3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation
Abstract
1. Introduction
2. Results
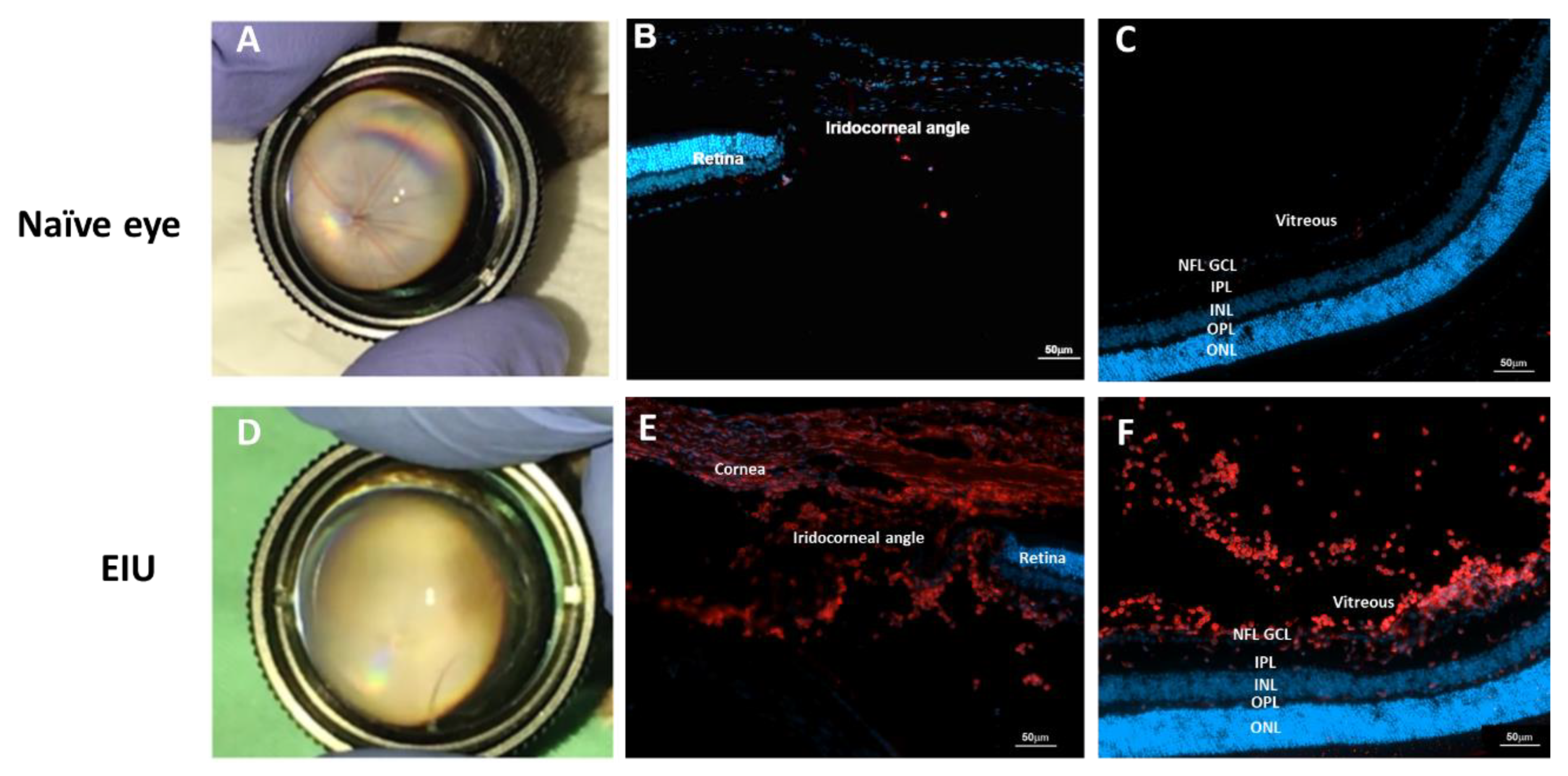
2.1. LPS-Induced EIU Causes Immune Cell Accumulation in Different Eye Compartments
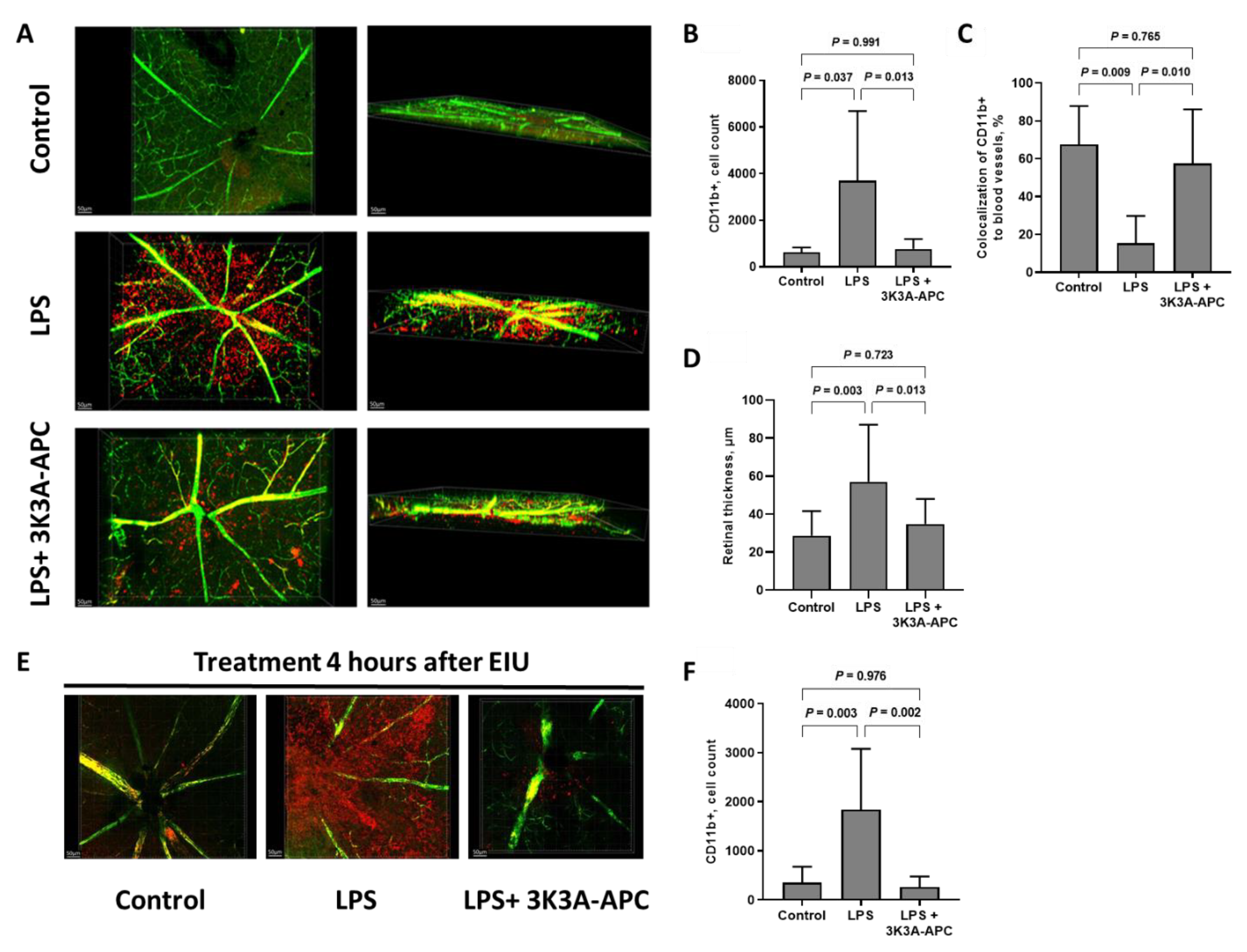
2.2. 3K3A-APC Inhibits Inflammatory Cell Extravasation into the Retinal Parenchyma and Increases Myeloid Retention in Blood Vessels
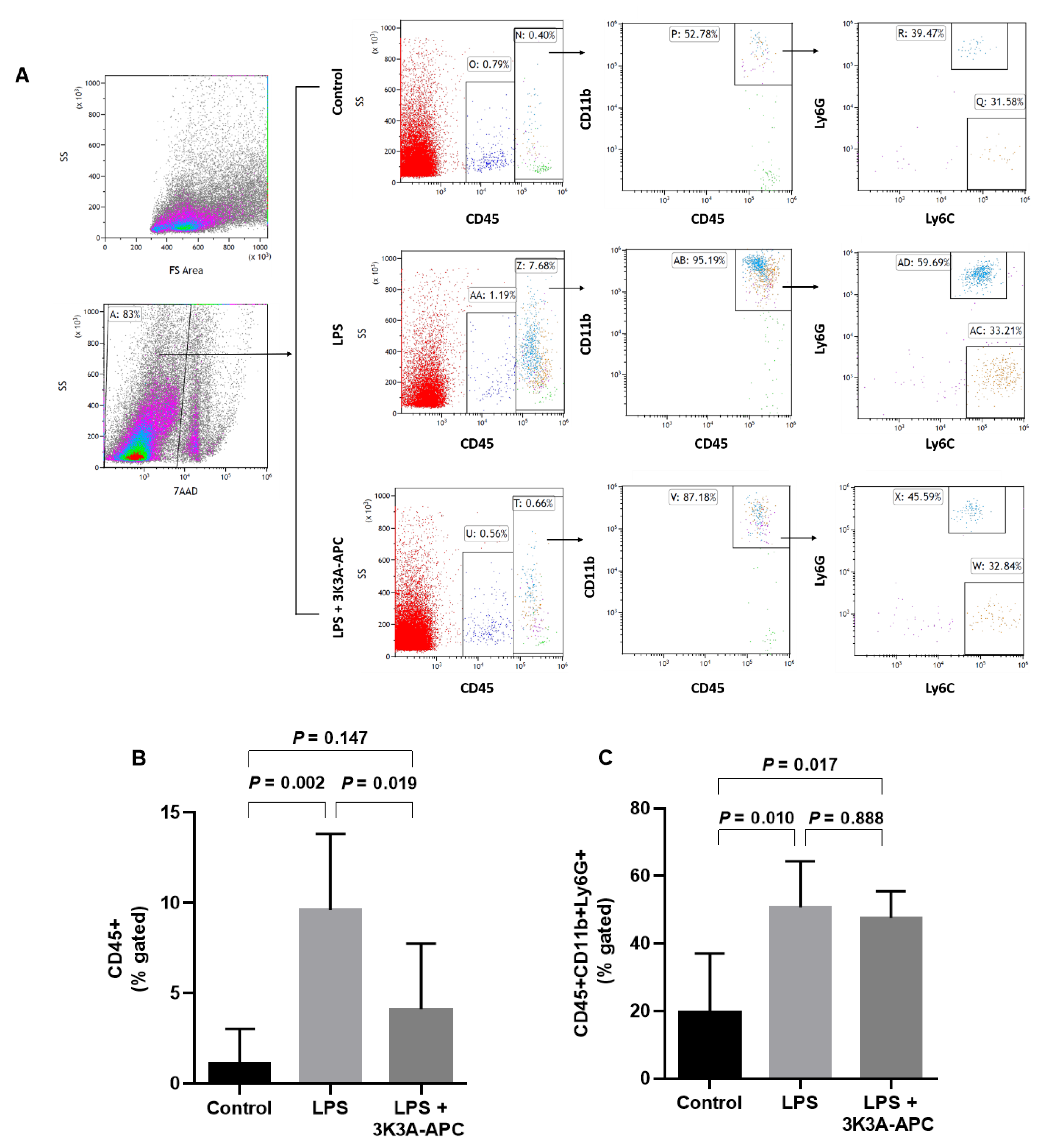
2.3. 3K3A-APC Reduces Recruitment of Leukocytes without Altering the Type of Inflammatory Cell Subpopulations after EIU Induction
2.4. 3K3A-APC Affects the Local Immune Environment by Inhibiting Resident Microglia Activation
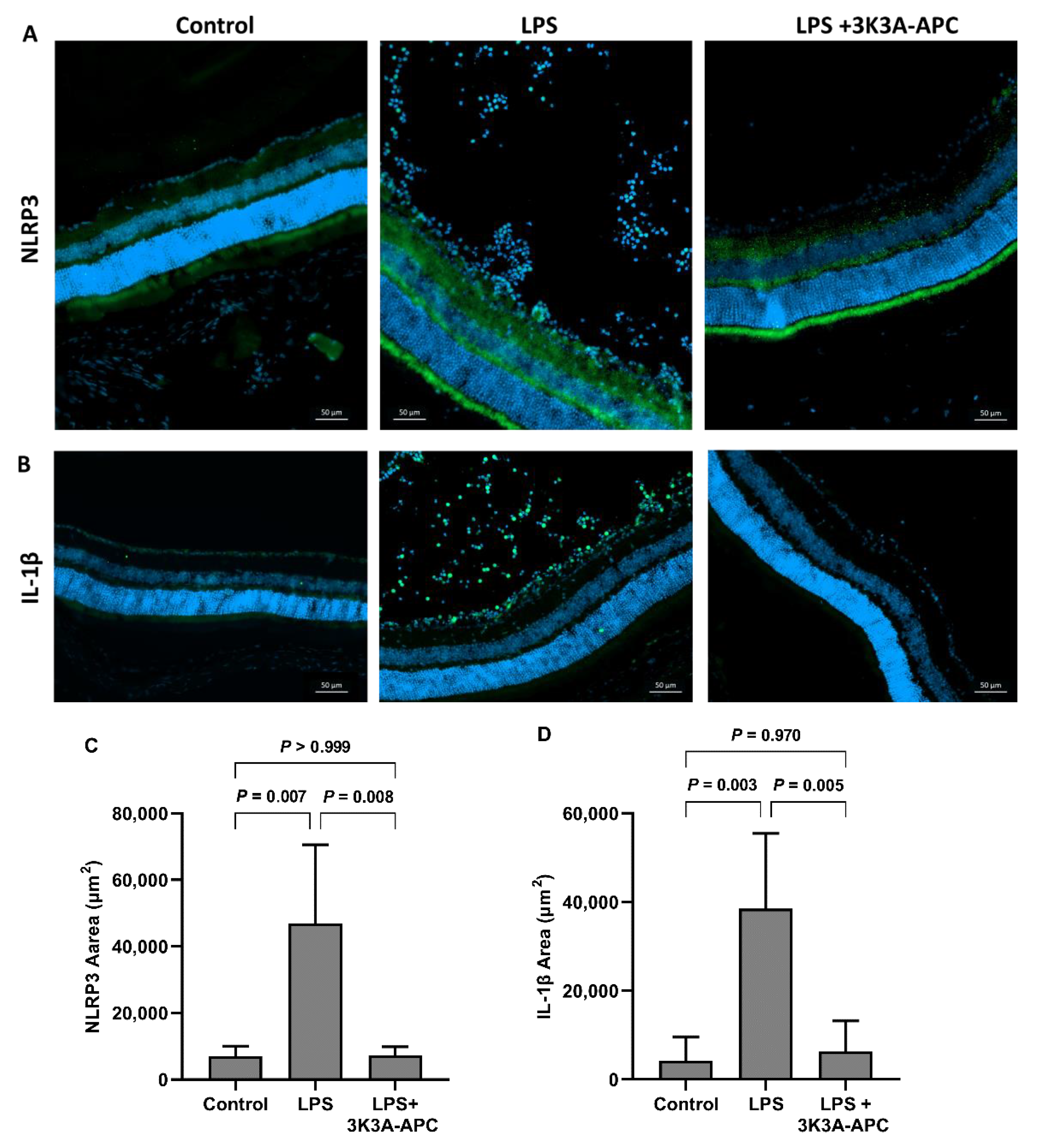
2.5. 3K3A-APC Reduces NLRP3 and Il1β Levels after EIU Induction
3. Discussion
4. Materials and Methods
4.1. Endotoxin-Induced Uveitis Animal Model
4.2. Vascular Imaging and Flatmount Immunostaining
4.3. Cryosection Histology and Immunofluorescence Staining
4.4. Retinal Dissociation and Flow Cytometry Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood 2018, 132, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C: Biased for translation. Blood 2015, 125, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Kohli, S.; Al-Dabet, M.M.; Isermann, B. Cell biology of activated protein C. Curr. Opin. Hematol. 2019, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, M.; Masterson, M.; David, M.; Rivard, G.E.; Muller, F.M.; Kreuz, W.; Beeg, T.; Minford, A.; Allgrove, J.; Cohen, J.D.; et al. Replacement therapy with a monoclonal antibody purified protein C concentrate in newborns with severe congenital protein C deficiency. Semin. Thromb. Hemost. 1995, 21, 371–381. [Google Scholar] [CrossRef]
