Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis
Abstract
1. Introduction
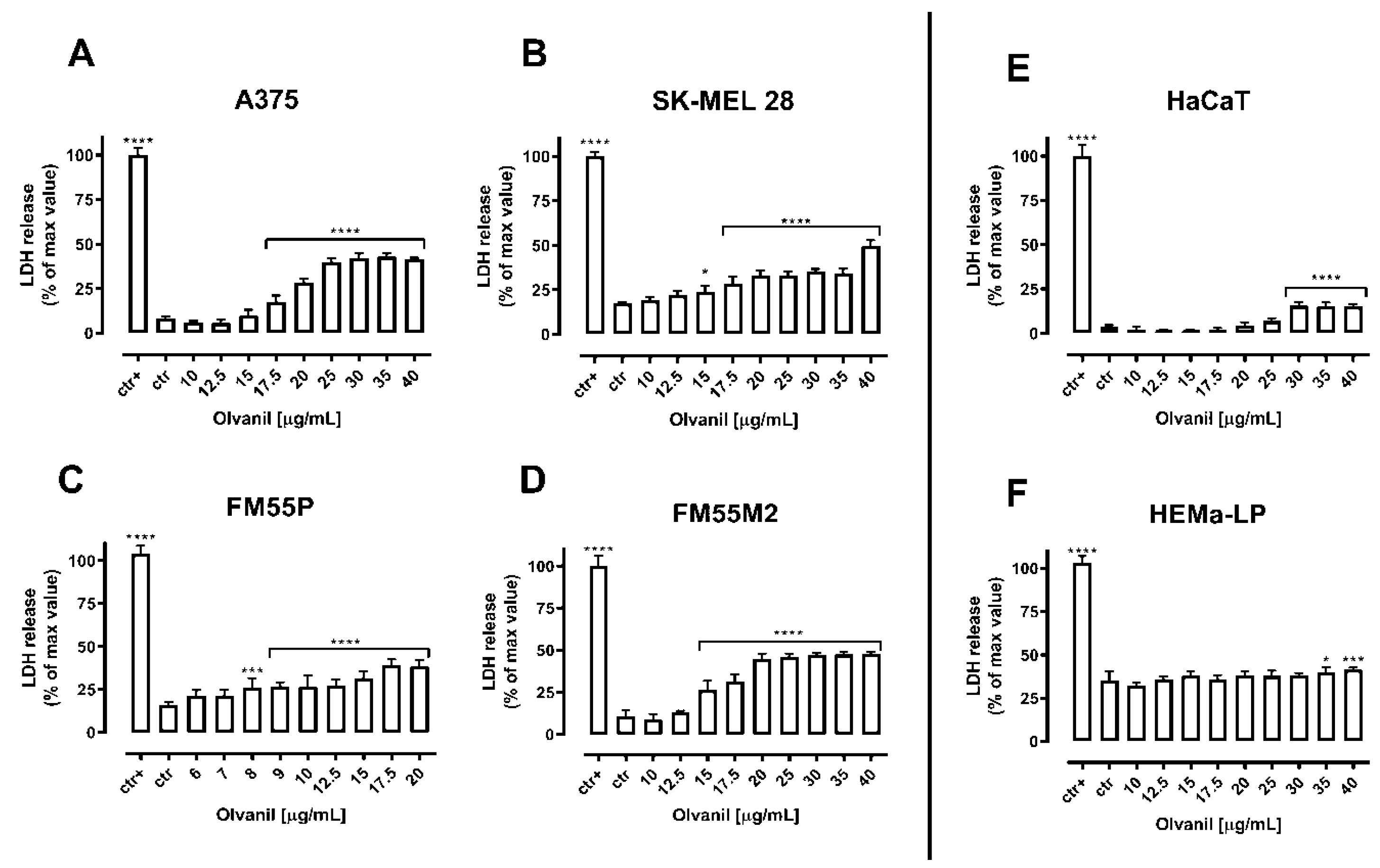
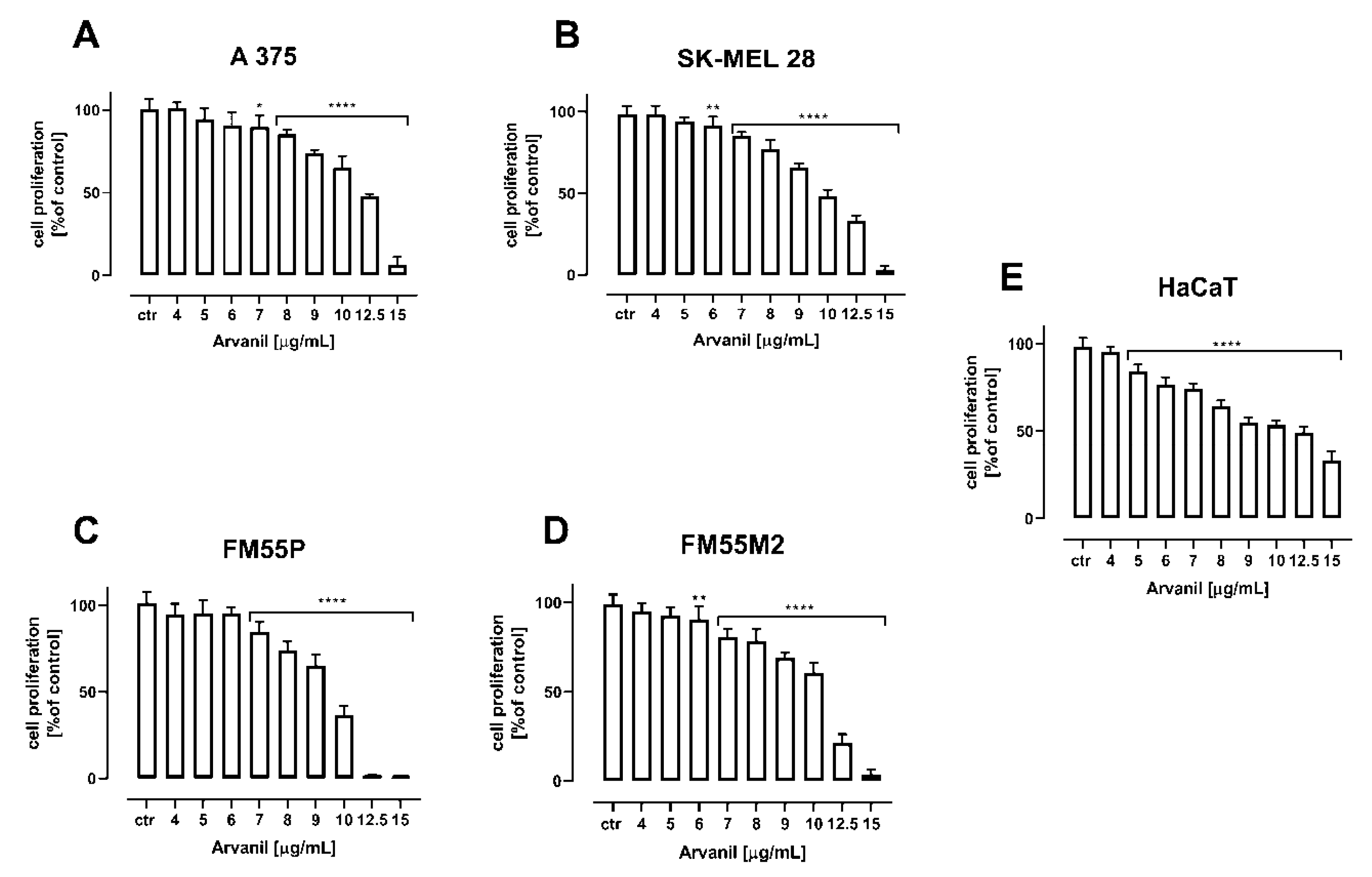
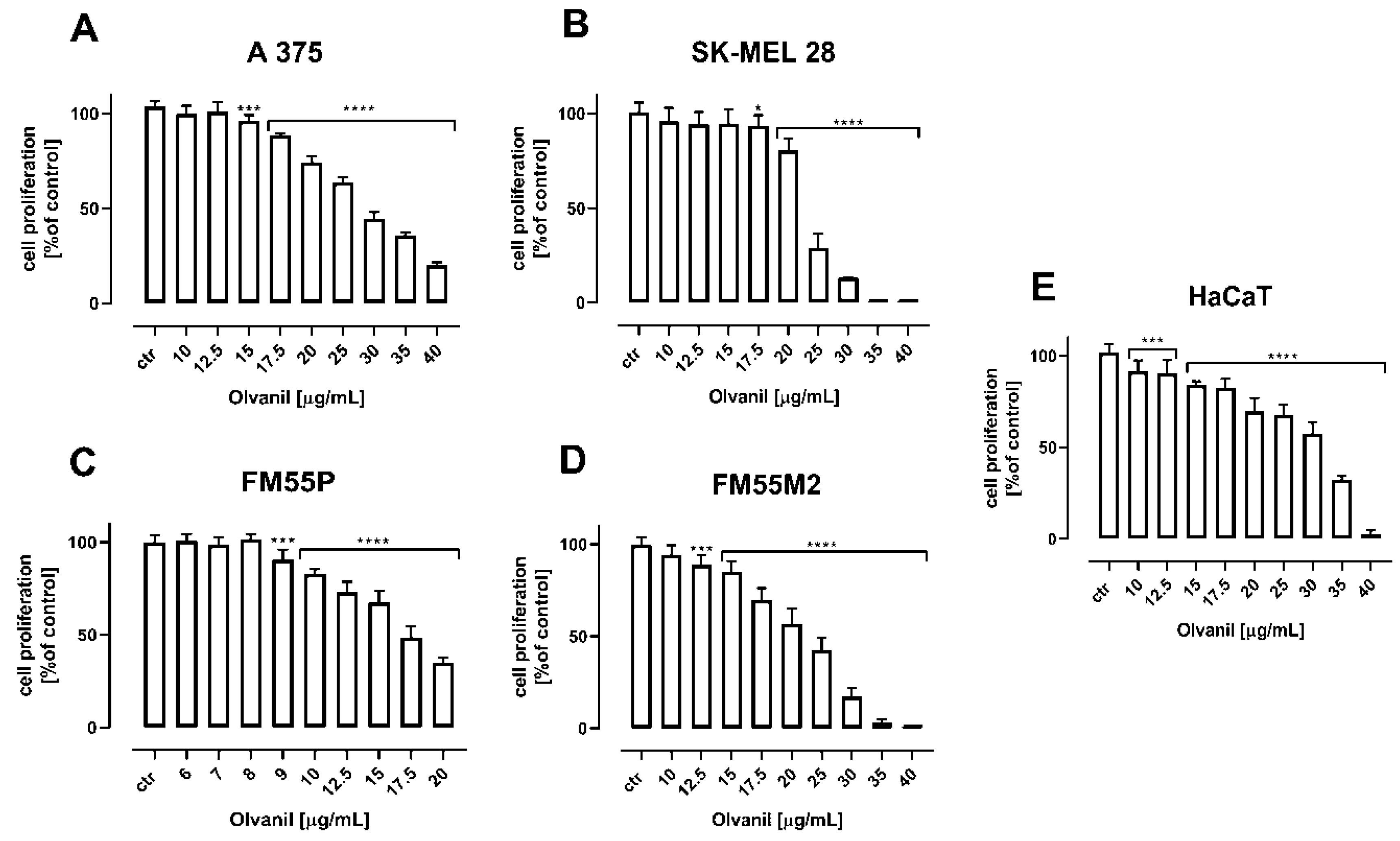
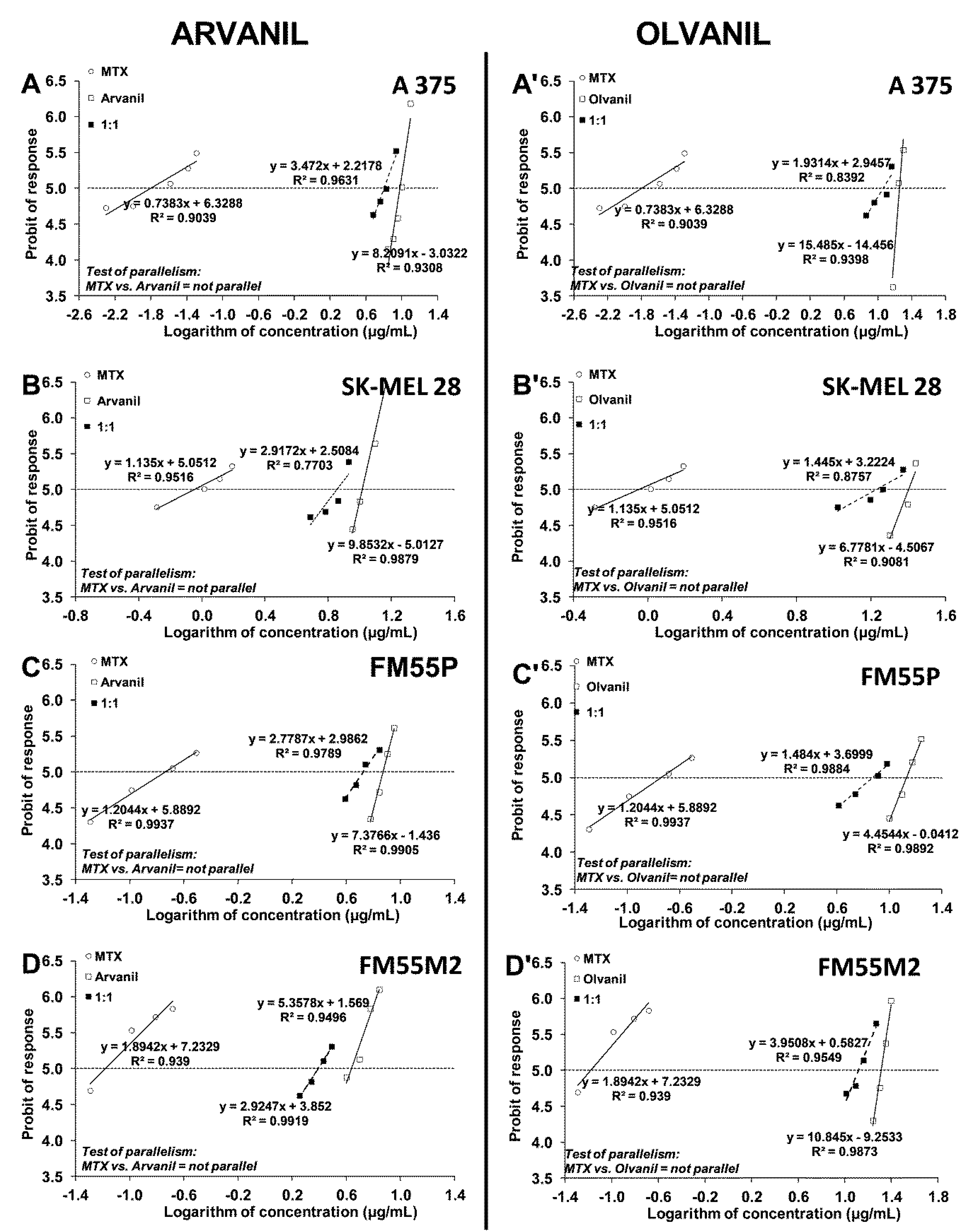
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Drugs
4.3. Cell Viability Assessment
4.4. Cytotoxicity Assessment—LDH Assay
4.5. Cell Proliferation Assay
4.6. Isobolographic Analysis of Interactions
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous Melanoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths among US Whites, 1986–2016. Am. J. Public Health. 2020, 110, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Di Marzo, V.; Breivogel, C.; Bisogno, T.; Melck, D.; Patrick, G.; Tao, Q.; Szallasi, A.; Razdan, R.K.; Martin, B.R. Neurobehavioral Activity in Mice of N-Vanillyl-Arachidonyl-Amide. Eur. J. Pharmacol. 2000, 406, 363–374. [Google Scholar] [CrossRef]
- Di Marzo, V.; Griffin, G.; De Petrocellis, L.; Brandi, I.; Bisogno, T.; Williams, W.; Grier, M.C.; Kulasegram, S.; Mahadevan, A.N.U.; Razdan, R.K.; et al. A Structure/Activity Relationship Study on Arvanil, an Endocannabinoid and Vanilloid Hybrid. J. Pharmacol. Exp. Ther. 2002, 300, 984–991. [Google Scholar] [CrossRef]
- Brooks, J.W.; Pryce, G.; Bisogno, T.; Jaggar, S.I.; Hankey, D.J.R.; Brown, P.; Bridges, D.; Ledent, C.; Bifulco, M.; Rice, A.S.C.; et al. Arvanil-Induced Inhibition of Spasticity and Persistent Pain: Evidence for Therapeutic Sites of Action Different from the Vanilloid VR1 Receptor and Cannabinoid CB(1)/CB(2) Receptors. Eur. J. Pharmacol. 2002, 439, 83–92. [Google Scholar] [CrossRef]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bisogno, T.; Melck, D.; Ross, R.; Brockie, H.; Stevenson, L.; Pertwee, R.; De Petrocellis, L. Interactions between Synthetic Vanilloids and the Endogenous Cannabinoid System. FEBS Lett. 1998, 436, 449–454. [Google Scholar] [CrossRef]
- Melck, D.; Bisogno, T.; De Petrocellis, L.; Chuang, H.H.; Julius, D.; Bifulco, M.; Di Marzo, V. Unsaturated Long-Chain N-Acyl-Vanillyl-Amides (N-AVAMs): Vanilloid Receptor Ligands That Inhibit Anandamide-Facilitated Transport and Bind to CB1 Cannabinoid Receptors. Biochem. Biophys. Res. Commun. 1999, 262, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, M.; Piomelli, D. Anandamide Transport Inhibition by the Vanilloid Agonist Olvanil. Eur. J. Pharmacol. 1999, 364, 75–78. [Google Scholar] [CrossRef]
