Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes
Abstract
1. Introduction
2. Socio-Demographic and Environmental Modifier Factors
3. Modifier Genes
3.1. Genetic Polymorphism
3.2. A Priori and Non-A Priori Approaches
3.3. Modifier Genes of the Lung Function
3.4. Modifier Genes of Pseudomonas aeruginosa Infection
3.5. Modifier Genes of Meconium Ileus
3.6. Modifier Genes of CF-Related Diabetes
3.7. Modifier Genes of CF-Liver Disease
4. Modifier Factors of CFTR Modulators Response
4.1. Modifier Genes and Response to Ivacaftor
4.2. Modifier Genes and Response to Lumacaftor-Ivacaftor
4.3. Modifier Genes and Response to Tezacaftor-Ivacaftor and Elexacaftor-Tezacaftor-Ivacaftor
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Corvol, H.; Taytard, J.; Tabary, O.; Le Rouzic, P.; Guillot, L.; Clement, A. Challenges of personalized medicine for cystic fibrosis. Arch. Pediatr. 2015, 22, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Corvol, H.; Thompson, K.E.; Tabary, O.; le Rouzic, P.; Guillot, L. Translating the genetics of cystic fibrosis to personalized medicine. Transl. Res. 2015, 168, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2014, 16, 45–56. [Google Scholar] [CrossRef]
- Paranjapye, A.; Ruffin, M.; Harris, A.; Corvol, H. Genetic variation in CFTR and modifier loci may modulate cystic fibrosis disease severity. J. Cyst. Fibros. 2020, 19 (Suppl. 1), S10–S14. [Google Scholar] [CrossRef]
- Rowntree, R.K.; Harris, A. The phenotypic consequences of CFTR mutations. Ann. Hum. Genet. 2003, 67, 471–485. [Google Scholar] [CrossRef]
- Santis, G.; Osborne, L.; Knight, R.A.; Hodson, M.E. Independent genetic determinants of pancreatic and pulmonary status in cystic fibrosis. Lancet 1990, 336, 1081–1084. [Google Scholar] [CrossRef]
- Mekus, F.; Ballmann, M.; Bronsveld, I.; Bijman, J.; Veeze, H.; Tummler, B. Categories of deltaF508 homozygous cystic fibrosis twin and sibling pairs with distinct phenotypic characteristics. Twin Res. 2000, 3, 277–293. [Google Scholar] [CrossRef]
- Collaco, J.M.; Blackman, S.M.; McGready, J.; Naughton, K.M.; Cutting, G.R. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J. Pediatr. 2010, 157, 802–807.e3. [Google Scholar] [CrossRef]
- Vanscoy, L.L.; Blackman, S.M.; Collaco, J.M.; Bowers, A.; Lai, T.; Naughton, K.; Algire, M.; McWilliams, R.; Beck, S.; Hoover-Fong, J.; et al. Heritability of lung disease severity in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Modifier genes in Mendelian disorders: The example of cystic fibrosis. Ann. N. Y. Acad. Sci. 2010, 1214, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Ferec, C.; Scotet, V.; Beucher, J.; Corvol, H. Genetics and modifier genes, atypical and rare forms. Arch. Pediatr. 2012, 19 (Suppl. 1), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Corey, M.; Edwards, L.; Levison, H.; Knowles, M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J. Pediatr. 1997, 131, 809–814. [Google Scholar] [CrossRef]
- Konstan, M.W.; Morgan, W.J.; Butler, S.M.; Pasta, D.J.; Craib, M.L.; Silva, S.J.; Stokes, D.C.; Wohl, M.E.; Wagener, J.S.; Regelmann, W.E.; et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J. Pediatr. 2007, 151, 134–139.e1. [Google Scholar] [CrossRef]
- Schaedel, C.; de Monestrol, I.; Hjelte, L.; Johannesson, M.; Kornfalt, R.; Lindblad, A.; Strandvik, B.; Wahlgren, L.; Holmberg, L. Predictors of deterioration of lung function in cystic fibrosis. Pediatr. Pulmonol. 2002, 33, 483–491. [Google Scholar] [CrossRef]
- Toledano, M.B.; Mukherjee, S.K.; Howell, J.; Westaby, D.; Khan, S.A.; Bilton, D.; Simmonds, N.J. The emerging burden of liver disease in cystic fibrosis patients: A UK nationwide study. PLoS ONE 2019, 14, e0212779. [Google Scholar] [CrossRef]
- Crull, M.R.; Somayaji, R.; Ramos, K.J.; Caldwell, E.; Mayer-Hamblett, N.; Aitken, M.L.; Nichols, D.P.; Rowhani-Rahbar, A.; Goss, C.H. Changing Rates of Chronic Pseudomonas aeruginosa Infections in Cystic Fibrosis: A Population-Based Cohort Study. Clin. Infect. Dis. 2018, 67, 1089–1095. [Google Scholar] [CrossRef]