- Ghassemi, F.; Abdi, F.; Esfahani, M. Ophthalmic manifestations of congenital protein C deficiency: A case report and mini review. BMC Ophthalmol. 2020, 20, 282. [Google Scholar] [CrossRef]
- Hara, C.; Kamei, M.; Sakaguchi, H.; Matsumura, N.; Sakimoto, S.; Suzuki, M.; Nishida, K.; Fukushima, Y.; Nishida, K. Activated Protein C for Ischemic Central Retinal Vein Occlusion: One-Year Results. Ophthalmol. Retin. 2019, 3, 93–94. [Google Scholar] [CrossRef]
- Hara, C.; Kamei, M.; Sakaguchi, H.; Matsumura, N.; Sakimoto, S.; Suzuki, M.; Nishida, K.; Fukushima, Y.; Nishida, K. Long-term outcomes of intravitreal activated protein C injection for ischemic central retinal vein occlusion: An extension trial. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2919–2927. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gardlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Gale, A.J.; Yegneswaran, S.; Griffin, J.H. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood 2004, 104, 1740–1744. [Google Scholar] [CrossRef]
- Kant, R.; Halder, S.K.; Fernandez, J.A.; Griffin, J.H.; Milner, R. Activated Protein C Attenuates Experimental Autoimmune Encephalomyelitis Progression by Enhancing Vascular Integrity and Suppressing Microglial Activation. Front. Neurosci. 2020, 14, 333. [Google Scholar] [CrossRef]
- Griffin, J.H.; Fernandez, J.A.; Lyden, P.D.; Zlokovic, B.V. Activated protein C promotes neuroprotection: Mechanisms and translation to the clinic. Thromb. Res. 2016, 141 (Suppl. S2), S62–S64. [Google Scholar] [CrossRef]