- Glaser, S.T.; Abumrad, N.A.; Fatade, F.; Kaczocha, M.; Studholme, K.M.; Deutsch, D.G. Evidence against the Presence of an Anandamide Transporter. Proc. Natl. Acad. Sci. USA 2003, 100, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, S.O.P.; Fowler, C.J. Characterization of Palmitoylethanolamide Transport in Mouse Neuro-2a Neuroblastoma and Rat RBL-2H3 Basophilic Leukaemia Cells: Comparison with Anandamide. Br. J. Pharmacol. 2001, 132, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Ursu, D.; Knopp, K.; Beattie, R.E.; Liu, B.; Sher, E. Pungency of TRPV1 Agonists Is Directly Correlated with Kinetics of Receptor Activation and Lipophilicity. Eur. J. Pharmacol. 2010, 641, 114–122. [Google Scholar] [CrossRef]
- Iida, T.; Moriyama, T.; Kobata, K.; Morita, A.; Murayama, N.; Hashizume, S.; Fushiki, T.; Yazawa, S.; Watanabe, T.; Tominaga, M. TRPV1 Activation and Induction of Nociceptive Response by a Non-Pungent Capsaicin-like Compound, Capsiate. Neuropharmacology 2003, 44, 958–967. [Google Scholar] [CrossRef]
- Kopczyńska, B. Role of VR1 and CB1 Receptors in Modelling of Cardio-Respiratory Response to Arvanil, an Endocannabinoid and Vanilloid Hybrid, in Rats. Life Sci. 2008, 83, 85–91. [Google Scholar] [CrossRef]
- Chu, K.M.; Ngan, M.P.; Wai, M.K.; Yeung, C.K.; Andrews, P.L.R.; Percie du Sert, N.; Rudd, J.A. Olvanil: A Non-Pungent TRPV1 Activator Has Anti-Emetic Properties in the Ferret. Neuropharmacology 2010, 58, 383–391. [Google Scholar] [CrossRef]
- Movahed, P.; Evilevitch, V.; Andersson, T.L.G.; Jönsson, B.A.G.; Wollmer, P.; Zygmunt, P.M.; Högestätt, E.D. Vascular Effects of Anandamide and N-Acylvanillylamines in the Human Forearm and Skin Microcirculation. Br. J. Pharmacol. 2005, 146, 171–179. