Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution
Abstract
:1. Introduction
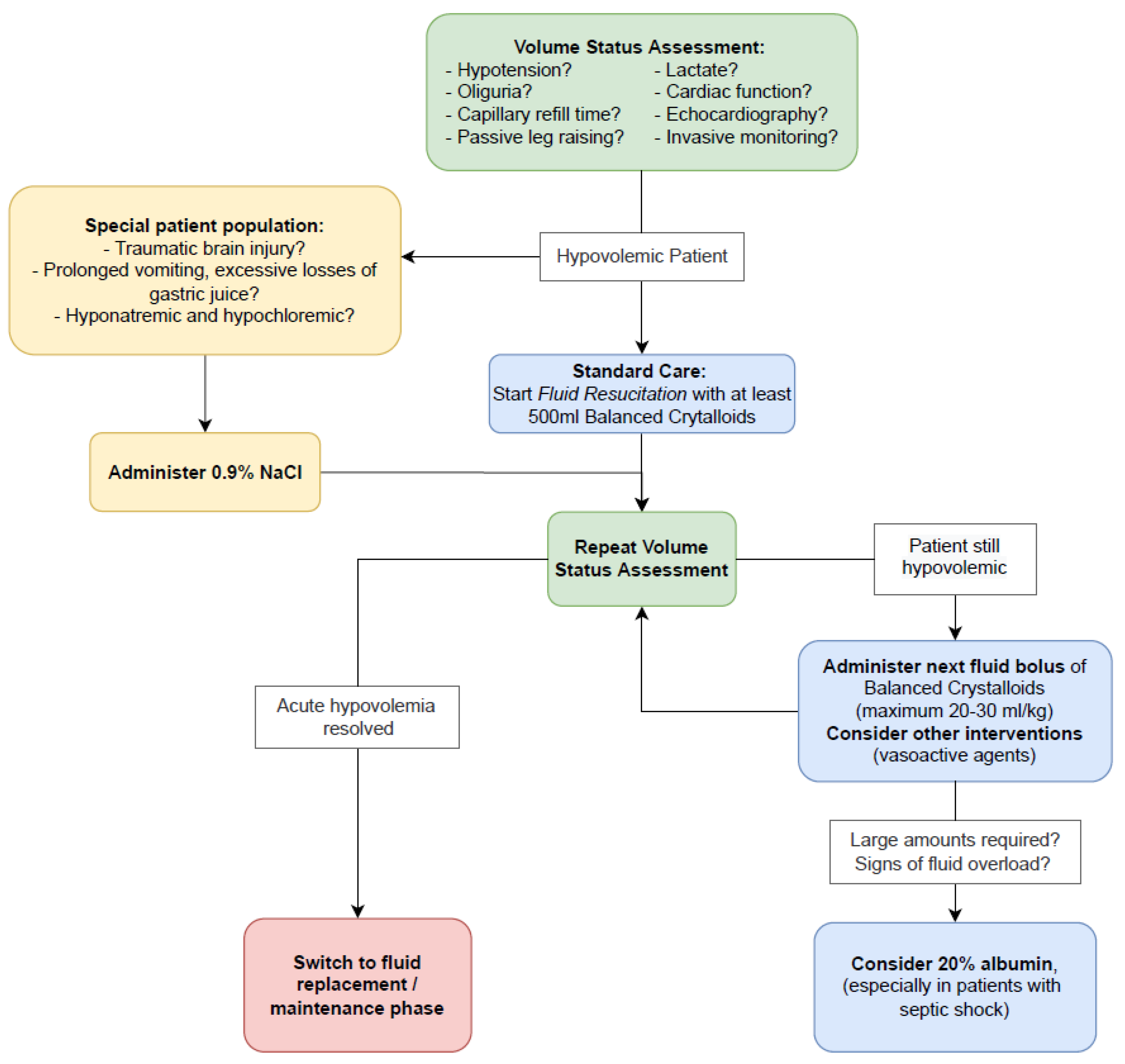
2. Evidence-Based Fluid Management of Hypovolemia in Critically Ill Patients
2.1. Safety Concerns with Semi-Synthetic Colloids
- Pharmaco-epidemiological studies on colloid solutions (HES, gelatin, dextran, and albumin) have shown that, until recently, the use of the semi-synthetic artificial colloids HES and gelatin was many times more frequent than the use of the natural colloid albumin, and overall, colloid was administered to more patients and during more episodes than crystalloid [10]. However, patterns of IV fluid resuscitation, which differed markedly across countries [10], changed because of longstanding controversial safety concerns that are well established and evident but still, practice significantly varied between geographical regions [11]. In light of the increasingly proven safety problems with HES solutions, this artificial colloid has issued warnings and has not been approved for use in intensive care units in recent years. Against this background, and in view of the lack of proof of efficacy for hard clinical endpoints in the indications used, the European Medicines Agency decided in 2022 to withdraw marketing authorization for HES-containing IV solutions. After a short transition period, HES solutions will no longer be available in the European Union [12].