- Lyden, P.; Pryor, K.E.; Coffey, C.S.; Cudkowicz, M.; Conwit, R.; Jadhav, A.; Sawyer, R.N., Jr.; Claassen, J.; Adeoye, O.; Song, S.; et al. Final Results of the RHAPSODY Trial: A Multi-Center, Phase 2 Trial Using a Continual Reassessment Method to Determine the Safety and Tolerability of 3K3A-APC, A Recombinant Variant of Human Activated Protein C, in Combination with Tissue Plasminogen Activator, Mechanical Thrombectomy or both in Moderate to Severe Acute Ischemic Stroke. Ann. Neurol. 2019, 85, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Livnat, T.; Weinberger, Y.; Fernandez, J.A.; Bashir, A.; Ben-David, G.; Palevski, D.; Levy-Mendelovich, S.; Kenet, G.; Budnik, I.; Nisgav, Y.; et al. Activated Protein C (APC) and 3K3A-APC-Induced Regression of Choroidal Neovascularization (CNV) Is Accompanied by Vascular Endothelial Growth Factor (VEGF) Reduction. Biomolecules 2021, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Livnat, T.; Weinberger, Y.; Budnik, I.; Deitch, I.; Dahbash, M.; Sella, R.; Dardik, R.; Kenet, G.; Nisgav, Y.; Weinberger, D. Activated protein C induces suppression and regression of choroidal neovascularization- A murine model. Exp. Eye Res. 2019, 186, 107695. [Google Scholar] [CrossRef]
- Healy, L.D.; Rigg, R.A.; Griffin, J.H.; McCarty, O.J.T. Regulation of immune cell signaling by activated protein C. J. Leukoc. Biol. 2018, 103, 1197–1203. [Google Scholar] [CrossRef]
- Nazir, S.; Gadi, I.; Al-Dabet, M.M.; Elwakiel, A.; Kohli, S.; Ghosh, S.; Manoharan, J.; Ranjan, S.; Bock, F.; Braun-Dullaeus, R.C.; et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood 2017, 130, 2664–2677. [Google Scholar] [CrossRef]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Yang, X.; Luo, C.; Cai, J.; Powell, D.W.; Yu, D.; Kuehn, M.H.; Tezel, G. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8442–8454. [Google Scholar] [CrossRef]