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Lim, S.Y.; Menzies, A.M.; Rizos, H. Mechanisms and Strategies to Overcome Resistance to Molecularly Targeted Therapyfor Melanoma. Cancer 2017, 123, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Evison, B.J.; Sleebs, B.E.; Watson, K.G.; Phillips, D.R.; Cutts, S.M. Mitoxantrone, More than Just Another Topoisomerase II Poison. Med. Res. Rev. 2016, 36, 248–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pandey, V.P.; Yadav, K.; Yadav, A.; Dwivedi, U.N. Natural Products as Anti-Cancerous Therapeutic Molecules Targeted towards Topoisomerases. Curr. Protein Pept. Sci. 2020, 21, 1103–1142. [Google Scholar] [CrossRef]
- Huang, R.Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram Analysis: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Gumbarewicz, E.; Luszczki, J.J.; Wawruszak, A.; Dmoszynska-Graniczka, M.; Grabarska, A.J.; Jarzab, A.M.; Polberg, K.; Stepulak, A. Isobolographic Analysis Demonstrates Additive Effect of Cisplatin and HDIs Combined Treatment Augmenting Their Anti-Cancer Activity in Lung Cancer Cell Lines. Am. J. Cancer Res. 2016, 6, 2831–2845. [Google Scholar]
- Marzęda, P.; Wróblewska-Łuczka, P.; Drozd, M.; Florek-Łuszczki, M.; Załuska-Ogryzek, K.; Łuszczki, J.J. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 6752. [Google Scholar] [CrossRef]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.J.; Wend, P.; Purfürst, B.; Nuber, U.A.; Gurok, U.; et al. Neural Precursor Cells Induce Cell Death of High-Grade Astrocytomas through Stimulation of TRPV1. Nat. Med. 2012, 18, 1232–1238. [Google Scholar] [CrossRef]
- Li, W.; Moore, B.M., II. Modern Chemistry & Applications the Effect of Arvanil on Prostate Cancer Cells Studied by Whole Cell High Resolution Magic Angle Spinning NMR. Mod. Chem. Appl. 2014, 2, 1. [Google Scholar] [CrossRef]
- Ḿarquez, N.; De Petrocellis, L.; Caballero, F.J.; Macho, A.; Schiano-Moriello, A.; Minassi, A.; Appendino, G.; Mũnoz, E.; Di Marzo, V. Iodinated N-Acylvanillamines: Potential “Multiple-Target” Anti-Inflammatory Agents Acting via the Inhibition of t-Cell Activation and Antagonism at Vanilloid TRPV1 Channels. Mol. Pharmacol. 2006, 69, 1373–1382. [Google Scholar] [CrossRef]