- Dextran solutions are no longer recommended for fluid management because of their numerous adverse effects [13].
- Because of the existing safety concerns with HES- and dextran-based colloids, it is not surprising that gelatin, which has been poorly studied, is being investigated for its safety [14]. A randomized controlled trial (RCT) on the use of gelatin in septic shock was initiated recently [15] but terminated prematurely by the Data Safety and Monitoring Board [16]. It is likely that the potential side effects of gelatin (kidney injury, bleeding, and allergy) are responsible for the early cessation of the RCT.
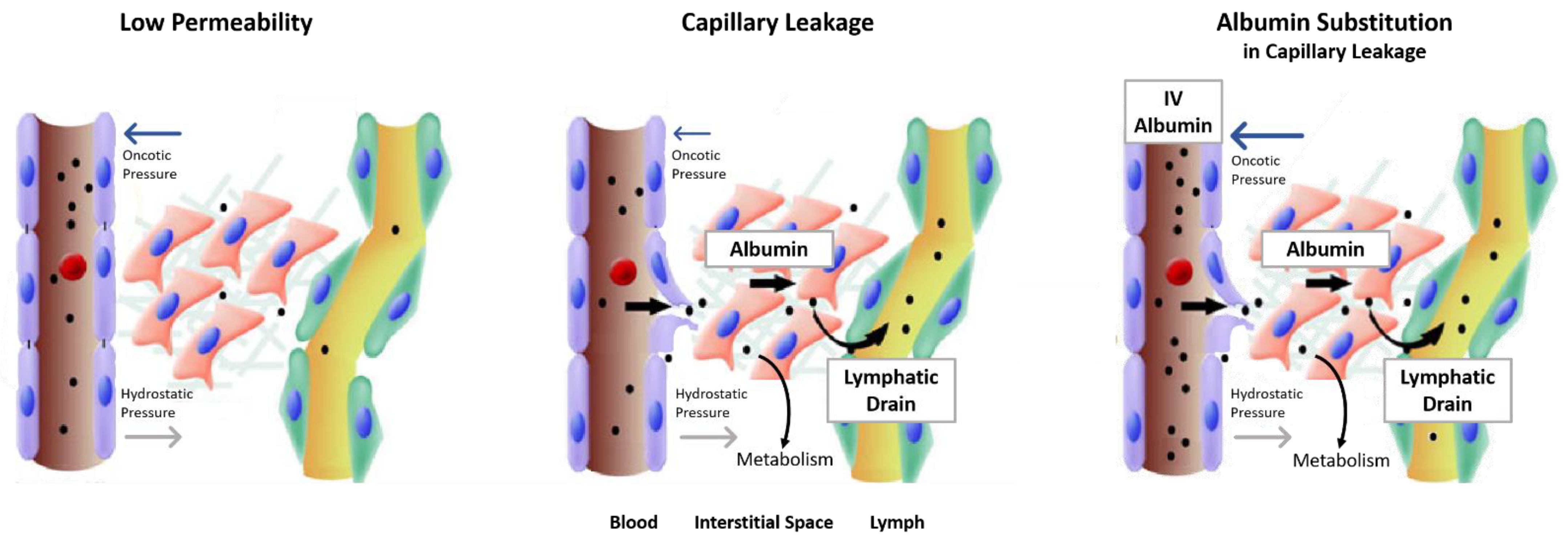
2.2. Safety Concerns with Human Albumin Solutions
3. Hypoalbuminemia as Effect Moderator of Resuscitation Fluids
3.1. Volume of Resuscitation Fluid Affected by Hypoalbuminemia in the Saline versus Albumin Fluid Evaluation (SAFE) Study
3.1.1. Volumes of Study Fluids Administered in the SAFE Study
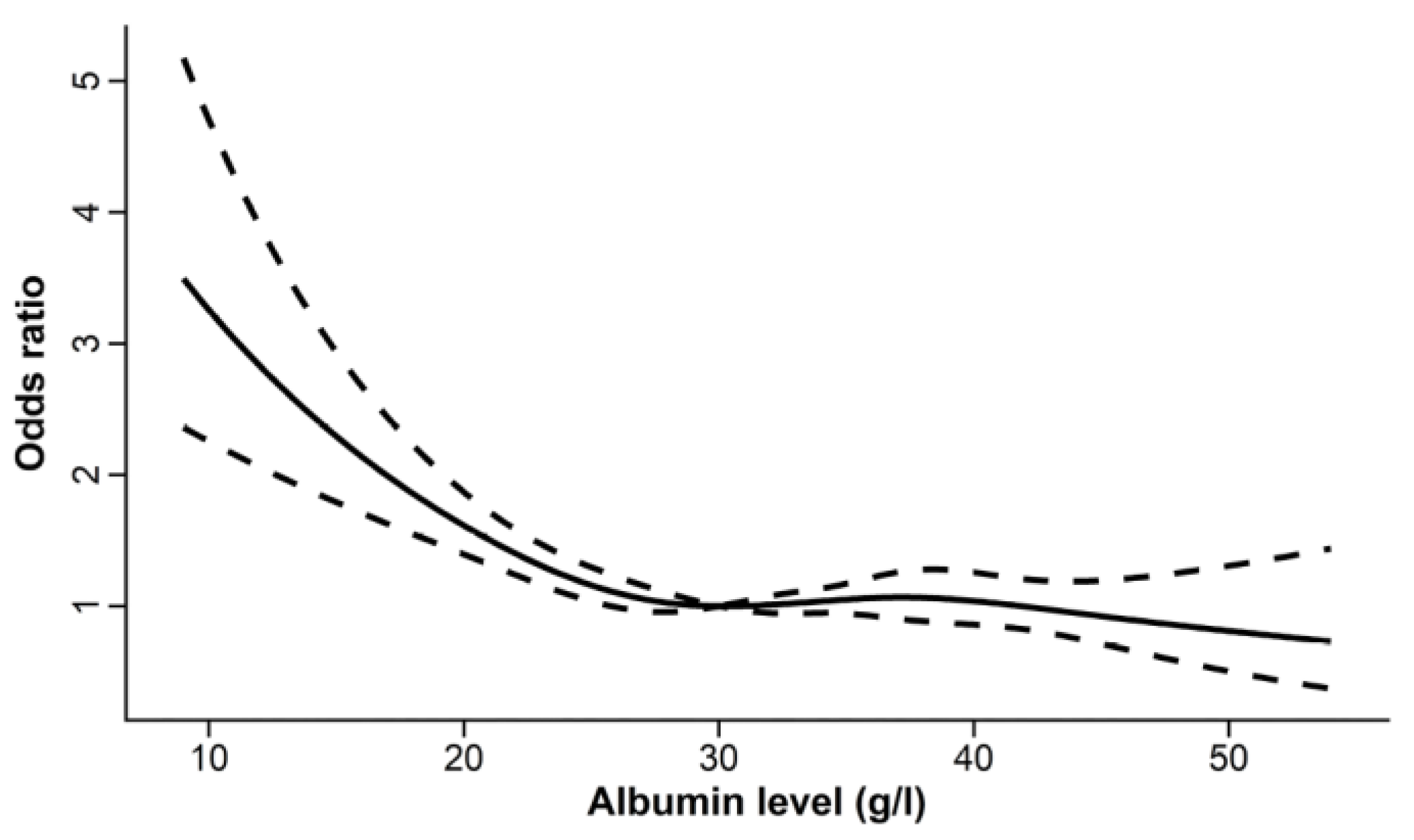
3.1.2. Effect of Baseline Serum Albumin Concentration on Outcomes
3.1.3. Effect of Baseline Serum Albumin Concentration on Ratios of the Volume of Albumin to the Volume of Saline Administered
3.2. Volume of Resuscitation Fluid in Randomized Controlled Trials on IV Albumin of Critically Ill Patients with Hypoalbuminemia
3.3. Prevention of Intradialytic Hypotension in End-Stage Kidney Disease Patients with Hypoalbuminemia by Concentrated IV Albumin Solution
