Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station
Abstract
:1. Introduction
2. Results
2.1. EU Set-Upg
2.2. Histology
2.3. Morphometric Analysis of Connective Tissue Fibers
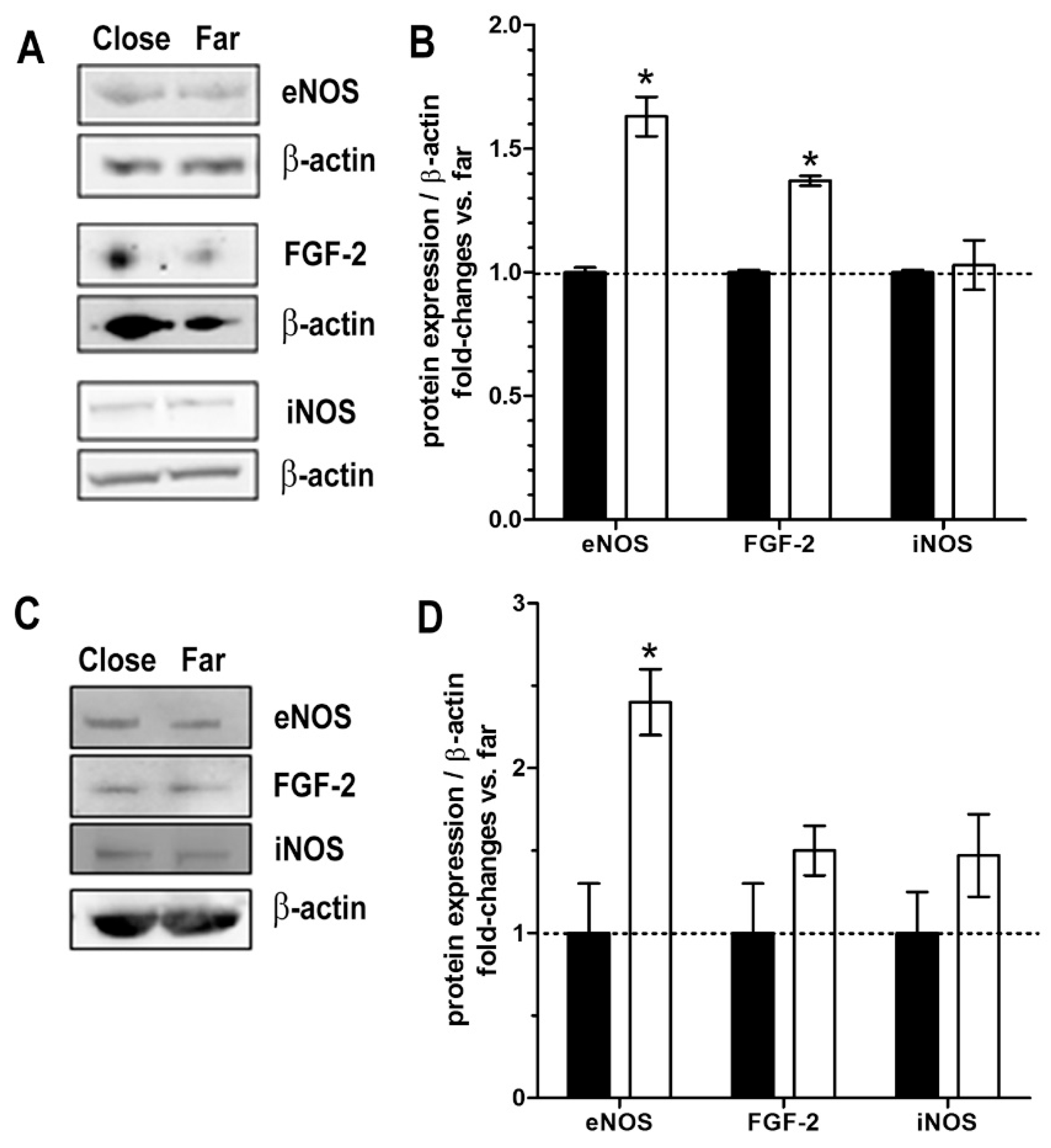
2.4. Blood Microvessels and Stromal Cells
2.5. Proliferating Epidermal Keratinocytes
2.6. Markers of Tissue Integrity and Functions
3. Discussion
4. Materials and Methods
4.1. Specimen Sampling and Handling
4.2. Bioreactor Development
4.3. Enriched Long-Term Incubation Media
4.4. Histology and Morphometric Evaluation of Collagen and Elastic Fibers
4.5. Fluorescent/Immunofluorescent Detection of Mast Cells, Blood Vessels and Fibroblasts
4.6. Evaluation of Proliferating Epidermal Keratinocytes
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekiguchi, C. Issues of health care under weightlessness. Acta Physiol. Scand. 1994, 616, 89–97. [Google Scholar]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O’Brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty years of human space travel: Implications for bone and calcium research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Braunstein, B.; Winnard, A.; Nasser, M.; Weber, T. Human biomechanical and cardiopulmonary responses to partial gravity—A systematic review. Front. Physiol. 2017, 8, 583. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, A.W.; Campbell, M.R.; Novinkov, O.L.; Goncharov, I.B.; Kovachevich, I.V. Blunt trauma and operative care in microgravity: A review of microgravity physiology and surgical investigations with implications for critical care and operative treatment in space. J. Am. Coll. Surg. 1997, 184, 441–453. [Google Scholar] [PubMed]
- Monici, M.; Cialdai, F.; Bani, D.; Bacci, S.; Morbidelli, L.; Norfini, A.; Balsamo, M.; van Loon, J.; Grimm, D.; Riwaldt, S.; et al. Suture in Space: Preparation of an experiment on the healing of sutured wounds on board the ISS. In Proceedings of the 72nd International Astronautical Congress (IAC), Dubai, United Arab Emirates, 25–29 October 2021. [Google Scholar]
- Bani, D. Recombinant human H2 relaxin (serelaxin) as a cardiovascular drug: Aiming at the right target. Drug Discov. Today 2020, 25, 1239. [Google Scholar] [CrossRef]
- Nistri, S.; Fiorillo, C.; Becatti, M.; Bani, D. Human relaxin-2 (serelaxin) attenuates oxidative stress in cardiac muscle cells exposed in vitro to hypoxia-reoxygenation. evidence for the involvement of reduced glutathione up-regulation. Antioxidants 2020, 9, 774. [Google Scholar] [CrossRef]