- Sancho, R.; De La Vega, L.; Appendino, G.; Di Marzo, V.; Macho, A.; Muñoz, E. The CB1/VR1 Agonist Arvanil Induces Apoptosis through an FADD/Caspase-8-Dependent Pathway. Br. J. Pharmacol. 2003, 140, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.D.; Akers, A.T.; Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Dasgupta, P. Non-Pungent Long Chain Capsaicin-Analogs Arvanil and Olvanil Display Better Anti-Invasive Activity than Capsaicin in Human Small Cell Lung Cancers. Cell Adhes. Migr. 2017, 11, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; De Petrocellis, L.; Bisogno, T. Cannabimimetic Fatty Acid Derivatives in Cancer and Inflammation. Prostaglandins Other Lipid Mediat. 2000, 61, 43–61. [Google Scholar] [CrossRef]
- Moles, E.G.; Friedman, J.R.; Miles, S.L.; Brown, K.C.; Hopper, K.J.; Chen, Y.C.; Dasgupta, P. Arvanil, a Synthetic Capsaicin Mimetic, Synergizes with Irinotecan to Trigger Enhanced Apoptosis in Cisplatin-Resistant Human Lung Cancer. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Bisogno, T.; Ligresti, A.; Bifulco, M.; Melck, D.; Di Marzo, V. Effect on Cancer Cell Proliferation of Palmitoylethanolamide, a Fatty Acid Amide Interacting with Both the Cannabinoid and Vanilloid Signalling Systems. Fundam. Clin. Pharmacol. 2002, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Akman, M.; Aliyev, E.; Tanrıöver, G.; Korcum, A.F. Olvanil Activates Sensory Nerve Fibers, Increases T Cell Response and Decreases Metastasis of Breast Carcinoma. Life Sci. 2022, 291, 120305. [Google Scholar] [CrossRef]
- Marzęda, P.; Drozd, M.; Wróblewska-Łuczka, P.; Łuszczki, J.J. Cannabinoids and Their Derivatives in Struggle against Melanoma. Pharmacol. Rep. 2021, 73, 1485–1496. [Google Scholar] [CrossRef]
- PDQ Supportive and Palliative Care Editorial Board. Nausea and Vomiting Related to Cancer Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Sharkey, K.A.; Cristino, L.; Oland, L.D.; Van Sickle, M.D.; Starowicz, K.; Pittman, Q.J.; Guglielmotti, V.; Davison, J.S.; Di Marzo, V. Arvanil, Anandamide and N-Arachidonoyl-Dopamine (NADA) Inhibit Emesis through Cannabinoid CB1 and Vanilloid TRPV1 Receptors in the Ferret. Eur. J. Neurosci. 2007, 25, 2773–2782. [Google Scholar] [CrossRef]
- Rudd, J.A.; Nalivaiko, E.; Matsuki, N.; Wan, C.; Andrews, P.L.R. The Involvement of TRPV1 in Emesis and Anti-Emesis. Temperature 2015, 2, 258–276. [Google Scholar] [CrossRef]