- Mésinèle, J.; Ruffin, M.; Kemgang, A.; Guillot, L.; Boëlle, P.Y.; Corvol, H. Risk factors for Pseudomonas aeruginosa airway infection and lung function decline in children with cystic fibrosis. J. Cyst. Fibros 2022, 21, 45–51. [Google Scholar] [CrossRef]
- van Horck, M.; van de Kant, K.; Winkens, B.; Wesseling, G.; Gulmans, V.; Hendriks, H.; van der Grinten, C.; Jobsis, Q.; Dompeling, E. Risk factors for lung disease progression in children with cystic fibrosis. Eur. Respir. J. 2018, 51, 1702509. [Google Scholar] [CrossRef]
- Pittman, J.E.; Noah, H.; Calloway, H.E.; Davis, S.D.; Leigh, M.W.; Drumm, M.; Sagel, S.D.; Accurso, F.J.; Knowles, M.R.; Sontag, M.K. Early childhood lung function is a stronger predictor of adolescent lung function in cystic fibrosis than early Pseudomonas aeruginosa infection. PLoS ONE 2017, 12, e0177215. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; O’Hea, U.; Williams, G.; Smyth, R.; Heaf, D. Passive smoking and impaired lung function in cystic fibrosis. Arch. Dis. Child 1994, 71, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Collaco, J.M.; Vanscoy, L.; Bremer, L.; McDougal, K.; Blackman, S.M.; Bowers, A.; Naughton, K.; Jennings, J.; Ellen, J.; Cutting, G.R. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 2008, 299, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Collaco, J.M.; McGready, J.; Green, D.M.; Naughton, K.M.; Watson, C.P.; Shields, T.; Bell, S.C.; Wainwright, C.E.; Group, A.S.; Cutting, G.R. Effect of temperature on cystic fibrosis lung disease and infections: A replicated cohort study. PLoS ONE 2011, 6, e27784. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Newsom, S.A.; Schildcrout, J.S.; Sheppard, L.; Kaufman, J.D. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004, 169, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Psoter, K.J.; De Roos, A.J.; Mayer, J.D.; Kaufman, J.D.; Wakefield, J.; Rosenfeld, M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann. Am. Thorac. Soc. 2015, 12, 385–391. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Kicinski, M.; Vermeulen, F.; Fierens, F.; De Boeck, K.; Nemery, B.; Nawrot, T.S.; Dupont, L.J. Impact of air pollution on cystic fibrosis pulmonary exacerbations: A case-crossover analysis. Chest 2013, 143, 946–954. [Google Scholar] [CrossRef]
- Psoter, K.J.; De Roos, A.J.; Wakefield, J.; Mayer, J.D.; Rosenfeld, M. Air pollution exposure is associated with MRSA acquisition in young U.S. children with cystic fibrosis. BMC Pulm. Med. 2017, 17, 106. [Google Scholar] [CrossRef]
- Carson, S.W.; Psoter, K.; Koehler, K.; Siklosi, K.R.; Montemayor, K.; Toporek, A.; West, N.E.; Lechtzin, N.; Hansel, N.N.; Collaco, J.M.; et al. Indoor air pollution exposure is associated with greater morbidity in cystic fibrosis. J. Cyst. Fibros. 2021, 21, e129–e135. [Google Scholar] [CrossRef]
- Psoter, K.J.; De Roos, A.J.; Wakefield, J.; Mayer, J.D.; Rosenfeld, M. Seasonality of acquisition of respiratory bacterial pathogens in young children with cystic fibrosis. BMC Infect. Dis. 2017, 17, 411. [Google Scholar] [CrossRef]
- Psoter, K.J.; DE Roos, A.J.; Wakefield, J.; Mayer, J.D.; Bryan, M.; Rosenfeld, M. Association of meteorological and geographical factors and risk of initial Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Epidemiol. Infect. 2015, 144, 1075–1083. [Google Scholar] [CrossRef]
- Curtis, J.R.; Burke, W.; Kassner, A.W.; Aitken, M.L. Absence of health insurance is associated with decreased life expectancy in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1997, 155, 1921–1924. [Google Scholar] [CrossRef]
- Schechter, M.S.; Shelton, B.J.; Margolis, P.A.; Fitzsimmons, S.C. The Association of Socioeconomic Status with Outcomes in Cystic Fibrosis Patients in the United States. Am. J. Respir. Crit. Care Med. 2001, 163, 1331–1337. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Vaincre-la-Mucoviscidose. Registre Français de la Mucoviscidose—Bilan des Données 2020; Vaincre La Mucovisidose: Paris, France, 2022; Available online: https://www.vaincrelamuco.org/sites/default/files/registre_francais_de_la_mucoviscidose_bilan_2020.pdf (accessed on 17 October 2022).