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Finfer, S.; Myburgh, J.; Bellomo, R. Intravenous Fluid Therapy in Critically Ill Adults. Nat. Rev. Nephrol. 2018, 14, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Mayerhöfer, T.; Shaw, A.D.; Wiedermann, C.J.; Joannidis, M. Fluids in the ICU: Which Is the Right One? Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2022, gfac279. [Google Scholar] [CrossRef]
- Vincent, J.-L. Fluid Management in the Critically Ill. Kidney Int. 2019, 96, 52–57. [Google Scholar] [CrossRef]
- Joannidis, M.; Wiedermann, C.J.; Ostermann, M. On Myths about Albumin and Misconceptions That Cause Confusion: Authors’ Reply to “What’s Wrong with the Ten Myths about Albumin: Three Layers for an Indisputable Dispute”. Intensive Care Med. 2022, 48, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.D.; Brown, R.M.; Semler, M.W. Resuscitation Fluids. Curr. Opin. Crit. Care 2018, 24, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.E.; Woodcock, T.M. Revised Starling Equation and the Glycocalyx Model of Transvascular Fluid Exchange: An Improved Paradigm for Prescribing Intravenous Fluid Therapy. Br. J. Anaesth. 2012, 108, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.L.; Hildebrand, K.L.; McCormick, S.A.; Bedel, M.J. The Effect of Intravenous Lactated Ringer’s Solution versus 0.9% Sodium Chloride Solution on Serum Osmolality in Human Volunteers. Anesth. Analg. 1999, 88, 999–1003. [Google Scholar] [CrossRef]