- Hoffmann, J.; Supronsinchai, W.; Andreou, A.P.; Summ, O.; Akerman, S.; Goadsby, P.J. Olvanil Acts on Transient Receptor Potential Vanilloid Channel 1 and Cannabinoid Receptors to Modulate Neuronal Transmission in the Trigeminovascular System. Pain 2012, 153, 2226–2232. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Chen, S.R.; Pan, H.L. Signaling Mechanisms of Down-Regulation of Voltage-Activated Ca2+ Channels by Transient Receptor Potential Vanilloid Type 1 Stimulation with Olvanil in Primary Sensory Neurons. Neuroscience 2006, 141, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kang, W.H.; Jin, Y.H.; Juhnn, Y.S. Expression of signal transducing G proteins in human melanoma cell lines. Exp. Mol. Med. 1997, 29, 223–227. [Google Scholar] [CrossRef]
- Benga, G. Basic studies on gene therapy of human malignant melanoma by use of the human interferon beta gene entrapped in cationic multilamellar liposomes. 1. Morphology and growth rate of six melanoma cell lines used in transfection experiments with the human interferon beta gene. J. Cell. Mol. Med. 2001, 5, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Real, L.M.; Jimenez, P.; Cantón, J.; Kirkin, A.; García, A.; Abril, E.; Zeuthen, J.; Ruiz-Cabello, F.; Garrido, F. In vivo and in vitro generation of a new altered HLA phenotype in melanoma-tumour-cell variants expressing a single HLA-class-I allele. Int. J. Cancer 1998, 75, 317–323. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Tallarida, R.J.; Porreca, F.; Cowan, A. Statistical Analysis of Drug-Drug and Site-Site Interactions with Isobolograms. Life Sci. 1989, 45, 947–961. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug Synergism: Its Detection and Applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]
- Bobiński, M.; Okła, K.; Łuszczki, J.; Bednarek, W.; Wawruszak, A.; Moreno-bueno, G.; Dmoszyńska-graniczka, M.; Tarkowski, R.; Kotarski, J. Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells. Cancers 2020, 12, 107. [Google Scholar] [CrossRef]
- Loewe, S. The Problem of Synergism and Antagonism of Combined Drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar]