- Corvol, H.; Blackman, S.M.; Boelle, P.Y.; Gallins, P.J.; Pace, R.G.; Stonebraker, J.R.; Accurso, F.J.; Clement, A.; Collaco, J.M.; Dang, H.; et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015, 6, 8382. [Google Scholar] [CrossRef]
- Sun, L.; Rommens, J.M.; Corvol, H.; Li, W.; Li, X.; Chiang, T.A.; Lin, F.; Dorfman, R.; Busson, P.F.; Parekh, R.V.; et al. Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat. Genet. 2012, 44, 562–569. [Google Scholar] [CrossRef]
- Gong, J.; Wang, F.; Xiao, B.; Panjwani, N.; Lin, F.; Keenan, K.; Avolio, J.; Esmaeili, M.; Zhang, L.; He, G.; et al. Genetic association and transcriptome integration identify contributing genes and tissues at cystic fibrosis modifier loci. PLoS Genet. 2019, 15, e1008007. [Google Scholar] [CrossRef]
- Miller, M.R.; Soave, D.; Li, W.; Gong, J.; Pace, R.G.; Boelle, P.Y.; Cutting, G.R.; Drumm, M.L.; Knowles, M.R.; Sun, L.; et al. Variants in Solute Carrier SLC26A9 Modify Prenatal Exocrine Pancreatic Damage in Cystic Fibrosis. J. Pediatr. 2015, 166, 1152–1157.e6. [Google Scholar] [CrossRef]
- Aksit, M.A.; Pace, R.G.; Vecchio-Pagán, B.; Ling, H.; Rommens, J.M.; Boelle, P.-Y.; Guillot, L.; Raraigh, K.S.; Pugh, E.; Zhang, P.; et al. Genetic Modifiers of Cystic Fibrosis-Related Diabetes Have Extensive Overlap with Type 2 Diabetes and Related Traits. J. Clin. Endocrinol. Metab. 2020, 105, 1401–1415. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Keenan, K.; Gong, J.; Panjwani, N.; Avolio, J.; Lin, F.; Adam, D.; Barrett, P.; Bégin, S.; Berthiaume, Y.; et al. Cystic fibrosis–related diabetes onset can be predicted using biomarkers measured at birth. Genet. Med. 2021, 23, 927–933. [Google Scholar] [CrossRef]
- Collaco, J.M.; Blackman, S.M.; Raraigh, K.S.; Corvol, H.; Rommens, J.M.; Pace, R.G.; Boelle, P.-Y.; McGready, J.; Sosnay, P.R.; Strug, L.J.; et al. Sources of Variation in Sweat Chloride Measurements in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 1375–1382. [Google Scholar] [CrossRef]
- Polineni, D.; Dang, H.; Gallins, P.J.; Jones, L.C.; Pace, R.G.; Stonebraker, J.R.; Commander, L.A.; Krenicky, J.E.; Zhou, Y.-H.; Corvol, H.; et al. Airway Mucosal Host Defense Is Key to Genomic Regulation of Cystic Fibrosis Lung Disease Severity. Am. J. Respir. Crit. Care Med. 2018, 197, 79–93. [Google Scholar] [CrossRef]
- Butnariu, L.I.; Țarcă, E.; Cojocaru, E.; Rusu, C.; Moisă, M.; Constantin, M.-M.L.; Gorduza, E.V.; Trandafir, L.M. Genetic Modifying Factors of Cystic Fibrosis Phenotype: A Challenge for Modern Medicine. J. Clin. Med. 2021, 10, 5821. [Google Scholar] [CrossRef]
- Sepahzad, A.; Morris-Rosendahl, D.; Davies, J. Cystic Fibrosis Lung Disease Modifiers and Their Relevance in the New Era of Precision Medicine. Genes 2021, 12, 562. [Google Scholar] [CrossRef]
- Wright, F.A.; Strug, L.J.; Doshi, V.K.; Commander, C.; Blackman, S.; Sun, L.; Berthiaume, Y.; Cutler, D.M.; Cojocaru, A.; Collaco, J.M.; et al. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat. Genet. 2011, 43, 539–546. [Google Scholar] [CrossRef]
- Gu, Y.; Harley, I.T.W.; Henderson, L.B.; Aronow, B.J.; Vietor, I.; Huber, L.A.; Harley, J.B.; Kilpatrick, J.R.; Langefeld, C.D.; Williams, A.H.; et al. Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature 2009, 458, 1039–1042. [Google Scholar] [CrossRef]
