DORN1 Is Involved in Drought Stress Tolerance through a Ca2+-Dependent Pathway
Abstract
:1. Introduction
2. Results
2.1. Creation and Identification of the Loss Function Mutants for DORN1
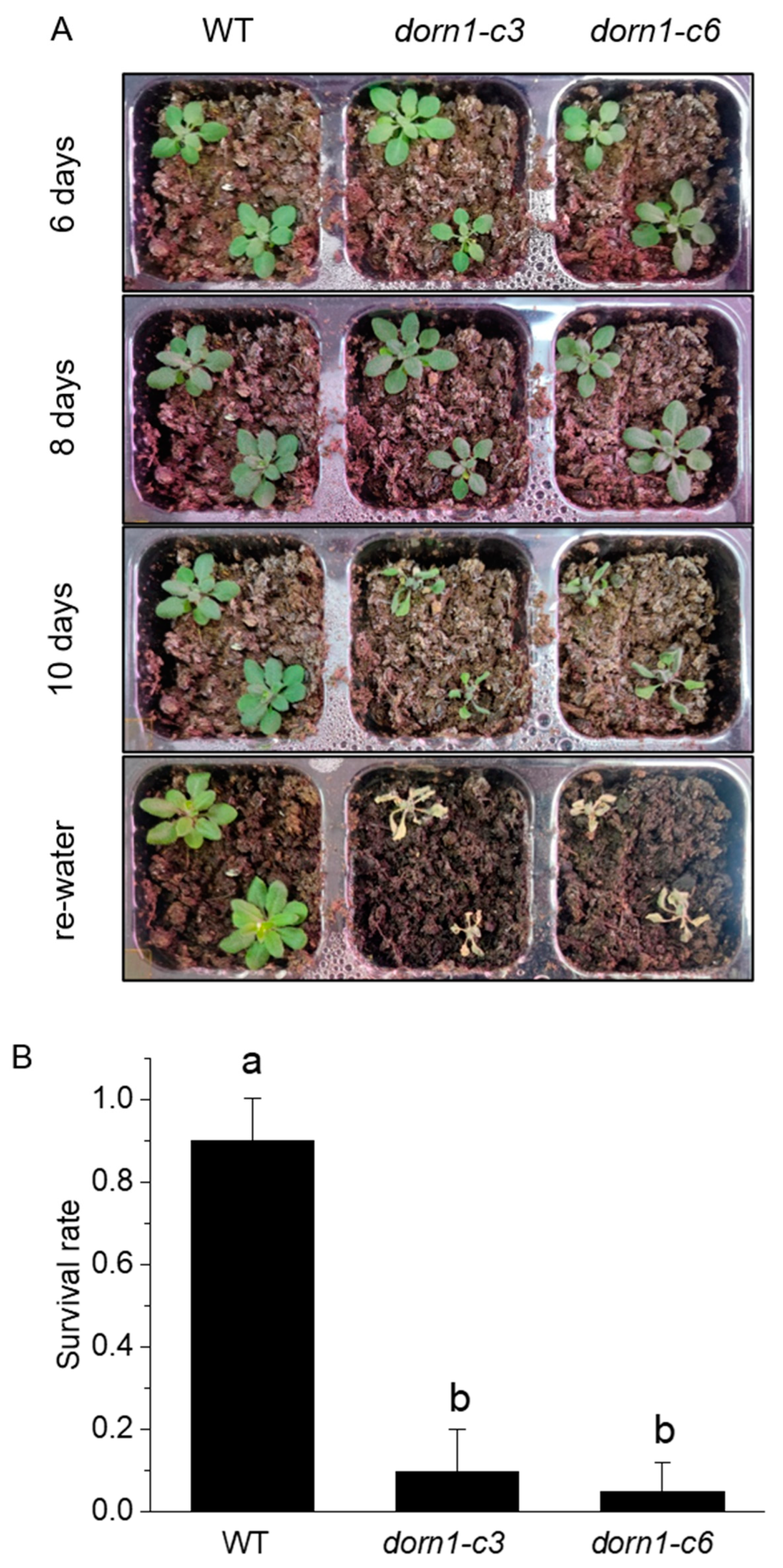
2.2. The Survival Rate Was Decreased at Day 10 of Soil drying in the dorn1 Mutant
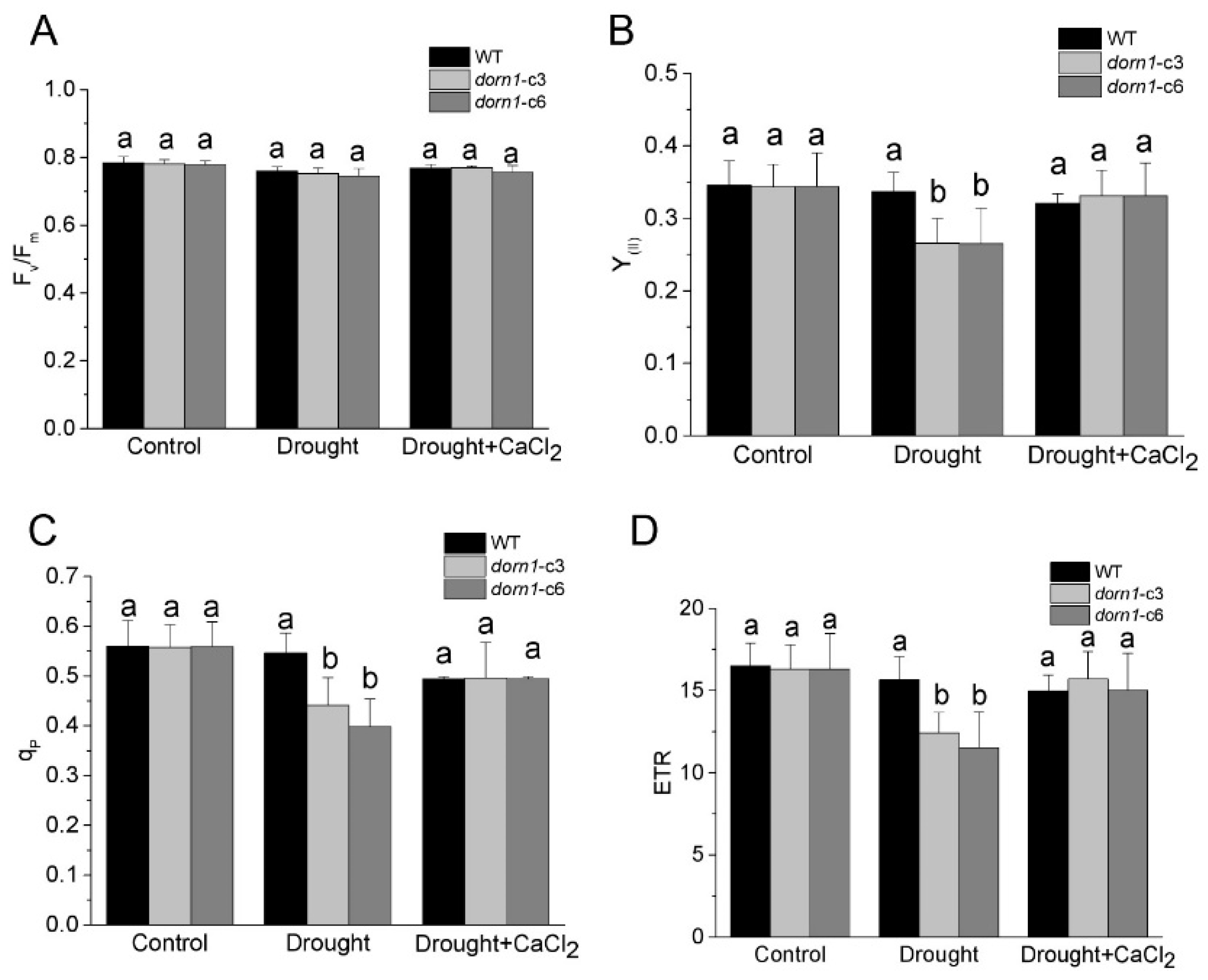
2.3. Loss Function of DORN1 Has No Obvious Influence on Fv/Fm Performance
