Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes
Abstract
1. Introduction
2. Results
2.1. Gene and Protein Expression of Sema3A and Nrp1 in OA Chondrocytes Compared with non-OA Chondrocytes
2.2. Effects of In Vitro Passaging on Nrp1 and Sema3A Expression in OA Chondrocytes
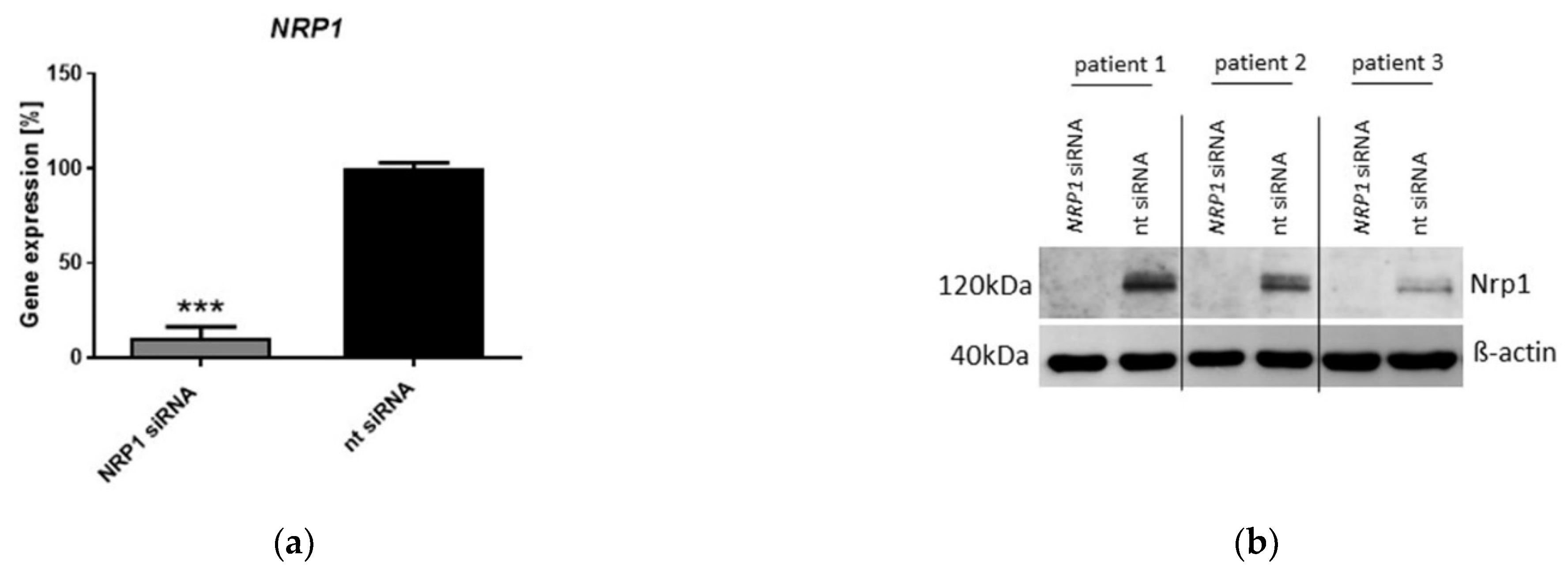
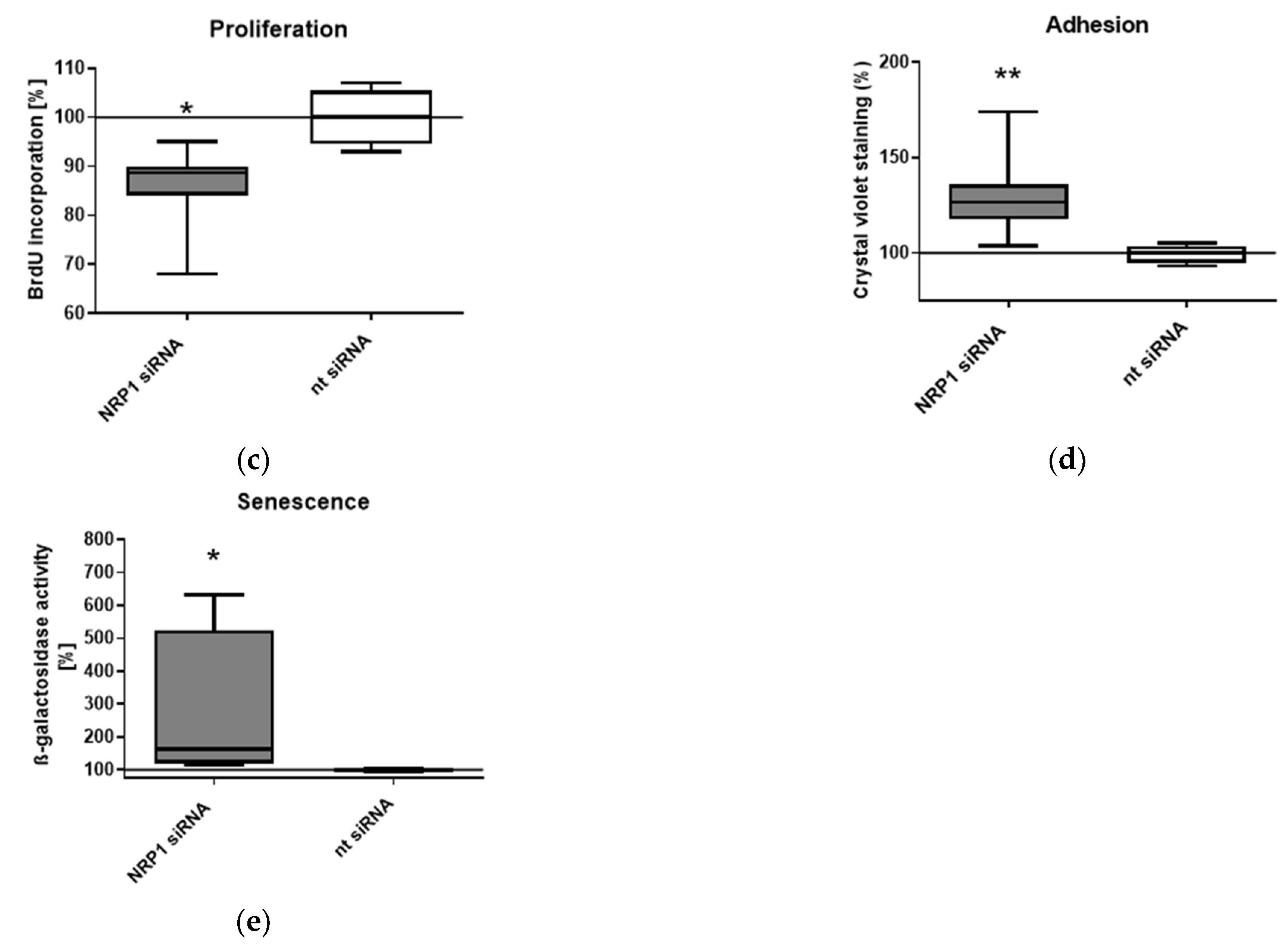
2.3. Nrp1 Knockdown Affects Proliferation, Adhesion and Senescence in OA-Chondrocytes
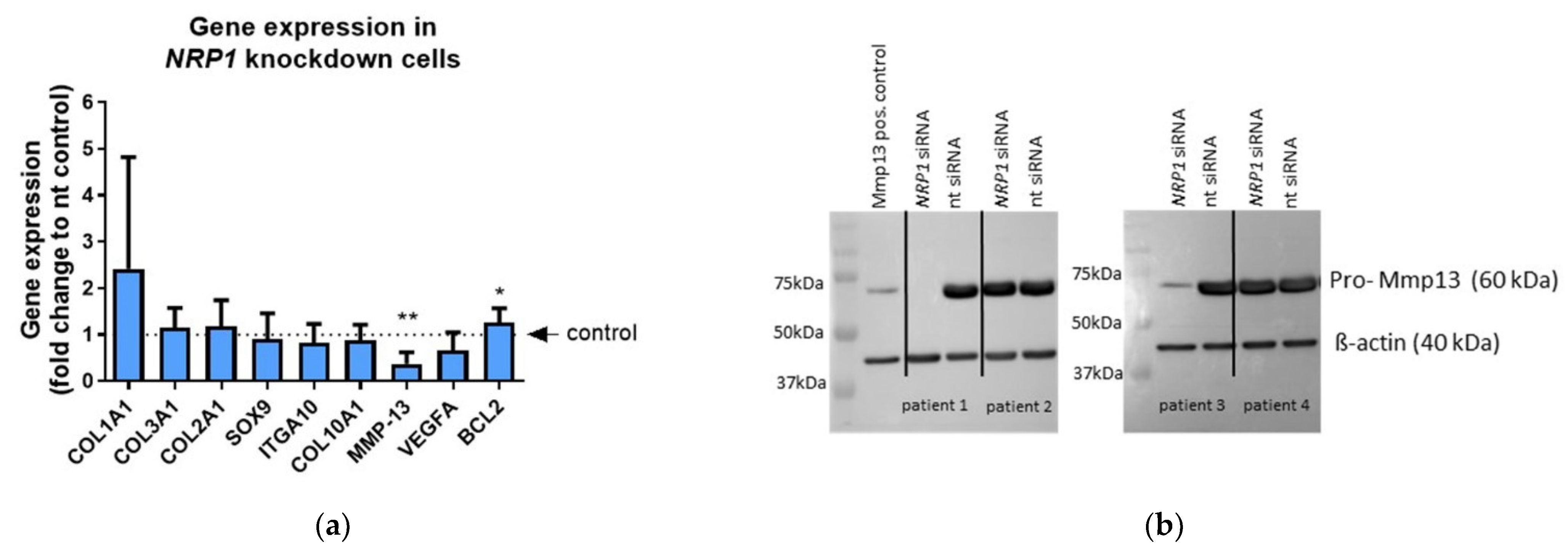
2.4. NRP1 Knockdown Affects MMP13 and BCL-2 Expression
2.5. Sema3A-Mediated Induction of MMP13 Gene Expression Can Be Blocked via NRP1 Knockdown
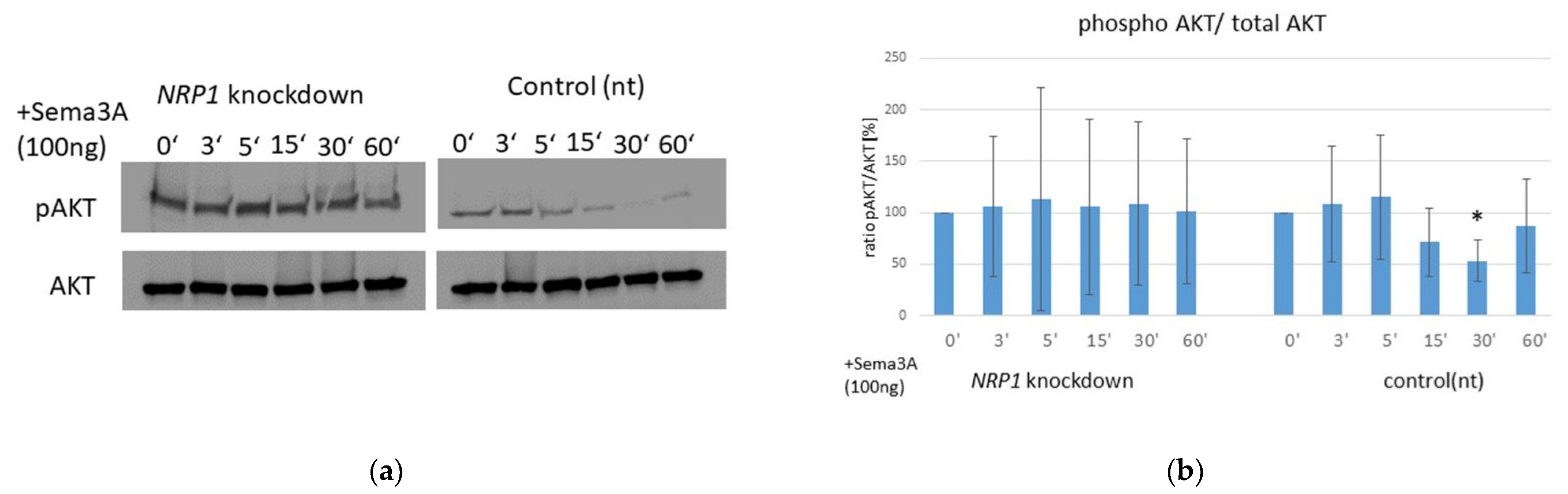
2.6. AKT Phosphorylation Is Impaired by Sema3A Stimulation in OA Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of OA- and Non-OA Chondrocytes
4.2. RNA Isolation and Real-Time RT-PCR from OA- and Non-OA Chondrocytes
4.3. Protein Extraction and Western Blot Analysis
4.4. Nrp1 siRNA Transfection
4.5. BrdU Proliferation Assay
4.6. Senescence-Associated (SA) β-Galactosidase Assay
4.7. Adhesion Assay
4.8. Caspase-3/7 Assay
4.9. Wound Healing (Migration) Assay
4.10. Sema3A Stimulation
4.11. Statistics
5. Conclusions and Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, J.; Jin, H.; Im, H.-J.; Sandy, J.; Chen, D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann. N. Y. Acad. Sci. 2011, 1240, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Muschter, D. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. Int. J. Mol. Sci. 2017, 18, 931. [Google Scholar] [CrossRef] [PubMed]
- Stöckl, S.; Eitner, A.; Bauer, R.J.; König, M.; Johnstone, B.; Grässel, S. Substance P and Alpha-Calcitonin Gene-Related Peptide Differentially Affect Human Osteoarthritic and Healthy Chondrocytes. Front. Immunol. 2021, 12, 722884. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Bahr, M. Growth and guidance cues for regenerating axons: Where have they gone? J. Neurobiol. 2004, 59, 162–180. [Google Scholar] [CrossRef]