- Green, D.M.; Collaco, J.M.; McDougal, K.E.; Naughton, K.M.; Blackman, S.M.; Cutting, G.R. Heritability of respiratory infection with Pseudomonas aeruginosa in cystic fibrosis. J. Pediatr. 2012, 161, 290–295.e1. [Google Scholar] [CrossRef]
- Emond, M.J.; Louie, T.; Emerson, J.; Chong, J.X.; Mathias, R.A.; Knowles, M.R.; Rieder, M.J.; Tabor, H.K.; Nickerson, D.A.; Barnes, K.C.; et al. Exome Sequencing of Phenotypic Extremes Identifies CAV2 and TMC6 as Interacting Modifiers of Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. PLoS Genet. 2015, 11, e1005273. [Google Scholar] [CrossRef]
- Emond, M.J.; Louie, T.; Emerson, J.; Zhao, W.; Mathias, R.A.; Knowles, M.R.; Wright, F.A.; Rieder, M.J.; Tabor, H.K.; Nickerson, D.A.; et al. Exome sequencing of extreme phenotypes identifies DCTN4 as a modifier of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Nat. Genet. 2012, 44, 886–889. [Google Scholar] [CrossRef]
- Li, W.; Soave, D.; Miller, M.R.; Keenan, K.; Lin, F.; Gong, J.; Chiang, T.; Stephenson, A.L.; Durie, P.; Rommens, J.; et al. Unraveling the complex genetic model for cystic fibrosis: Pleiotropic effects of modifier genes on early cystic fibrosis-related morbidities. Hum. Genet. 2014, 133, 151–161. [Google Scholar] [CrossRef]
- Castaldo, A.; Cernera, G.; Iacotucci, P.; Cimbalo, C.; Gelzo, M.; Comegna, M.; Di Lullo, A.M.; Tosco, A.; Carnovale, V.; Raia, V.; et al. TAS2R38 is a novel modifier gene in patients with cystic fibrosis. Sci. Rep. 2020, 10, 5806. [Google Scholar] [CrossRef]
- Tesse, R.; Cardinale, F.; Santostasi, T.; Polizzi, A.; Mappa, L.; Manca, A.; De Robertis, F.; Silecchia, O.; Armenio, L. Association of interleukin-10 gene haplotypes with Pseudomonas aeruginosa airway colonization in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 329–332. [Google Scholar] [CrossRef]
- Coutinho, C.A.; Al Marson, F.; Marcelino, A.R.; Bonadia, L.C.; Carlin, M.P.; Ribeiro, A.F.; Ribeiro, J.D.; Bertuzzo, C.S. TNF-alpha polymorphisms as a potential modifier gene in the cystic fibrosis. Int. J. Mol. Epidemiol. Genet. 2014, 5, 87–99. [Google Scholar]
- Trevisiol, C.; Boniotto, M.; Giglio, L.; Poli, F.; Morgutti, M.; Crovella, S. MBL2 polymorphisms screening in a regional Italian CF Center. J. Cyst. Fibros. 2005, 4, 189–191. [Google Scholar] [CrossRef][Green Version]
- McDougal, K.E.; Green, D.M.; Vanscoy, L.L.; Fallin, M.D.; Grow, M.; Cheng, S.; Blackman, S.M.; Collaco, J.M.; Henderson, L.B.; Naughton, K.; et al. Use of a modeling framework to evaluate the effect of a modifier gene (MBL2) on variation in cystic fibrosis. Eur. J. Hum. Genet. 2010, 18, 680–684. [Google Scholar] [CrossRef]
- Blackman, S.M.; Hsu, S.; Ritter, S.E.; Naughton, K.M.; Wright, F.A.; Drumm, M.L.; Knowles, M.R.; Cutting, G.R. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009, 52, 1858–1865. [Google Scholar] [CrossRef]
- Blackman, S.M.; Commander, C.W.; Watson, C.; Arcara, K.M.; Strug, L.J.; Stonebraker, J.R.; Wright, F.A.; Rommens, J.M.; Sun, L.; Pace, R.G.; et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 2013, 62, 3627–3635. [Google Scholar] [CrossRef]
- Lam, A.N.; Aksit, M.A.; Vecchio-Pagan, B.; Shelton, C.A.; Osorio, D.L.; Anzmann, A.F.; Goff, L.A.; Whitcomb, D.C.; Blackman, S.M.; Cutting, G.R. Increased expression of anion transporter SLC26A9 delays diabetes onset in cystic fibrosis. J. Clin. Investig. 2020, 130, 272–286. [Google Scholar] [CrossRef]