- Van Aken, H.K.; Kampmeier, T.G.; Ertmer, C.; Westphal, M. Fluid Resuscitation in Patients with Traumatic Brain Injury: What Is a SAFE Approach? Curr. Opin. Anaesthesiol. 2012, 25, 563–565. [Google Scholar] [CrossRef]
- Mårtensson, J.; Bihari, S.; Bannard-Smith, J.; Glassford, N.J.; Lloyd-Donald, P.; Cioccari, L.; Luethi, N.; Tanaka, A.; Crisman, M.; Rey de Castro, N.; et al. Small Volume Resuscitation with 20% Albumin in Intensive Care: Physiological Effects: The SWIPE Randomised Clinical Trial. Intensive Care Med. 2018, 44, 1797–1806. [Google Scholar] [CrossRef]
- Finfer, S.; Liu, B.; Taylor, C.; Bellomo, R.; Billot, L.; Cook, D.; Du, B.; McArthur, C.; Myburgh, J.; SAFE TRIPS Investigators. Resuscitation Fluid Use in Critically Ill Adults: An International Cross-Sectional Study in 391 Intensive Care Units. Crit. Care Lond. Engl. 2010, 14, R185. [Google Scholar] [CrossRef]
- Hammond, N.E.; Taylor, C.; Finfer, S.; Machado, F.R.; An, Y.; Billot, L.; Bloos, F.; Bozza, F.; Cavalcanti, A.B.; Correa, M.; et al. Patterns of Intravenous Fluid Resuscitation Use in Adult Intensive Care Patients between 2007 and 2014: An International Cross-Sectional Study. PLoS ONE 2017, 12, e0176292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Hydroxyethyl Starch (HES) Solutions for Infusion: Suspension of Marketing Authorisations Due to Continued Use in Contraindicated Patient Populations with Increased Risk Serious Harm. Available online: https://www.ema.europa.eu/en/medicines/dhpc/hydroxyethyl-starch-hes-solutions-infusion-suspension-marketing-authorisations-due-continued-use (accessed on 13 November 2022).