- Boehnert, M.U.; Armbruster, F.P.; Hilbig, H. Relaxin as a protective substance in preservation solutions for organ transplantation, as shown in an isolated perfused rat liver model. Transplant. Proc. 2008, 40, 978–980. [Google Scholar] [CrossRef]
- Monti, M.; Solito, R.; Puccetti, L.; Pasotti, L.; Roggeri, R.; Monzani, E.; Casella, L.; Morbidelli, L. Protective effects of novel metal-nonoates on the cellular components of the vascular system. J. Pharmacol. Exp. Ther. 2014, 351, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Hyseni, I.; Pacini, A.; Monzani, E.; Casella, L.; Morbidelli, L. Cross-talk between endogenous H2S and NO accounts for vascular protective activity of the metal-nonoate Zn(PipNONO)Cl. Biochem. Pharmacol. 2018, 152, 143–152. [Google Scholar] [CrossRef]
- Jones, P.H. Epithelial stem cells. BioEssays 1997, 19, 683–690. [Google Scholar] [CrossRef]
- Coulombe, P.A. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem. Biophys. Res. Commun. 1997, 236, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelization in wound healing a comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacci, S.; Bani, D. The epidermis in microgravity and unloading conditions and their effects on wound healing. Front. Bioeng. Biotechnol. 2022, 10, 666434. [Google Scholar] [CrossRef]
- Nagata, K. Hsp47: A collagen-specific molecular chaperone. Trends Biochem. Sci. 1996, 21, 22–26. [Google Scholar] [CrossRef]
- Wang, J.F.; Olson, M.E.; Winkfein, R.J.; Kulyk, W.M.; Wright, J.B.; Hart, D.A. Molecular and cell biology of porcine HSP47 during wound healing: Complete cDNA sequence and regulation of gene expression. Wound Repair Regen. 2002, 10, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Gasper, W.J.; Rahman, A.S.; Conte, M.S. Vein graft failure. J. Vasc. Surg. 2015, 61, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautier, A.; Sabatier, F.; Stellmann, P.; Andrac, L.; Nouaille de Gorce, Y.; Dignat-George, F.; Magalon, G. Assessment of organ culture for the conservation of human skin allografts. Cell Tissue Bank 2008, 9, 19–29. [Google Scholar] [CrossRef]
- Goodwin, T.J.; Christofidou-Solomidou, M. Oxidative stress and space biology: An organ-based approach. Int. J. Mol Sci. 2018, 19, 959. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wounds. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [Green Version]