- Stonebraker, J.R.; Ooi, C.Y.; Pace, R.G.; Corvol, H.; Knowles, M.R.; Durie, P.R.; Ling, S.C. Features of severe liver disease with portal hypertension in patients with cystic fibrosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1207–1215.e3. [Google Scholar] [CrossRef]
- Debray, D.; Kelly, D.; Houwen, R.; Strandvik, B.; Colombo, C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J. Cyst. Fibros. 2011, 10 (Suppl. 2), S29–S36. [Google Scholar] [CrossRef]
- Boelle, P.Y.; Debray, D.; Guillot, L.; Clement, A.; Corvol, H.; on behalf of the French CF Modifier Gene Study Investigators. Cystic Fibrosis Liver Disease: Outcomes and Risk Factors in a Large Cohort of French Patients. Hepatology 2019, 69, 1648–1656. [Google Scholar] [CrossRef]
- Frangolias, D.D.; Ruan, J.; Wilcox, P.J.; Davidson, A.G.; Wong, L.T.; Berthiaume, Y.; Hennessey, R.; Freitag, A.; Pedder, L.; Corey, M.; et al. Alpha 1-antitrypsin deficiency alleles in cystic fibrosis lung disease. Am. J. Respir. Cell Mol. Biol. 2003, 29, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, P.D.; Pravica, V.; Geraghty, P.J.; Super, M.; Webb, A.K.; Schwarz, M.; Hutchinson, I.V. End-organ dysfunction in cystic fibrosis: Association with angiotensin I converting enzyme and cytokine gene polymorphisms. Am. J. Respir. Crit. Care Med. 2003, 167, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Henrion-Caude, A.; Flamant, C.; Roussey, M.; Housset, C.; Flahault, A.; Fryer, A.A.; Chadelat, K.; Strange, R.C.; Clement, A. Liver disease in pediatric patients with cystic fibrosis is associated with glutathione S-transferase P1 polymorphism. Hepatology 2002, 36, 913–917. [Google Scholar] [CrossRef]
- Gabolde, M.; Hubert, D.; Guilloud-Bataille, M.; Lenaerts, C.; Feingold, J.; Besmond, C. The mannose binding lectin gene influences the severity of chronic liver disease in cystic fibrosis. J. Med. Genet. 2001, 38, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, R.; Degiorgio, D.; Coviello, D.A.; Baccarelli, A.; Elce, A.; Raia, V.; Motta, V.; Seia, M.; Castaldo, G.; Colombo, C. An MBL2 haplotype and ABCB4 variants modulate the risk of liver disease in cystic fibrosis patients: A multicentre study. Dig. Liver Dis. 2009, 41, 817–822. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Friedman, K.J.; Ling, S.C.; Pace, R.G.; Bell, S.C.; Bourke, B.; Castaldo, G.; Castellani, C.; Cipolli, M.; Colombo, C.; et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009, 302, 1076–1083. [Google Scholar] [CrossRef]
- Boelle, P.Y.; Debray, D.; Guillot, L.; Corvol, H.; on behalf of the French CF Modifier Gene Study Investigators. SERPINA1 Z allele is associated with cystic fibrosis liver disease. Genet. Med. 2019, 21, 2151–2155. [Google Scholar] [CrossRef]
- Trouve, P.; Genin, E.; Ferec, C. In silico search for modifier genes associated with pancreatic and liver disease in Cystic Fibrosis. PLoS ONE 2017, 12, e0173822. [Google Scholar] [CrossRef]
- HAS. Protocole national de diagnostic et de soins (PNDS) Muocviscidose. In Guide—Affection de Longue Durée; HAS: Paris, France, 2017; Available online: https://www.has-sante.fr/jcms/c_2792719/fr/mucoviscidose (accessed on 17 October 2022).