- Mugisho, O.O.; Green, C.R. The NLRP3 inflammasome in age-related eye disease: Evidence-based connexin hemichannel therapeutics. Exp. Eye Res. 2022, 215, 108911. [Google Scholar] [CrossRef]
- Yadav, U.C.; Ramana, K.V. Endotoxin-induced uveitis in rodents. Methods Mol. Biol. 2013, 1031, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Klaska, I.P.; Forrester, J.V. Mouse models of autoimmune uveitis. Curr. Pharm. Des. 2015, 21, 2453–2467. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; McDevitt, H.O.; Guss, R.B.; Egbert, P.R. Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980, 286, 611–613. [Google Scholar] [CrossRef]
- Chu, C.J.; Gardner, P.J.; Copland, D.A.; Liyanage, S.E.; Gonzalez-Cordero, A.; Kleine Holthaus, S.M.; Luhmann, U.F.; Smith, A.J.; Ali, R.R.; Dick, A.D. Multimodal analysis of ocular inflammation using the endotoxin-induced uveitis mouse model. Dis. Model. Mech. 2016, 9, 473–481. [Google Scholar] [CrossRef]
- Kokona, D.; Ebneter, A.; Escher, P.; Zinkernagel, M.S. Colony-stimulating factor 1 receptor inhibition prevents disruption of the blood-retina barrier during chronic inflammation. J. Neuroinflam. 2018, 15, 340. [Google Scholar] [CrossRef]
- Lin, F.L.; Ho, J.D.; Cheng, Y.W.; Chiou, G.C.Y.; Yen, J.L.; Chang, H.M.; Lee, T.H.; Hsiao, G. Theissenolactone C Exhibited Ocular Protection of Endotoxin-Induced Uveitis by Attenuating Ocular Inflammatory Responses and Glial Activation. Front. Pharm. 2018, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.E.; Gardner, P.J.; Ribeiro, J.; Cristante, E.; Sampson, R.D.; Luhmann, U.F.; Ali, R.R.; Bainbridge, J.W. Flow cytometric analysis of inflammatory and resident myeloid populations in mouse ocular inflammatory models. Exp. Eye Res. 2016, 151, 160–170. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, T.H.; Won, Y.K.; Shin, Y.I.; Kim, J.Y. Characteristics of retinal layer thickness in acute anterior uveitis: An optical coherence tomography study. Acta Ophthalmol. 2020, 98, e50–e55. [Google Scholar] [CrossRef]
- Noailles, A.; Fernandez-Sanchez, L.; Lax, P.; Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J. Neuroinflam. 2014, 11, 186. [Google Scholar] [CrossRef]
- Lyden, P.D.; Pryor, K.E.; Minigh, J.; Davis, T.P.; Griffin, J.H.; Levy, H.; Zlokovic, B.V. Stroke Treatment With PAR-1 Agents to Decrease Hemorrhagic Transformation. Front. Neurol. 2021, 12, 593582. [Google Scholar] [CrossRef]
- Huuskonen, M.T.; Wang, Y.; Nikolakopoulou, A.M.; Montagne, A.; Dai, Z.; Lazic, D.; Sagare, A.P.; Zhao, Z.; Fernandez, J.A.; Griffin, J.H.; et al. Protection of ischemic white matter and oligodendrocytes in mice by 3K3A-activated protein C. J. Exp. Med. 2022, 219, e20211372. [Google Scholar] [CrossRef]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2021. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef]
- Shibata, M.; Kumar, S.R.; Amar, A.; Fernandez, J.A.; Hofman, F.; Griffin, J.H.; Zlokovic, B.V. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 2001, 103, 1799–1805. [Google Scholar] [CrossRef]
- Ebneter, A.; Casson, R.J.; Wood, J.P.; Chidlow, G. Microglial activation in the visual pathway in experimental glaucoma: Spatiotemporal characterization and correlation with axonal injury. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6448–6460. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef]