- Parrinello, S.; Noon, L.A.; Harrisingh, M.C.; Digby, P.W.; Rosenberg, L.H.; Cremona, C.A.; Echave, P.; Flanagan, A.M.; Parada, L.F.; Lloyd, A.C. NF1 loss disrupts Schwann cell–axonal interactions: A novel role for semaphorin 4F. Genes Dev. 2008, 22, 3335–3348. [Google Scholar] [CrossRef]
- Cooper, H.M. Axon guidance receptors direct growth cone pathfinding: Rivalry at the leading edge. Int. J. Dev. Biol. 2002, 46, 621–631. [Google Scholar]
- Gomez, C.; Burt-Pichat, B.; Mallein-Gerin, F.; Merle, B.; Delmas, P.D.; Skerry, T.M.; Vico, L.; Malaval, L.; Chenu, C. Expression of Semaphorin-3A and its receptors in endochondral ossification: Potential role in skeletal development and innervation. Dev. Dyn. 2005, 234, 393–403. [Google Scholar] [CrossRef]
- Behar, O.; Golden, J.A.; Mashimo, H.; Schoen, F.J.; Fishman, M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 1996, 383, 525–528. [Google Scholar] [CrossRef]
- Okubo, M.; Kimura, T.; Fujita, Y.; Mochizuki, S.; Niki, Y.; Enomoto, H.; Suda, Y.; Toyama, Y.; Okada, Y. Semaphorin 3A is expressed in human osteoarthritic cartilage and antagonizes vascular endothelial growth factor 165-promoted chondrocyte migration: An implication for chondrocyte cloning. Arthritis Care Res. 2011, 63, 3000–3009. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.Y.; Chae, B.C.; Jang, J.; Lee, E.; Son, Y. Fully Dedifferentiated Chondrocytes Expanded in Specific Mesenchymal Stem Cell Growth Medium with FGF2 Obtains Mesenchymal Stem Cell Phenotype In Vitro but Retains Chondrocyte Phenotype In Vivo. Cell Transpl. 