- Hubert, D.; Bui, S.; Marguet, C.; Colomb-Jung, V.; Murris-Espin, M.; Corvol, H.; Munck, A. New therapies for cystic fibrosis targeting the CFTR gene or the CFTR protein. Rev. Mal. Respir. 2016, 33, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.F.; Borowitz, D.; Drevinek, P.; Griese, M.; Konstan, M.W.; Wainwright, C.; Ratjen, F.; Sermet-Gaudelus, I.; Plant, B.; Munck, A.; et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: A phase 3, open-label extension study (PERSIST). Lancet Respir. Med. 2014, 2, 902–910. [Google Scholar] [CrossRef]
- Corvol, H.; Mésinèle, J.; Douksieh, I.-H.; Strug, L.J.; Boëlle, P.-Y.; Guillot, L. SLC26A9 Gene Is Associated With Lung Function Response to Ivacaftor in Patients With Cystic Fibrosis. Front. Pharmacol. 2018, 9, 828. [Google Scholar] [CrossRef]
- Strug, L.J.; Gonska, T.; He, G.; Keenan, K.; Ip, W.; Boelle, P.Y.; Lin, F.; Panjwani, N.; Gong, J.; Li, W.; et al. Cystic fibrosis gene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum. Mol. Genet. 2016, 25, 4590–4600. [Google Scholar] [CrossRef]
- Eastman, A.C.; Pace, R.G.; Dang, H.; Aksit, M.A.; Vecchio-Pagan, B.; Lam, A.N.; O’Neal, W.K.; Blackman, S.M.; Knowles, M.R.; Cutting, G.R. SLC26A9 SNP rs7512462 is not associated with lung disease severity or lung function response to ivacaftor in cystic fibrosis patients with G551D-CFTR. J. Cyst. Fibros. 2021, 20, 851–856. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Jones, A.M.; Barry, P.J. Lumacaftor/ivacaftor for patients homozygous for Phe508del-CFTR: Should we curb our enthusiasm? Thorax 2015, 70, 615–616. [Google Scholar] [CrossRef][Green Version]
- Jennings, M.T.; Dezube, R.; Paranjape, S.; West, N.E.; Hong, G.; Braun, A.; Grant, J.; Merlo, C.A.; Lechtzin, N. An Observational Study of Outcomes and Tolerances in Patients with Cystic Fibrosis Initiated on Lumacaftor/Ivacaftor. Ann. Am. Thorac. Soc. 2017, 14, 1662–1666. [Google Scholar] [CrossRef]
- Konstan, M.W.; McKone, E.F.; Moss, R.B.; Marigowda, G.; Tian, S.; Waltz, D.; Huang, X.; Lubarsky, B.; Rubin, J.; Millar, S.J.; et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017, 5, 107–118. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Munck, A.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; et al. Real-Life Safety and Effectiveness of Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Jain, M.; Barto, T.L.; Haddad, T.; Atkinson, J.; Tian, S.; Tang, R.; Marigowda, G.; Waltz, D.; Pilewski, J. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J. Cyst. Fibros. 