- Barron, M.E.; Wilkes, M.M.; Navickis, R.J. A Systematic Review of the Comparative Safety of Colloids. Arch. Surg. 2004, 139, 552–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, C.; Fleischmann, C.; Thomas-Rueddel, D.; Vlasakov, V.; Rochwerg, B.; Theurer, P.; Gattinoni, L.; Reinhart, K.; Hartog, C.S. How Safe Is Gelatin? A Systematic Review and Meta-Analysis of Gelatin-Containing Plasma Expanders vs. Crystalloids and Albumin. J. Crit. Care 2016, 35, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Zacharowski, K.; Ichai, C.; Asehnoune, K.; Černý, V.; Dembinski, R.; Ferrer Roca, R.; Fries, D.; Molnar, Z.; Rosenberger, P.; et al. Efficacy and Safety of Early Target-Controlled Plasma Volume Replacement with a Balanced Gelatine Solution versus a Balanced Electrolyte Solution in Patients with Severe Sepsis/Septic Shock: Study Protocol, Design, and Rationale of a Prospective, Randomized, Controlled, Double-Blind, Multicentric, International Clinical Trial: GENIUS-Gelatine Use in ICU and Sepsis. Trials 2021, 22, 376. [Google Scholar] [CrossRef]
- Braun, B.; Melsungen, A.G. Prospective, Controlled, Double-Blind, Randomized Multicentric Study on the Efficacy and Safety of an Early Target Controlled Plasma Volume Replacement Therapy with a Balanced Gelatine Solution vs. a Balanced Electrolyte Solution in Patients with Severe Sepsis; clinicaltrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2021.
- Gosling, P. Albumin: Friend or Foe? Trauma 2000, 2, 125–134. [Google Scholar] [CrossRef]
- Cochrane Injuries Group Albumin Reviewers. Human Albumin Administration in Critically Ill Patients: Systematic Review of Randomised Controlled Trials. BMJ 1998, 317, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, M.M.; Navickis, R.J. Patient Survival after Human Albumin Administration. A Meta-Analysis of Randomized, Controlled Trials. Ann. Intern. Med. 2001, 135, 149–164. [Google Scholar] [CrossRef]
- Boldt, J. Human Albumin on the Intensive Care Unit: Can We Live without It? In Proceedings of the Yearbook of Intensive Care and Emergency Medicine 2000; Vincent, J.-L., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 467–475. [Google Scholar]
- Wiedermann, C.J. Ethical Publishing in Intensive Care Medicine: A Narrative Review. World J. Crit. Care Med. 2016, 5, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R.; SAFE Study Investigators. A Comparison of Albumin and Saline for Fluid Resuscitation in the Intensive Care Unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar] [CrossRef] [Green Version]
- Wiedermann, C.J. Phases of Fluid Management and the Roles of Human Albumin Solution in Perioperative and Critically Ill Patients. Curr. Med. Res. Opin. 2020, 36, 1961–1973. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-T.; Liu, J.; Hu, B.; Wang, R.-L.; Yang, X.-H.; Shang, X.-L.; Wang, G.; Wang, C.-S.; Li, B.-L.; Gong, Y.; et al. Expert Consensus on the Use of Human Serum Albumin in Critically Ill Patients. Chin. Med. J. 2021, 134, 1639–1654. [Google Scholar] [CrossRef] [PubMed]