2017, 26, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Boppart, M.D.; Burkin, D.J.; Kaufman, S.J. Activation of AKT signaling promotes cell growth and survival in alpha7beta1 integrin-mediated alleviation of muscular dystrophy. Biochim. Biophys. Acta 2011, 1812, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, X.; Wang, Z.; Liu, Y.; Lu, J.; Lu, Y.; Cui, M.; Zhang, X.; Li, F. Inflammatory milieu cultivated Sema3A signaling promotes chondrocyte apoptosis in knee osteoarthritis. J. Cell. Biochem. 2017, 119, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Salikhova, A.; Wang, L.; Lanahan, A.A.; Liu, M.; Simons, M.; Leenders, W.P.; Mukhopadhyay, D.; Horowitz, A. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ. Res. 2008, 103, e71–e79. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, M.; Tosato, G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood 2006, 107, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sato, T.; Ushida, T.; Hirochika, R.; Tateishi, T. Redifferentiation of dedifferentiated bovine chondrocytes when cultured in vitro in a PLGA-collagen hybrid mesh. FEBS Lett. 2003, 542, 95–99. [Google Scholar] [CrossRef]
- Kino-Oka, M.; Yashiki, S.; Ota, Y.; Mushiaki, Y.; Sugawara, K.; Yamamoto, T.; Takezawa, T.; Taya, M. Subculture of Chondrocytes on a Collagen Type I-Coated Substrate with Suppressed Cellular Dedifferentiation. Tissue Eng. 2005, 11, 597–608. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Retaining Zonal Chondrocyte Phenotype by Means of Novel Growth Environments. Tissue Eng. 2005, 11, 395–403. [Google Scholar] [CrossRef]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A.; Stokes, D. Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum. 2002, 46, 683–693. [Google Scholar] [CrossRef]