- Kollmannsberger, P.; Bidan, C.M.; Dunlop, J.; Fratzl, P.; Vogel, V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 2018, 4, eaao4881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.H.; Ogawa, R. Keloid research: Current status and future directions. Scars Burn. Heal. 2019, 5, 2059513119868659. [Google Scholar] [CrossRef] [Green Version]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Bergstresser, P.R.; Tigelaar, R.E.; Tharp, M.D. Conjugated avidin identifies cutaneous rodent and human mast cells. J. Investig. Dermatol. 1984, 83, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Greene, A.S.; Lombard, J.H.; Cowley, A.W.; Hansen-Smith, F.M. Microvessel changes in hypertension measured by Griffonia Simplicifolia I lectin. Hypertension 1990, 15, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Tajima, S. HSP47 is a useful marker for skin fibroblasts in formalin-fixed, paraffin-embedded tissue specimens. J. Cutan. Pathol. 2004, 31, 241–246. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Terzuoli, E.; Ristori, E.; Filippelli, A.; Ziche, M.; Morbidelli, L.; Donnini, S. ALDH1A1 overexpression in melanoma cells promotes tumor angiogenesis by activating the IL-8/Notch signaling cascade. Int. J. Mol. Med. 2022, 50, 99. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cialdai, F.; Bacci, S.; Zizi, V.; Norfini, A.; Balsamo, M.; Ciccone, V.; Morbidelli, L.; Calosi, L.; Risaliti, C.; Vanhelden, L.; et al. Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station. Int. J. Mol. Sci. 2022, 23, 14123. https://doi.org/10.3390/ijms232214123
Cialdai F, Bacci S, Zizi V, Norfini A, Balsamo M, Ciccone V, Morbidelli L, Calosi L, Risaliti C, Vanhelden L, et al. Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station. International Journal of Molecular Sciences. 2022; 23(22):14123. https://doi.org/10.3390/ijms232214123
Chicago/Turabian StyleCialdai, Francesca, Stefano Bacci, Virginia Zizi, Aleandro Norfini, Michele Balsamo, Valerio Ciccone, Lucia Morbidelli, Laura Calosi, Chiara Risaliti, Lore Vanhelden, and et al. 2022. "Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station" International Journal of Molecular Sciences 23, no. 22: 14123. https://doi.org/10.3390/ijms232214123
APA StyleCialdai, F., Bacci, S., Zizi, V., Norfini, A., Balsamo, M., Ciccone, V., Morbidelli, L., Calosi, L., Risaliti, C., Vanhelden, L., Pantalone, D., Bani, D., & Monici, M. (2022). Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station. International Journal of Molecular Sciences, 23(22), 14123. https://doi.org/10.3390/ijms232214123








