, R. Regulation of epithelial and lymphocyte cell adhesion by adenosine deaminase-CD26 interaction. Biochem. J. 2002, 361, 203–209. [Google Scholar] [CrossRef]
- Muramatsu, K.; Zheng, M.; Yoshimoto, N.; Ito, T.; Ujiie, I.; Iwata, H.; Shimizu, H.; Ujiie, H. Regulatory T cell subsets in bullous pemphigoid and dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. J. Dermatol. Sci. 2020, 100, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gronow, M.; Grenett, H.E.; Weber, M.R.; Gawdi, G.; Pizzo, S.V. Interaction of plasminogen with dipeptidyl peptidase IV initiates a signal transduction mechanism which regulates expression of matrix metalloproteinase-9 by prostate cancer cells. Biochem. J. 2001, 355, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Nishie, W.; Lamer, S.; Schlosser, A.; Licarete, E.; Franzke, C.W.; Hofmann, S.C.; Jackow, J.; Sitaru, C.; Bruckner-Tuderman, L. Ectodomain shedding generates Neoepitopes on collagen XVII, the major autoantigen for bullous pemphigoid. J. Immunol. 2010, 185, 4938–4947. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, A.; Bruckner-Tuderman, L.; Chapple, I.L.C.; Fine, J.D.; Harper, N.; Has, C.; Magin, T.M.; Marinkovich, M.P.; Marshall, J.F.; McGrath, J.A.; et al. Epidermolysis bullosa. Nat. Rev. Dis. Primers 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Liu, L.; Bolling, M.C.; Charlesworth, A.V.; El Hachem, M.; Escamez, M.J.; Fuentes, I.; Buchel, S.; Hiremagalore, R.; Pohla-Gubo, G.; et al. Clinical practice guidelines for laboratory diagnosis of epidermolysis bullosa. Br. J. Dermatol. 2020, 182, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Egami, S.; Yamagami, J.; Amagai, M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J. Allergy Clin. Immunol. 2020, 145, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Ellebrecht, C.T.; Maseda, D.; Payne, A.S. Pemphigus and Pemphigoid: From Disease Mechanisms to Druggable Pathways. J. Investig. Dermatol. 2022, 142, 907–914. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Kowalski, E.H.; Kneibner, D.; Kridin, K.; Amber, K.T. Serum and blister fluid levels of cytokines and chemokines in pemphigus and bullous pemphigoid. Autoimmun. Rev. 2019, 18, 526–534. [Google Scholar] [CrossRef]
- Schmidt, E.; Reimer, S.; Kruse, N.; Jainta, S.; Brocker, E.B.; Marinkovich, M.P.; Giudice, G.J.; Zillikens, D. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J. Investig. Dermatol. 2000, 115, 842–848. [Google Scholar] [CrossRef]
- Sasaoka, T.; Ujiie, H.; Nishie, W.; Iwata, H.; Ishikawa, M.; Higashino, H.; Natsuga, K.; Shinkuma, S.; Shimizu, H. Intravenous IgG Reduces Pathogenic Autoantibodies, Serum IL-6 Levels, and Disease Severity in Experimental Bullous Pemphigoid Models. J. Investig. Dermatol. 2018, 138, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xu, Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef] [PubMed]