- Gafni, R.I.; Weise, M.; Robrecht, D.T.; Meyers, J.L.; Barnes, K.M.; De-Levi, S.; Baron, J. Catch-Up Growth Is Associated with Delayed Senescence of the Growth Plate in Rabbits. Pediatr. Res. 2001, 50, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Hemmel, M.; Neureiter, D.; Gebhard, P.M.; Zeiler, G.; Kirchner, T.; McKenna, L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: A study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001, 44, 1304–1312. [Google Scholar] [PubMed]
- Raper, J.A. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 2000, 10, 88–94. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, S.; Wang, R.; Zhang, Y.; Dong, J.; Li, Y. Mechanistic Insight Into the Roles of Integrins in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 693484. [Google Scholar] [CrossRef]
- Neuhold, L.A.; Killar, L.; Zhao, W.; Sung, M.-L.A.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 2001, 107, 35–44. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]






| Gene | Forward | Reverse |
|---|---|---|
| SEMA3A | GGAGACTTGGTATGATTTAGAAGAGG | TGCTGAAATAGCAGTCGGTTC |
| NRP1 | GCCACAGTGGAACAGGTGAT | CTATGACCGTGGGCTTTTCT |
| COL1A1 | ACGTCCTGGTGAAGTTGGTC | ACCAGGGAAGCCTCTCTCTC |
| COL3A1 | CTTCTCTCCAGCCGAGCTTC | TGTGTTTCGTGCAACCATCC |
| COL2A1 | CCAGATGACCTTCCTACGCC | TTCAGGGCAGTGTACGTGAAC |
| SOX9 | GTACCCGCACTTGCACAAC | TCTCGCTCTCGTTCAGAAGTC |
| ITGA10 | GTGTGGATGCTTCATTCCAG | GCCATCCAAGACAATGACAA |
| COL10A1 | CCC TCT TGT TAG TGC CAA CC | AGA TTC CAG TCC TTG GGT CA |
| MMP13 | GACTGGTAATGGCATCAAGGGA | CACCGGCAAAAGCCACTTTA |
| VEGFA | CTTGCCTTGCTGCTCTAC | ACCACTTCGTGATGATTCTG |
| BCL2 | ATGTGTGTGGAGAGCGTCAA | ACAGTTCCACAAAGGCATCC |
| GAPDH | CTGACTTCAACAGCGACACC | CCCTGTTGCTGTAGCCAAAT |
| TBP | GAACATCATGGATCAGAACAACA | ATAGGGATTCCGGGAGTCAT |
| 18s | CTGGATACCGCAGCTAGGAA | GAATTTCACCTCTAGCGGCG |
| TFRC | ATC GGT TGG TGC CAC TGA AT | TTG CTG GTA CCA AGA ACC GC |
| Antibody | Company | Dilution |
|---|---|---|