- Fan, W.; Huang, W.; Chen, J.; Li, N.; Mao, L.; Hou, S. Retinal microglia: Functions and diseases. Immunology 2022, 166, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Lazic, D.; Sagare, A.P.; Nikolakopoulou, A.M.; Griffin, J.H.; Vassar, R.; Zlokovic, B.V. 3K3A-activated protein C blocks amyloidogenic BACE1 pathway and improves functional outcome in mice. J. Exp. Med. 2019, 216, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Merida, S.; Palacios, E.; Navea, A.; Bosch-Morell, F. Macrophages and Uveitis in Experimental Animal Models. Mediat. Inflamm. 2015, 2015, 671417. [Google Scholar] [CrossRef]
- Ardeljan, D.; Chan, C.C. Aging is not a disease: Distinguishing age-related macular degeneration from aging. Prog. Retin. Eye Res. 2013, 37, 68–89. [Google Scholar] [CrossRef]
- Zeng, X.X.; Ng, Y.K.; Ling, E.A. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis. Neurosci. 2000, 17, 463–471. [Google Scholar] [CrossRef]
- Healy, L.D.; Fernandez, J.A.; Mosnier, L.O.; Griffin, J.H. Activated protein C and PAR1-derived and PAR3-derived peptides are anti-inflammatory by suppressing macrophage NLRP3 inflammasomes. J. Thromb. Haemost. JTH 2021, 19, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.; Khan, M.N.; Chemaly, M.; Callaghan, B.; Doyle, C.; Willoughby, C.E.; Atkinson, S.D.; Gregory-Ksander, M.; McGilligan, V. Targeting the NLRP3 Inflammasome in Glaucoma. Biomolecules 2021, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, J.; Qin, T.; Bao, J.; Dong, H.; Zhou, X.; Hou, S.; Mao, L. The role of the inflammasomes in the pathogenesis of uveitis. Exp. Eye Res. 2021, 208, 108618. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.K.; Ahmed, Z.; Singh, S.; Kumar, A. Essential Role of NLRP3 Inflammasome in Mediating IL-1beta Production and the Pathobiology of Staphylococcus aureus Endophthalmitis. Infect. Immun. 