- Egi, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Iba, T.; Kakihana, Y.; Kawasaki, T.; Kushimoto, S.; Kuroda, Y.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J. Intensive Care 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Wiedermann, C.J.; Ostermann, M. Ten Myths about Albumin. Intensive Care Med. 2022, 48, 602–605. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Wiedermann, W.; Joannidis, M. Hypoalbuminemia and Acute Kidney Injury: A Meta-Analysis of Observational Clinical Studies. Intensive Care Med. 2010, 36, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Iatrogenic Hypoalbuminemia Due to Hydroxyethyl Starch 130/0.4: A Risk Factor for Acute Kidney Injury? Anesth. Analg. 2010, 110, 1242, author reply 1243. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Sun, L.; Zhang, J.; Gao, Y.; Li, R.; Ren, J.; Hou, Y.; Su, D.; Liu, J.; et al. Prognostic Value of Serum Albumin Level in Critically Ill Patients: Observational Data From Large Intensive Care Unit Databases. Front. Nutr. 2022, 9, 1187. [Google Scholar] [CrossRef]
- Martin, G.S.; Bassett, P. Crystalloids vs. Colloids for Fluid Resuscitation in the Intensive Care Unit: A Systematic Review and Meta-Analysis. J. Crit. Care 2019, 50, 144–154. [Google Scholar] [CrossRef]
- Ye, Z.; Gao, M.; Ge, C.; Lin, W.; Zhang, L.; Zou, Y.; Peng, Q. Association between Albumin Infusion and Septic Patients with Coronary Heart Disease: A Retrospective Study Based on Medical Information Mart for Intensive Care III Database. Front. Cardiovasc. Med. 2022, 9, 982969. [Google Scholar] [CrossRef]
- Bannard-Smith, J.; Alexander, P.; Glassford, N.; Chan, M.J.; Lee, M.; Wong, B.T.; Crawford, G.; Bailey, M.; Bellomo, R. Haemodynamic and Biochemical Responses to Fluid Bolus Therapy with Human Albumin Solution, 4% versus 20%, in Critically Ill Adults. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2015, 17, 122–128. [Google Scholar]
- Zdolsek, M.; Hahn, R.G.; Sjöberg, F.; Zdolsek, J.H. Plasma Volume Expansion and Capillary Leakage of 20% Albumin in Burned Patients and Volunteers. Crit. Care Lond. Engl. 2020, 24, 191. [Google Scholar] [CrossRef]
- SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health; Myburgh, J.; Cooper, D.J.; Finfer, S.; Bellomo, R.; Norton, R.; Bishop, N.; et al. Saline or Albumin for Fluid Resuscitation in Patients with Traumatic Brain Injury. N. Engl. J. Med. 2007, 357, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedermann, C.J. Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited-A Narrative Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2662. [Google Scholar] [CrossRef]
- Briegel, J. Albumin in traumatic brain injury-osmolarity is what matters! Med. Klin. Intensivmed. Notf. 2022, 117, 69–70. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Amêndola, C.P.; Serpa-Neto, A.; Paranhos, J.L.R.; et al. Effect of Intravenous Fluid Treatment with a Balanced Solution vs. 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021, 326, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Alderson, P.; Bunn, F.; Lefebvre, C.; Li, W.P.A.; Li, L.; Roberts, I.; Schierhout, G.; Albumin Reviewers. Human Albumin Solution for Resuscitation and Volume Expansion in Critically Ill Patients. Cochrane Database Syst. Rev. 2004, CD001208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAFE Study Investigators; Finfer, S.; Bellomo, R.; McEvoy, S.; Lo, S.K.; Myburgh, J.; Neal, B.; Norton, R. Effect of Baseline Serum Albumin Concentration on Outcome of Resuscitation with Albumin or Saline in Patients in Intensive Care Units: Analysis of Data from the Saline versus Albumin Fluid Evaluation (SAFE) Study. BMJ 2006, 333, 1044. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.J.; Orellana-Jimenez, C.; Melot, C.; De Backer, D.; Berre, J.; Leeman, M.; Brimioulle, S.; Appoloni, O.; Creteur, J.; Vincent, J.L. Albumin Administration Improves Organ Function in Critically Ill Hypoalbuminemic Patients: A Prospective, Randomized, Controlled, Pilot Study. Crit. Care Med. 2006, 34, 2536–2540. [Google Scholar] [CrossRef]