| rabbit monoclonal anti-p44/42 MAPK (ERK1/2) | Cell Signaling Technology (CST), Danvers, MA, USA; #4695 | 1:2000 |
| rabbit monoclonal anti-phospho-p44/42 MAPK (p-ERK1/2) | Cell Signaling Technology (CST), Danvers, MA, USA; #4370 | 1:1000 |
| rabbit monoclonal anti-phospho-Akt (Ser473) (D9E) XP® | Cell Signaling Technology (CST), Danvers, MA, USA; #4060 | 1:2000 |
| rabbit monoclonal anti-Akt (pan) (C67E7) | Cell Signaling Technology (CST), Danvers, MA, USA; #4691 | 1:1000 |
| Semaphorin 3A | Abcam, Cambridge, UK; ab199475 | 1:1000 |
| Neuropilin-1 | Abcam, Cambridge, UK; ab81321 | 1:2000 |
| MMP13 | Abcam, Cambridge, UK; ab39012 | 1:3000 |
| rabbit polyclonal anti-beta actin | Abcam, Cambridge, UK; #ab8227 | 1:5000 |
| rabbit HRP (horseradish peroxidase)-coupled (secondary) antibody | Jackson Immuno Research, West Grove, PA, USA; #AB_2313567 | 1: 10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stöckl, S.; Reichart, J.; Zborilova, M.; Johnstone, B.; Grässel, S. Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2022, 23, 14180. https://doi.org/10.3390/ijms232214180
Stöckl S, Reichart J, Zborilova M, Johnstone B, Grässel S. Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. International Journal of Molecular Sciences. 2022; 23(22):14180. https://doi.org/10.3390/ijms232214180
Chicago/Turabian StyleStöckl, Sabine, Johanna Reichart, Magdalena Zborilova, Brian Johnstone, and Susanne Grässel. 2022. "Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes" International Journal of Molecular Sciences 23, no. 22: 14180. https://doi.org/10.3390/ijms232214180
APA StyleStöckl, S., Reichart, J., Zborilova, M., Johnstone, B., & Grässel, S. (2022). Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. International Journal of Molecular Sciences, 23(22), 14180. https://doi.org/10.3390/ijms232214180