2018, 17, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; Marguet, C.; et al. Clinical response to lumacaftor-ivacaftor in patients with cystic fibrosis according to baseline lung function. J. Cyst. Fibros. 2020, 20, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Aalbers, B.; Groot, K.D.W.-D.; Arets, H.; Hofland, R.; de Kiviet, A.; Ven, M.V.O.-V.D.; Kruijswijk, M.; Schotman, S.; Michel, S.; van der Ent, C.; et al. Clinical effect of lumacaftor/ivacaftor in F508del homozygous CF patients with FEV1 ≥ 90% predicted at baseline. J. Cyst. Fibros. 2020, 19, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Ramsey, B.W.; Boyle, M.P.; Konstan, M.W.; Huang, X.; Marigowda, G.; Waltz, D.; Wainwright, C.E. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: A pooled analysis. Lancet Respir. Med. 2016, 4, 617–626. [Google Scholar] [CrossRef]
- Mésinèle, J.; Ruffin, M.; Guillot, L.; Boëlle, P.-Y.; Corvol, H.; on behalf of the French CF Modifier Gene Study Investigators. Factors Predisposing the Response to Lumacaftor/Ivacaftor in People with Cystic Fibrosis. J. Pers. Med. 2022, 12, 252. [Google Scholar] [CrossRef]
- Pereira, S.V.-N.; Ribeiro, J.D.; Bertuzzo, C.S.; Marson, F.A.L. Association of clinical severity of cystic fibrosis with variants in the SLC gene family (SLC6A14, SLC26A9, SLC11A1 and SLC9A3). Gene 2017, 629, 117–126. [Google Scholar] [CrossRef]
- Ruffin, M.; Mercier, J.; Calmel, C.; Mésinèle, J.; Bigot, J.; Sutanto, E.N.; Kicic, A.; Corvol, H.; Guillot, L. Update on SLC6A14 in lung and gastrointestinal physiology and physiopathology: Focus on cystic fibrosis. Cell. Mol. Life Sci. 2020, 77, 3311–3323. [Google Scholar] [CrossRef]
- Ruffin, M.; Mercier, J.; Calmel, C.; Mésinèle, J.; Corvol, H.; Guillot, L. SLC6A14, un gène modificateur dans la mucoviscidose. Rev. Mal. Respir. 2020, 37, 218–221. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor–Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Durieu, I.; Chiron, R.; Ramel, S.; Danner-Boucher, I.; Prevotat, A.; Grenet, D.; Marguet, C.; Reynaud-Gaubert, M.; Macey, J.; et al. Rapid Improvement after Starting Elexacaftor–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and Advanced Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Saint-Criq, V.; Wang, Y.; Delpiano, L.; Lin, J.; Sheppard, D.N.; Gray, M.A. Extracellular phosphate enhances the function of F508del-CFTR rescued by CFTR correctors. J. Cyst. Fibros. 2021, 20, 843–850. [Google Scholar] [CrossRef]
- Pinto, M.C.; Quaresma, M.C.; Silva, I.A.L.; Railean, V.; Ramalho, S.S.; Amaral, M.D. Synergy in Cystic Fibrosis Therapies: Targeting SLC26A9. Int. J. Mol. Sci. 2021, 22, 13064. [Google Scholar] [CrossRef]
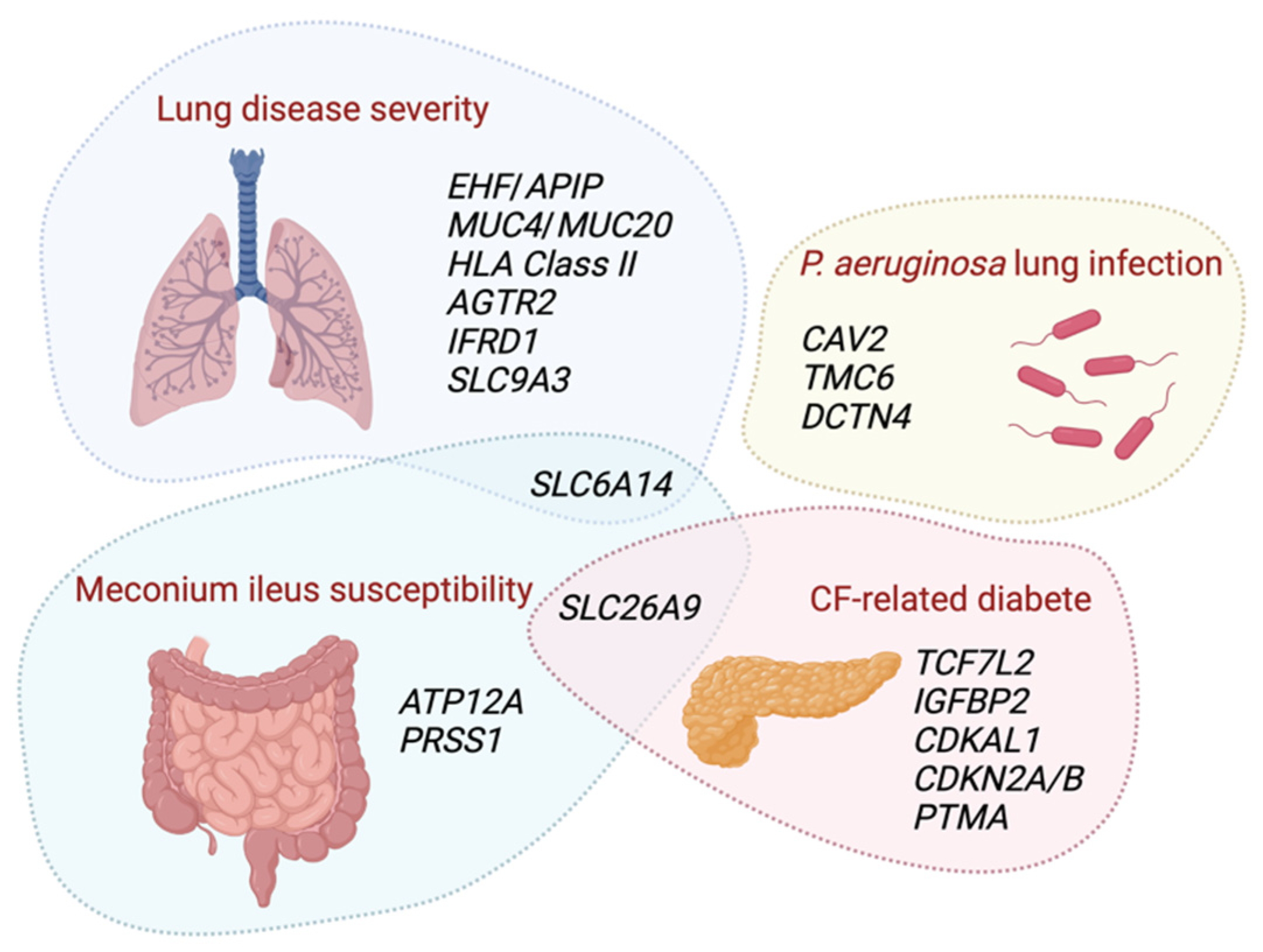