- Luszczki, J.J. Isobolographic Analysis of Interaction between Drugs with Nonparallel Dose-Response Relationship Curves: A Practical Application. Naunyn. Schmiedebergs. Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Czuczwar, S.J. Biphasic Characteristic of Interactions between Stiripentol and Carbamazepine in the Mouse Maximal Electroshock-Induced Seizure Model: A Three-Dimensional Isobolographic Analysis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006, 374, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C. What Is Synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [PubMed]
- Pöch, G.; Vychodil-Kahr, S.; Petru, E. Sigmoid Model versus Median-Effect Analysis for Obtaining Dose-Response Curves for in Vitro Chemosensitivity Testing. Int. J. Clin. Pharmacol. Ther. 1999, 37, 189–192. [Google Scholar] [PubMed]
- Alexa, V.T.; Galuscan, A.; Soica, C.M.; Cozma, A.; Coricovac, D.; Borcan, F.; Popescu, I.; Mioc, A.; Szuhanek, C.; Dehelean, C.A.; et al. In Vitro Assessment of the Cytotoxic and Antiproliferative Profile of Natural Preparations Containing Bergamot, Orange and Clove Essential Oils. Molecules 2022, 27, 990. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, A.; Arteta, B.; Duranti, A.; Alonso-Alconada, D. The Synthetic Cannabinoid URB447 Exerts Antitumor and Antimetastatic Effect in Melanoma and Colon Cancer. Pharmaceuticals 2022, 15, 1166. [Google Scholar] [CrossRef]
- Furuya, A.; Nozawa, M.; Gotoh, J.; Jingu, S.; Akimoto, M.; Higuchi, S.; Suwa, T.; Ogata, H. Pharmacokinetic and Pharmacodynamic Analysis of TS-943, a Selective Non-Peptide Platelet Glycoprotein-IIb/IIIa (GPIIb/IIIa) Receptor Antagonist, Using a Nonlinear Mixed Effect Model in Dogs. J. Pharm. Pharmacol. 2002, 54, 921–927. [Google Scholar] [CrossRef]
- Hassan, S.B.; Jonsson, E.; Larsson, R.; Karlsson, M.O. Model for Time Dependency of Cytotoxic Effect of CHS 828 in Vitro Suggests Two Different Mechanisms of Action. J. Pharmacol. Exp. Ther. 2001, 299, 1140–1147. [Google Scholar]
- Dawson, D.A.; Genco, N.; Bensinger, H.M.; Guinn, D.; Il’giovine, Z.J.; Wayne Schultz, T.; Pöch, G. Evaluation of an Asymmetry Parameter for Curve-Fitting in Single-Chemical and Mixture Toxicity Assessment. Toxicology 2012, 292, 156–161. [Google Scholar] [CrossRef]