- SAFE Study Investigators; Finfer, S.; McEvoy, S.; Bellomo, R.; McArthur, C.; Myburgh, J.; Norton, R. Impact of Albumin Compared to Saline on Organ Function and Mortality of Patients with Severe Sepsis. Intensive Care Med. 2011, 37, 86–96. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Bauer, M.; Nierhaus, A.; Kluge, S.; Schumacher, U.; Putensen, C.; Fichtner, F.; Petros, S.; Scheer, C.; Jaschinski, U.; et al. Randomized Controlled Multicentre Study of Albumin Replacement Therapy in Septic Shock (ARISS): Protocol for a Randomized Controlled Trial. Trials 2020, 21, 1002. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Radhakrishnan, Y.; Petnak, T.; Qureshi, F.; Mao, M.A.; Kashani, K.B. Impact of Hypoalbuminemia on Mortality in Critically Ill Patients Requiring Continuous Renal Replacement Therapy. J. Crit. Care 2022, 68, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.J.; Jiang, W.; Pan, L.; Pan, J. Reduced Serum Albumin as a Risk Factor for Poor Prognosis in Critically Ill Patients Receiving Renal Replacement Therapy. BMC Nephrol. 2021, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Z.; Shen, B.; Teng, J.; Zou, J.; Ding, X. Intradialytic Hypotension as an Independent Risk Factor for Long-Term Mortality in Maintaining Hemodialysis Patients: A 5-Year Follow-Up Cohort Study. Blood Purif. 2018, 45, 320–326. [Google Scholar] [CrossRef]
- Chou, J.A.; Kalantar-Zadeh, K.; Mathew, A.T. A Brief Review of Intradialytic Hypotension with a Focus on Survival. Semin. Dial. 2017, 30, 473–480. [Google Scholar] [CrossRef]
- Knoll, G.A.; Grabowski, J.A.; Dervin, G.F.; O’Rourke, K. A Randomized, Controlled Trial of Albumin versus Saline for the Treatment of Intradialytic Hypotension. J. Am. Soc. Nephrol. JASN 2004, 15, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Jardin, F.; Prost, J.F.; Ozier, Y.; Margairaz, A. Hemodialysis in Septic Patients: Improvements in Tolerance of Fluid Removal with Concentrated Albumin as the Priming Fluid. Crit. Care Med. 1982, 10, 650–652. [Google Scholar] [CrossRef]
- Hryciw, N.; Joannidis, M.; Hiremath, S.; Callum, J.; Clark, E.G. Intravenous Albumin for Mitigating Hypotension and Augmenting Ultrafiltration during Kidney Replacement Therapy. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 820–828. [Google Scholar] [CrossRef]
- O’Brien, Z.; Finnis, M.; Gallagher, M.; Bellomo, R.; on behalf of the RENAL Study Investigators and the Australian and New Zealand Intensive Care Clinical Trials Group. Hyperoncotic Albumin Solution in Continuous Renal Replacement Therapy Patients. Blood Purif. 2022, 51, 590–599. [Google Scholar] [CrossRef]
- Macedo, E.; Karl, B.; Lee, E.; Mehta, R.L. A Randomized Trial of Albumin Infusion to Prevent Intradialytic Hypotension in Hospitalized Hypoalbuminemic Patients. Crit. Care Lond. Engl. 2021, 25, 18. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiedermann, C.J. Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution. Int. J. Mol. Sci. 2022, 23, 14175. https://doi.org/10.3390/ijms232214175
Wiedermann CJ. Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution. International Journal of Molecular Sciences. 2022; 23(22):14175. https://doi.org/10.3390/ijms232214175
Chicago/Turabian StyleWiedermann, Christian J. 2022. "Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution" International Journal of Molecular Sciences 23, no. 22: 14175. https://doi.org/10.3390/ijms232214175
APA StyleWiedermann, C. J. (2022). Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution. International Journal of Molecular Sciences, 23(22), 14175. https://doi.org/10.3390/ijms232214175