| Modifier Gene or Locus | Physiological Role | Ref. |
|---|---|---|
| Angiotensin II receptor type 2 (AGTR2)/Solute carrier family 6 member A14 (SLC6A14) Locus chrXq22-q23 | AGTR2 encodes the angiotensin, which is part of the renin-angiotensin system, a major control system for blood pressure and fluid balance. SLC6A14 imports and concentrates neutral amino acids and the two cationic acids lysine and arginine into the cytoplasm of different cell types. | [37] |
| ETS homologous factor (EHF)/APAF1 interacting protein (APIP) Locus chr11p12-p13 | EHF transcription factor modulates epithelial tight junctions and wound repair. APIP is a methionine salvage pathway enzyme associated with apoptosis and systemic inflammatory responses. | [37] [47] |
| Human leukocyte antigen (HLA) Class II Locus chr6p21.3 | HLA plays a central role in immunity by presenting peptides derived from extracellular proteins. | [37] |
| Interferon related developmental regulator 1 (IFRD1) Locus chr11p12-p13 | IFRD1 is as a transcriptional co-activator/repressor regulating cellular growth and differentiation during tissue development and regeneration. | [48] |
| Locus chr 20q13.2 | The five genes located in this locus are expressed in respiratory epithelial cells. Among these genes, the melanocortin 3 receptor (MC3R) is implicated in weight maintenance and regulation of energy balance. Some of these genes encode proteins involved in cell adhesion, migration and phagocytosis of bacterial pathogens. | [47] |
| Mucin 4 (MUC4)/Mucin 20 (MUC20) Locus chr3q29 | Mucins are highly glycosylated mucus proteins. | [37] |
| Solute carrier family 9 member A3 (SLC9A3) | SLC9A3 is a sodium–hydrogen exchanger. | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mésinèle, J.; Ruffin, M.; Guillot, L.; Corvol, H. Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes. Int. J. Mol. Sci. 2022, 23, 14205. https://doi.org/10.3390/ijms232214205
Mésinèle J, Ruffin M, Guillot L, Corvol H. Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes. International Journal of Molecular Sciences. 2022; 23(22):14205. https://doi.org/10.3390/ijms232214205
Chicago/Turabian StyleMésinèle, Julie, Manon Ruffin, Loïc Guillot, and Harriet Corvol. 2022. "Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes" International Journal of Molecular Sciences 23, no. 22: 14205. https://doi.org/10.3390/ijms232214205
APA StyleMésinèle, J., Ruffin, M., Guillot, L., & Corvol, H. (2022). Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes. International Journal of Molecular Sciences, 23(22), 14205. https://doi.org/10.3390/ijms232214205