- De Lago, E.; Urbani, P.; Ramos, J.A.; Di Marzo, V.; Fernández-Ruiz, J. Arvanil, a Hybrid Endocannabinoid and Vanilloid Compound, Behaves as an Antihyperkinetic Agent in a Rat Model of Huntington’s Disease. Brain Res. 2005, 1050, 210–216. [Google Scholar] [CrossRef]
- Buchwald, P. Quantification of Receptor Binding from Response Data Obtained at Different Receptor Levels: A Simple Individual Sigmoid Fitting and a Unified SABRE Approach. Sci. Rep. 2022, 12, 18833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, K.; Liu, F.; Li, Y.; Zhong, Z.; Hong, S.; Liu, X.; Liu, L. Predicting Antitumor Effect of Deoxypodophyllotoxin in NCI-H460 Tumor-Bearing Mice on the Basis of In Vitro Pharmacodynamics and a Physiologically Based Pharmacokinetic-Pharmacodynamic Model. Drug Metab. Dispos. 2018, 46, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.; Czerwonka, A.; Okon, E.; Stepulak, A. Assessment of Pharmacological Interactions between SIRT2 Inhibitor AGK2 and Paclitaxel in Different Molecular Subtypes of Breast Cancer Cells. Cells 2022, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
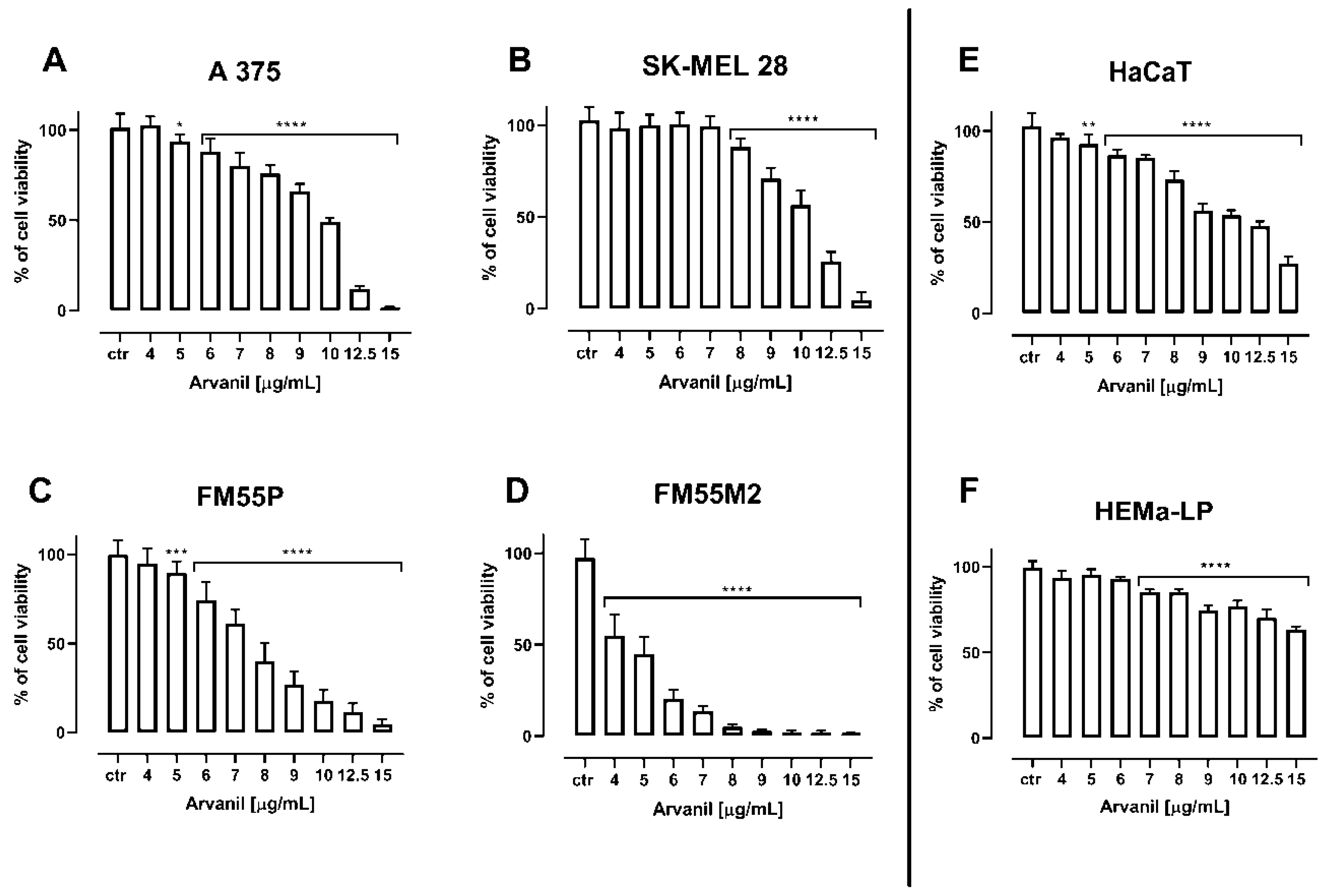
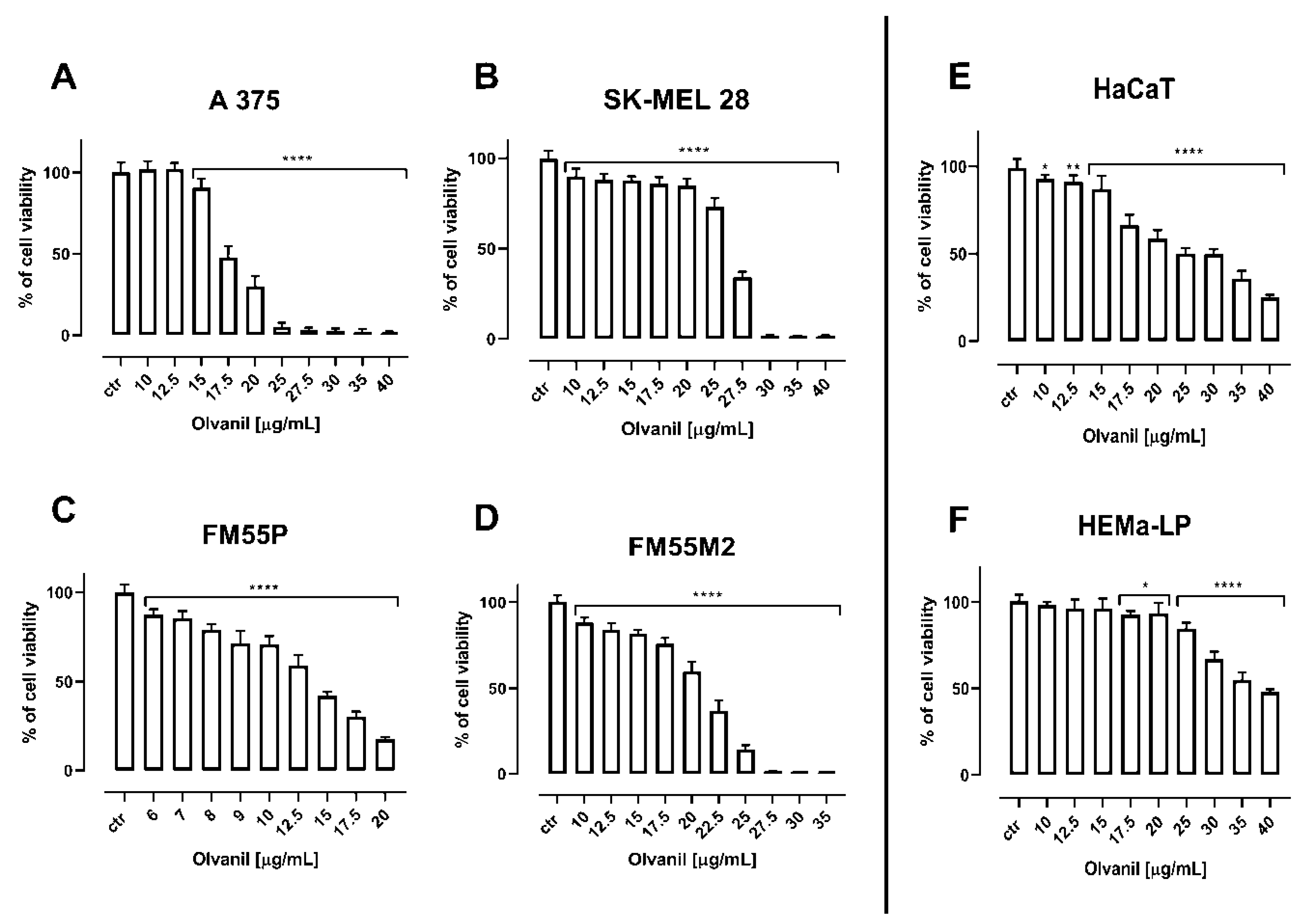
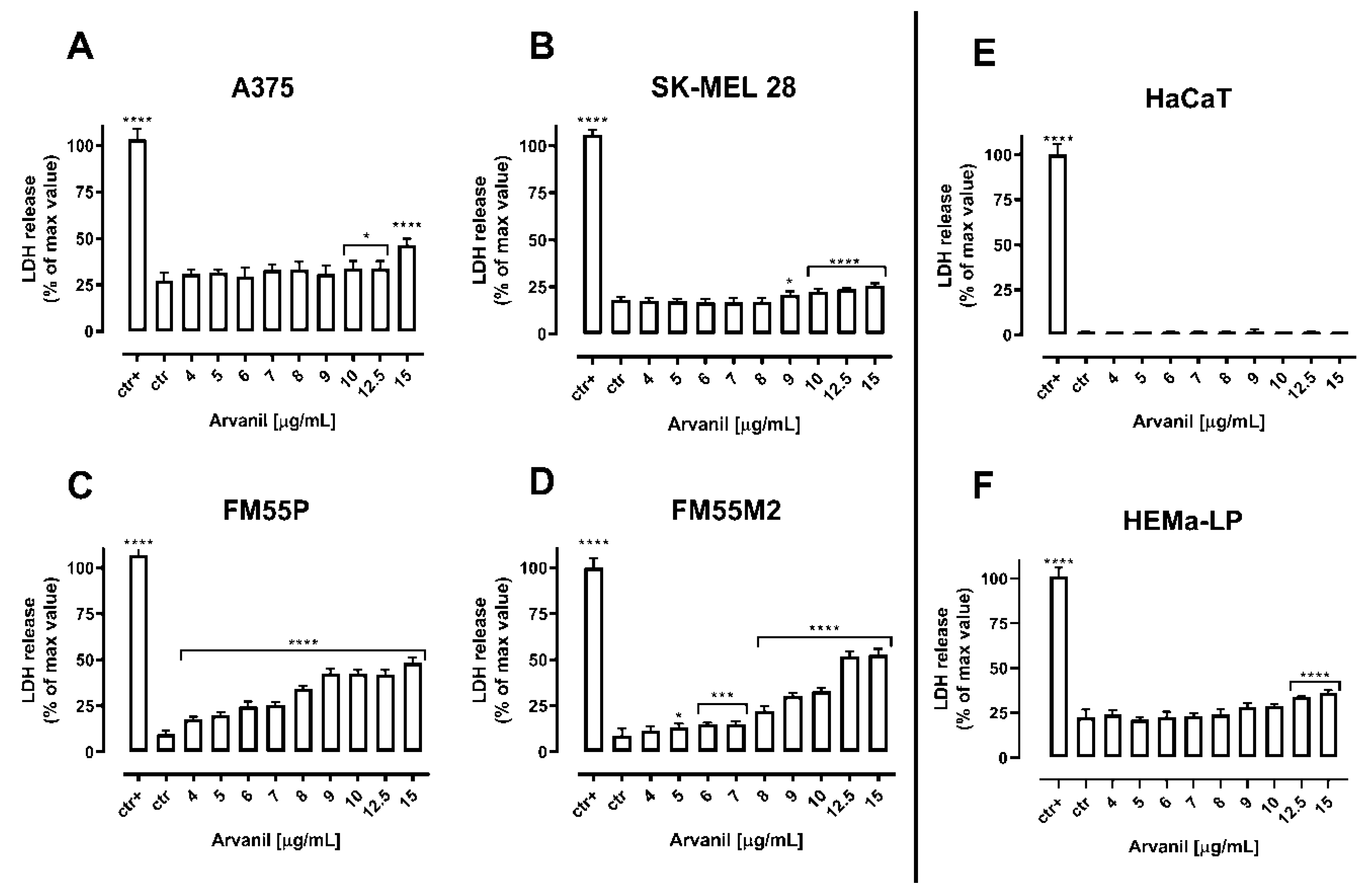

| Cell Line | Arvanil (µg/mL) | Arvanil (µM) | Olvanil (µg/mL) | Olvanil (µM) |
|---|---|---|---|---|
| A375 | 9.52 ± 0.30 | 21.65 ± 0.68 | 18.05 ± 0.40 | 43.22 ± 0.96 |
| SK–MEL28 | 10.38 ± 0.40 | 23.64 ± 0.91 | 25.27 ± 1.43 | 60.51 ± 3.42 |
| FM55P | 7.46 ± 0.39 | 16.97 ± 0.89 | 13.54 ± 1.17 | 32.42 ± 2.80 |
| FM55M2 | 4.37 ± 0.31 | 9.94 ± 0.71 | 20.62 ± 0.73 | 49.37 ± 1.75 |
| Drug Combination | Cell Line | IC50mix (μg/mL) | nmix | Lower IC50add (μg/mL) | nadd | Upper IC50add (μg/mL) | Interaction |
|---|---|---|---|---|---|---|---|
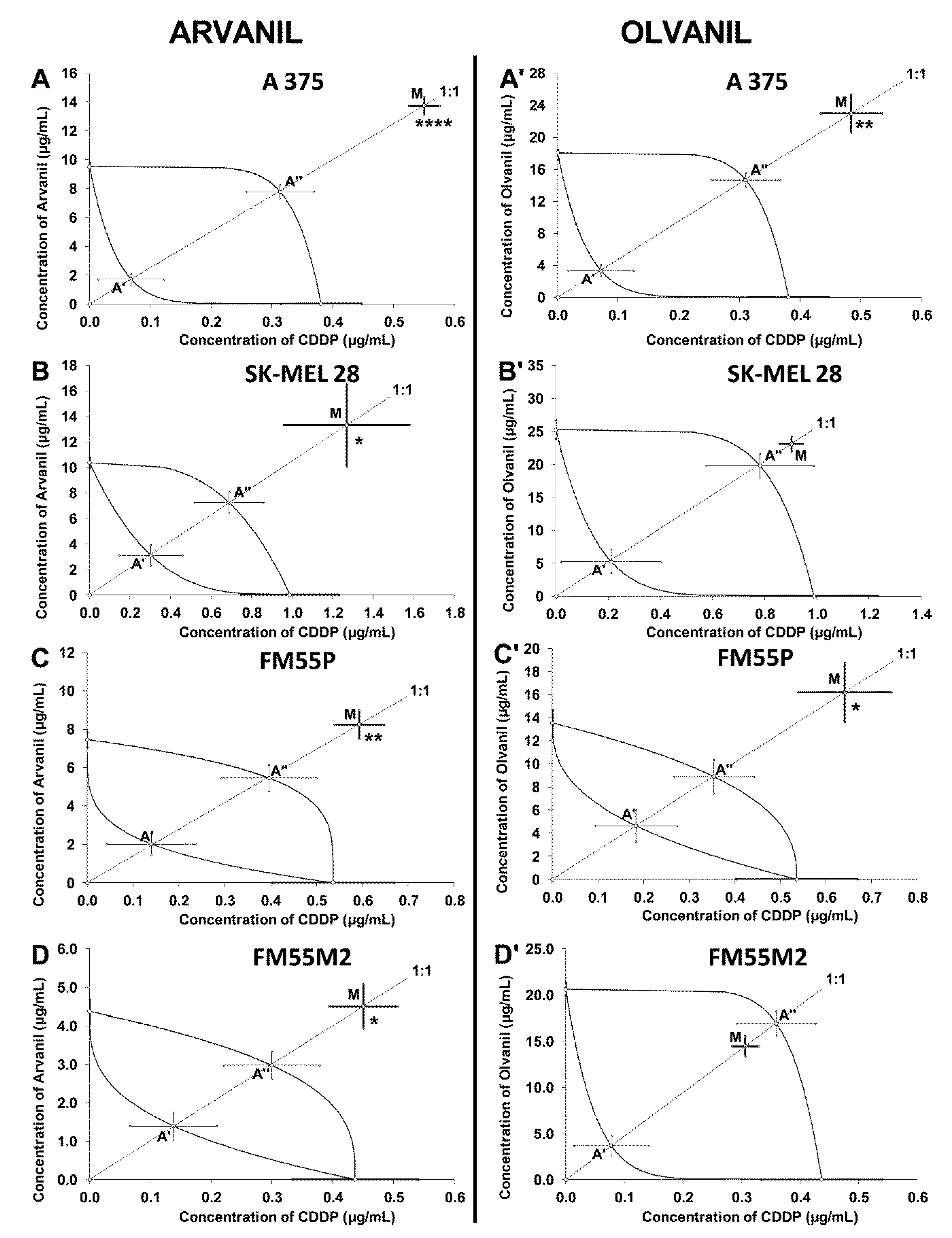
| Arvanil + CDDP | A375 | 14.30 ± 0.65 **** | 96 | 1.77 ± 0.44 | 356 | 8.09 ± 0.52 | Antagonism |
| SK–MEL 28 | 14.60 ± 3.08 * | 96 | 3.42 ± 0.96 | 140 | 7.93 ± 1.00 | Antagonism | |
| FM55P | 8.84 ± 0.81 ** | 72 | 2.15 ± 0.66 | 140 | 5.84 ± 0.79 | Antagonism | |