- Murrell, D.F.; Daniel, B.S.; Joly, P.; Borradori, L.; Amagai, M.; Hashimoto, T.; Caux, F.; Marinovic, B.; Sinha, A.A.; Hertl, M.; et al. Definitions and outcome measures for bullous pemphigoid: Recommendations by an international panel of experts. J. Am. Acad. Dermatol 2012, 66, 479–485. [Google Scholar] [CrossRef]
- Roufosse, F.; Weller, P.F. Practical approach to the patient with hypereosinophilia. J. Allergy Clin. Immunol. 2010, 126, 39–44. [Google Scholar] [CrossRef] [PubMed]
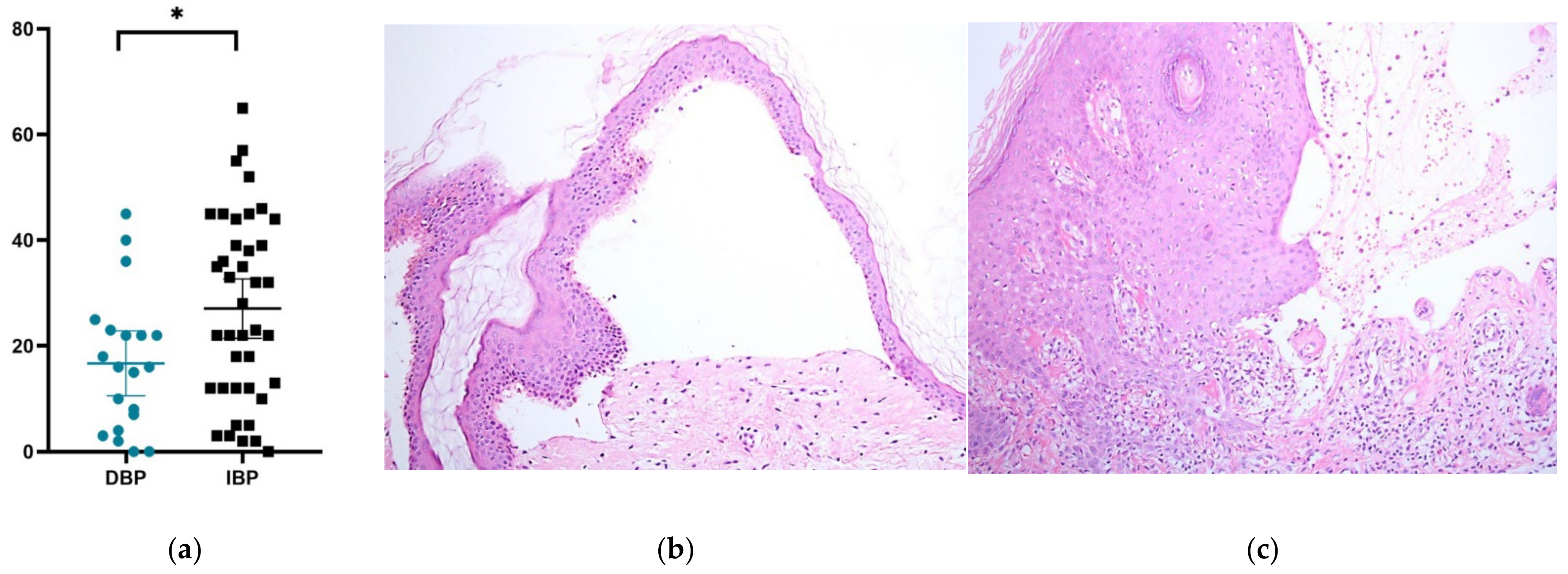



| Characteristics | DBP | IBP | p |
|---|---|---|---|
| Total | 20 | 40 | - |
| Age at diagnosis, mean (SD), years | 79.25(10.9) | 79.93 (10.6) | 0.8187 |
| Male, n (%) | 13 (65) | 20 (50) | 0.2787 |
| Pruritus, n (%) | 19 (95) | 40 (100) | 0.1591 |
| Mucosa involved, n (%) | 2 (10) | 4 (10) | - |
| BPDAI scores, mean (range) | |||
| Overall | 37.1 (5–105) | 36.33 (5–53) | 0.8927 |
| Erosions and blisters | 24.6 (3–65) | 16.68 (3–42) | 0.0189 |
| Urticaria and erythema | 12 (0–40) | 19.05 (2–46) | 0.0183 |
| Mucosal lesions | 0.5 (0–5) | 0.6 (0–7) | 0.8359 |
| Hematological involvements, n (%) | |||
| Anemia | 15 (75) | 21 (52.5) | 0.0966 |
| Thrombocytopenia | 1 (5) | 5 (12.5) | 0.3698 |
| AEC, median (range), cells/μl | 780 (0–3138) | 470 (0–1672) | 0.4216 |
| AEC ≥ 500 cells/μL, n (%) | 7 (35) | 17 (42.5) | 0.5837 |
| AEC ≥ 1500 cells/μL, n (%) | 2 (10) | 1 (2.5) | 0.2156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-T.; Chang, Y.-L.; Wang, W.-M. Dipeptidyl Peptidase-4 Inhibitor-Related Bullous Pemphigoid: Clinical, Laboratory, and Histological Features, and Possible Pathogenesis. Int. J. Mol. Sci. 2022, 23, 14101. https://doi.org/10.3390/ijms232214101
Hung C-T, Chang Y-L, Wang W-M. Dipeptidyl Peptidase-4 Inhibitor-Related Bullous Pemphigoid: Clinical, Laboratory, and Histological Features, and Possible Pathogenesis. International Journal of Molecular Sciences. 2022; 23(22):14101. https://doi.org/10.3390/ijms232214101
Chicago/Turabian StyleHung, Chih-Tsung, Yung-Lung Chang, and Wei-Ming Wang. 2022. "Dipeptidyl Peptidase-4 Inhibitor-Related Bullous Pemphigoid: Clinical, Laboratory, and Histological Features, and Possible Pathogenesis" International Journal of Molecular Sciences 23, no. 22: 14101. https://doi.org/10.3390/ijms232214101
APA StyleHung, C.-T., Chang, Y.-L., & Wang, W.-M. (2022). Dipeptidyl Peptidase-4 Inhibitor-Related Bullous Pemphigoid: Clinical, Laboratory, and Histological Features, and Possible Pathogenesis. International Journal of Molecular Sciences, 23(22), 14101. https://doi.org/10.3390/ijms232214101