2022, 90, e0010322. [Google Scholar] [CrossRef]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Arta, A.; Eriksen, A.Z.; Melander, F.; Kempen, P.; Larsen, M.; Andresen, T.L.; Urquhart, A.J. Endothelial Protein C-Targeting Liposomes Show Enhanced Uptake and Improved Therapeutic Efficacy in Human Retinal Endothelial Cells. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2119–2132. [Google Scholar] [CrossRef]
- Goldberg, Z.; Sher, I.; Qassim, L.; Chapman, J.; Rotenstreich, Y.; Shavit-Stein, E. Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers. Int. J. Mol. Sci. 2022, 23, 984. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Reiser, G. Two types of protease-activated receptors (PAR-1 and PAR-2) mediate calcium signaling in rat retinal ganglion cells RGC-5. Brain Res. 2005, 1047, 159–167. [Google Scholar] [CrossRef]
- Lee-Rivera, I.; Lopez, E.; Carranza-Perez, M.G.; Lopez-Colome, A.M. PKC-zeta Regulates Thrombin-Induced Proliferation of Human Muller Glial Cells. Invest. Ophthalmol. Vis. Sci. 2016, 57, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.A.; Xu, X.; Liu, D.; Zlokovic, B.V.; Griffin, J.H. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol. Dis. 2003, 30, 271–276. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Singh, I.; Liu, D.; Fernandez, J.A.; Griffin, J.H.; Chow, N.; Zlokovic, B.V. Species-dependent neuroprotection by activated protein C mutants with reduced anticoagulant activity. J. Neurochem. 2009, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, J.D.; Toguri, J.T.; Gupta, R.R.; Samad, A.; O’Brien, D.M.; Dickinson, J.; Cruess, A.; Kelly, M.E.M.; Seamone, M.E. Effects of intravitreal bevacizumab in Gram-positive and Gram-negative models of ocular inflammation. Clin. Exp. Ophthalmol. 