| FM55M2 | 4.96 ± 0.63 * | 96 | 1.53 ± 0.42 | 132 | 3.27 ± 0.44 | Antagonism | |
| Olvanil + CDDP | A375 | 23.44 ± 2.46 ** | 96 | 3.42 ± 0.73 | 286 | 14.95 ± 0.96 | Antagonism |
| SK–MEL 28 | 23.99 ± 1.18 | 96 | 5.48 ± 1.93 | 140 | 20.56 ± 2.03 | Additivity | |
| FM55P | 16.84 ± 2.70 * | 72 | 4.80 ± 1.48 | 140 | 9.25 ± 1.56 | Antagonism | |
| FM55M2 | 14.77 ± 1.12 | 96 | 3.75 ± 1.12 | 132 | 17.28 ± 1.40 | Additivity | |
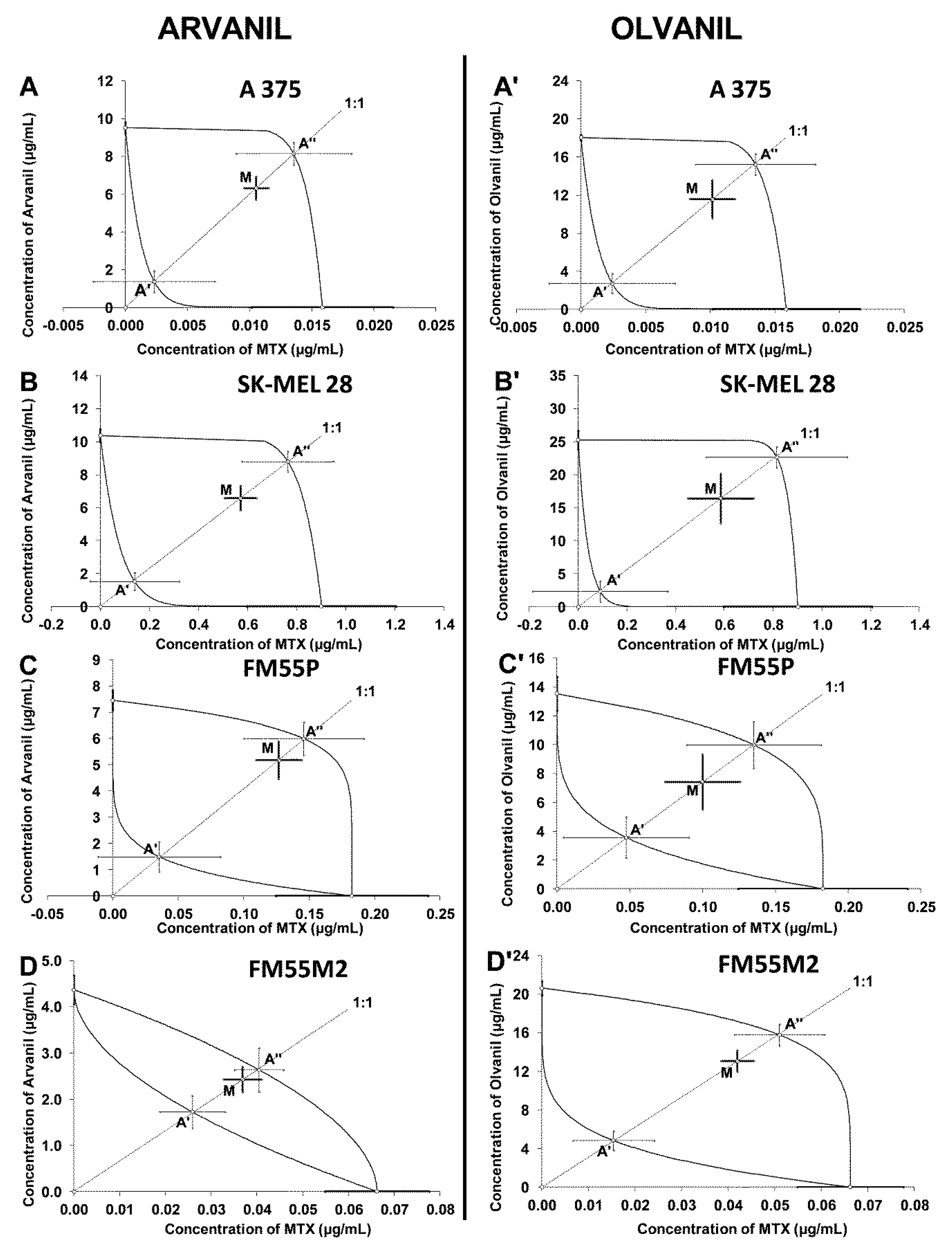
| Arvanil + MTX | A375 | 6.33 ± 0.81 | 96 | 1.37 ± 0.57 | 306 | 8.16 ± 0.59 | Additivity |
| SK–MEL 28 | 7.15 ± 0.81 | 96 | 1.66 ± 0.64 | 140 | 9.57 ± 0.96 | Additivity | |
| FM55P | 5.31 ± 0.73 | 72 | 1.53 ± 0.60 | 140 | 6.14 ± 0.67 | Additivity | |
| FM55M2 | 2.47 ± 0.28 | 96 | 1.75 ± 0.36 | 168 | 2.68 ± 0.48 | Additivity | |
| Olvanil + MTX | A375 | 11.58 ± 1.99 | 96 | 2.72 ± 1.03 | 306 | 15.23 ± 1.11 | Additivity |
| SK–MEL 28 | 16.99 ± 3.90 | 96 | 2.42 ± 1.83 | 140 | 23.46 ± 1.83 | Additivity | |
| FM55P | 7.52 ± 1.94 | 72 | 3.61 ± 1.46 | 140 | 10.12 ± 1.65 | Additivity | |
| FM55M2 | 13.12 ± 1.10 | 96 | 4.82 ± 1.00 | 168 | 15.82 ± 1.10 | Additivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzęda, P.; Wróblewska-Łuczka, P.; Florek-Łuszczki, M.; Drozd, M.; Góralczyk, A.; Łuszczki, J.J. Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 14192. https://doi.org/10.3390/ijms232214192
Marzęda P, Wróblewska-Łuczka P, Florek-Łuszczki M, Drozd M, Góralczyk A, Łuszczki JJ. Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. International Journal of Molecular Sciences. 2022; 23(22):14192. https://doi.org/10.3390/ijms232214192
Chicago/Turabian StyleMarzęda, Paweł, Paula Wróblewska-Łuczka, Magdalena Florek-Łuszczki, Małgorzata Drozd, Agnieszka Góralczyk, and Jarogniew J. Łuszczki. 2022. "Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis" International Journal of Molecular Sciences 23, no. 22: 14192. https://doi.org/10.3390/ijms232214192
APA StyleMarzęda, P., Wróblewska-Łuczka, P., Florek-Łuszczki, M., Drozd, M., Góralczyk, A., & Łuszczki, J. J. (2022). Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. International Journal of Molecular Sciences, 23(22), 14192. https://doi.org/10.3390/ijms232214192