2019, 47, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, H.L.; Woods, A.; Clowers, J.S.; Planck, S.R.; Rosenbaum, J.T. The NLRP3 inflammasome is active but not essential in endotoxin-induced uveitis. Inflamm. Res. 2012, 61, 225–231. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palevski, D.; Ben-David, G.; Weinberger, Y.; Haj Daood, R.; Fernández, J.A.; Budnik, I.; Levy-Mendelovich, S.; Kenet, G.; Nisgav, Y.; Weinberger, D.; et al. 3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation. Int. J. Mol. Sci. 2022, 23, 14196. https://doi.org/10.3390/ijms232214196
Palevski D, Ben-David G, Weinberger Y, Haj Daood R, Fernández JA, Budnik I, Levy-Mendelovich S, Kenet G, Nisgav Y, Weinberger D, et al. 3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation. International Journal of Molecular Sciences. 2022; 23(22):14196. https://doi.org/10.3390/ijms232214196
Chicago/Turabian StylePalevski, Dahlia, Gil Ben-David, Yehonatan Weinberger, Rabeei Haj Daood, José A. Fernández, Ivan Budnik, Sarina Levy-Mendelovich, Gili Kenet, Yael Nisgav, Dov Weinberger, and et al. 2022. "3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation" International Journal of Molecular Sciences 23, no. 22: 14196. https://doi.org/10.3390/ijms232214196
APA StylePalevski, D., Ben-David, G., Weinberger, Y., Haj Daood, R., Fernández, J. A., Budnik, I., Levy-Mendelovich, S., Kenet, G., Nisgav, Y., Weinberger, D., Griffin, J. H., & Livnat, T. (2022). 3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation. International Journal of Molecular Sciences, 23(22), 14196. https://doi.org/10.3390/ijms232214196