2.4. Exogenous CaCl2 Promotes the Photochemical Reaction Efficiency Performance in dorn1 Plants under Drought Stress
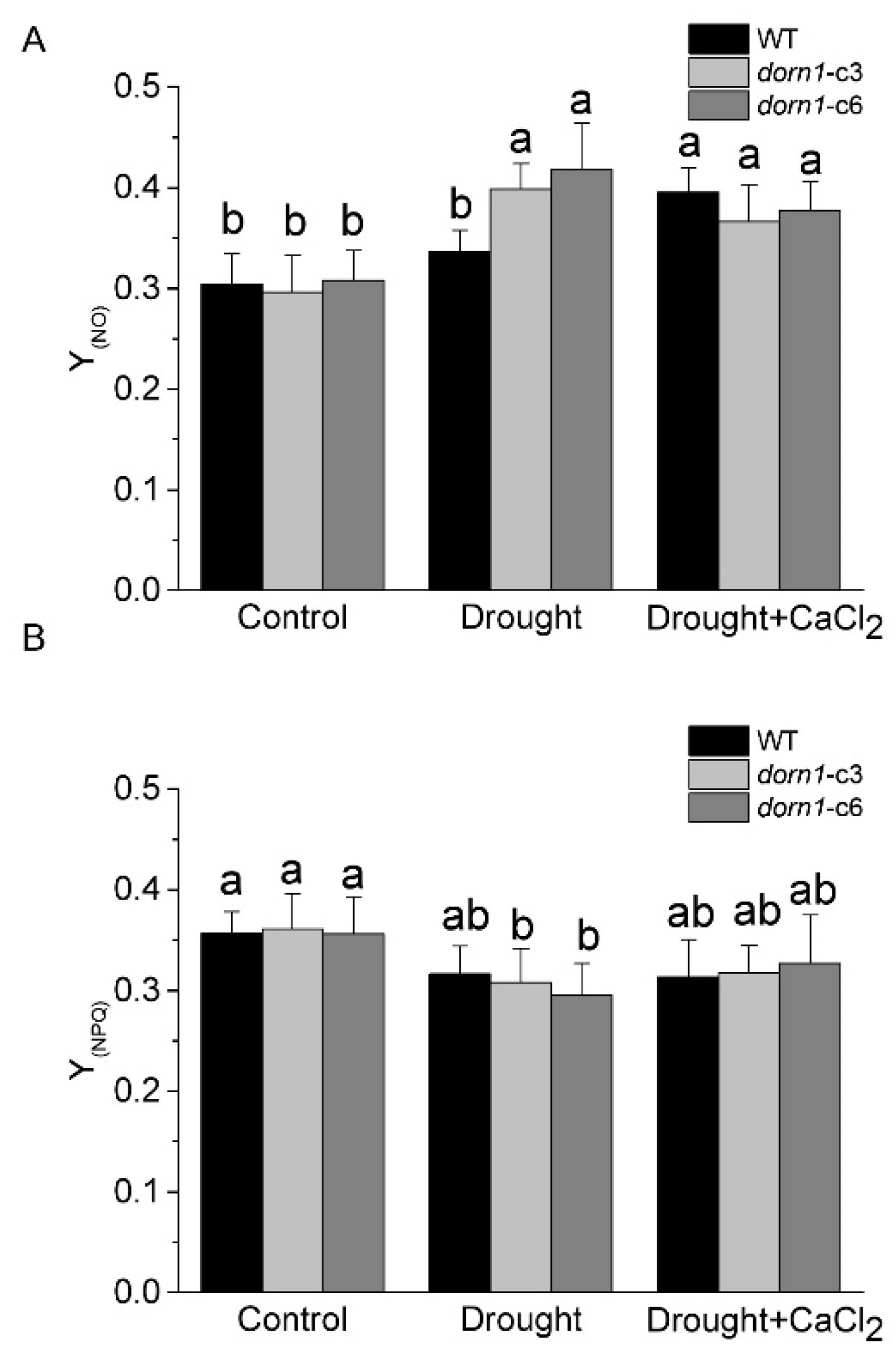
2.5. Photooxidative Damage Increased in the dorn1 Plant under Drought Stress
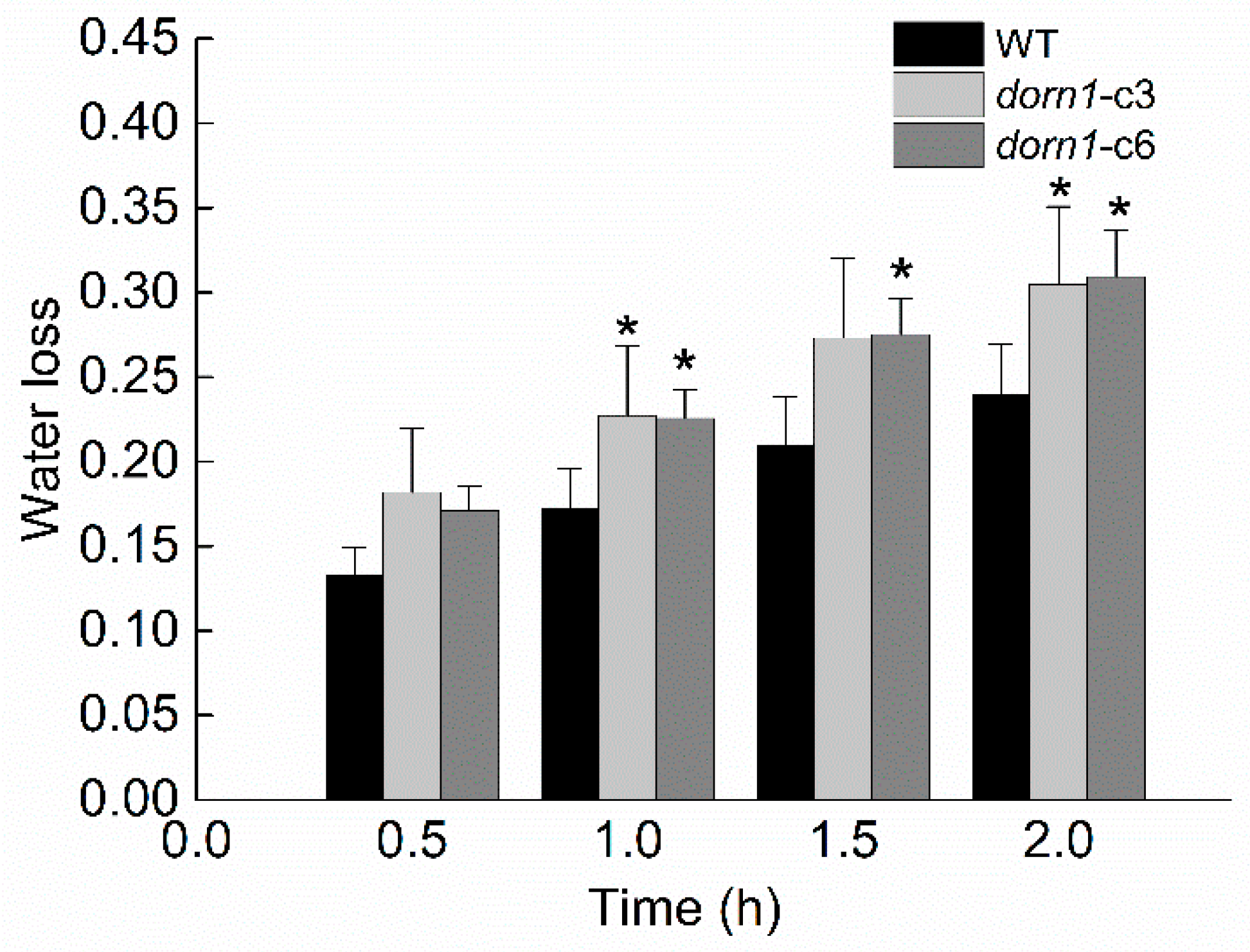
2.6. The dorn1 Plants Have a Greater Transpirational Water Loss
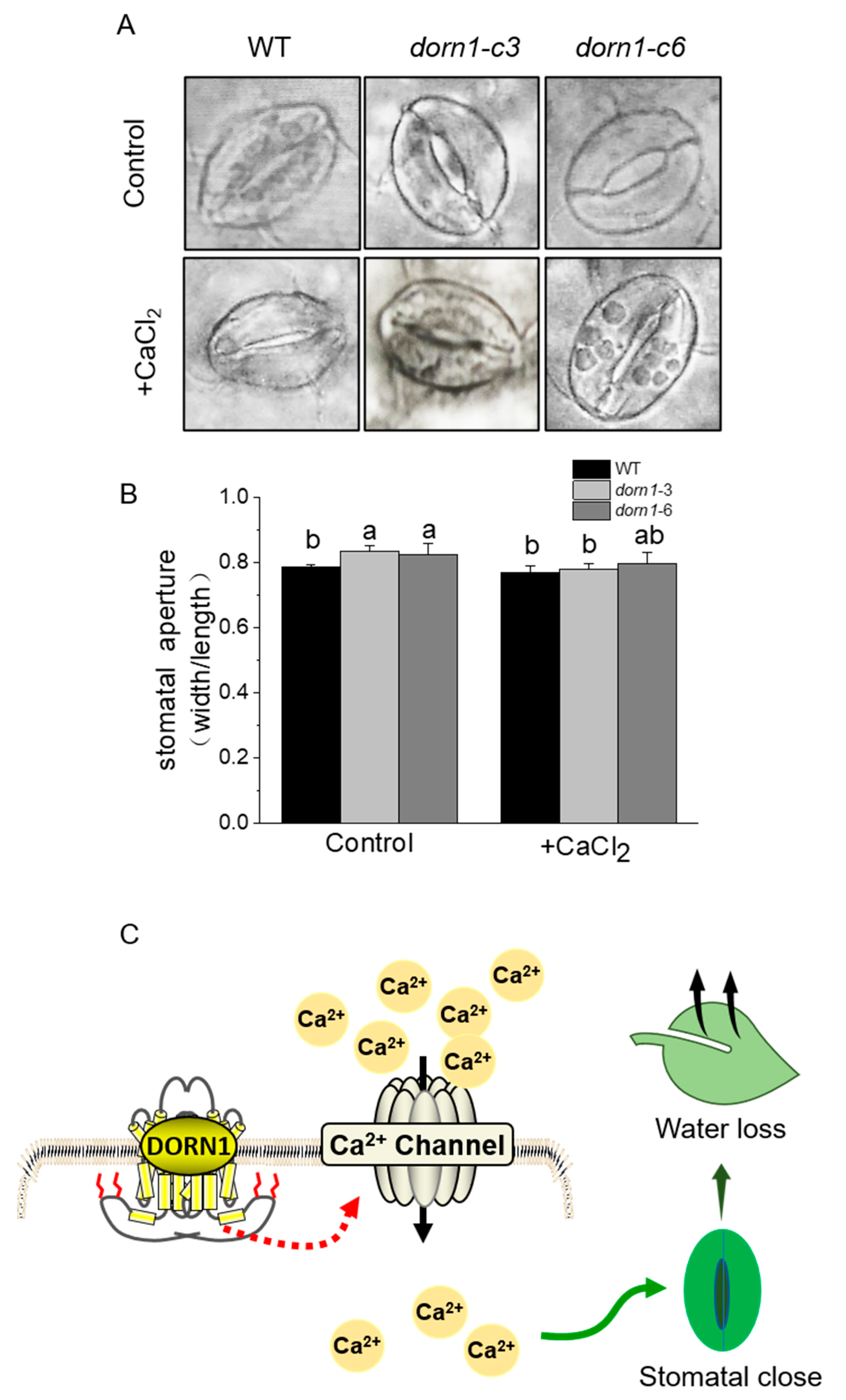
2.7. Exogenous CaCl2 Could Relieve Excessive Stomatal Opening in the dorn1 Mutants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Construction of CRISPR/Cas9 Plant Expression Vectors
4.3. Identification of the Homozygous Mutant Plants
4.4. Measurement of Chlorophyll Fluorescence
4.5. Measurement of Transpirational Water Loss
4.6. Measurement of Stomatal Closure in Response to CaCl2 and Drought Treatment
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roelfsema, M.R.G.; Hedrich, R. Making sense out of Ca2+ signals: Their role in regulating stomatal movements. Plant Cell Environ. 2010, 33, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Chen, J.; Liu, T.W.; Chen, J.; Han, A.D.; Simon, M.; Dong, X.J.; He, J.X.; Zheng, H.L. Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis. J. Exp. Bot. 2014, 65, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, C.Y.; Mano, N.; Finkler, A.; Weng, H.; Day, I.S.; Reddy, A.S.N.; Poovaiah, B.W.; Fromm, H.; Hasegawa, P.M.; Mickelbart, M.V. A Ca2+/CaM-regulated transcriptional switch modulates stomatal development in response to water deficit. Sci. Rep. 2019, 9, 12282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, S.J.; Steinebrunner, I. Extracellular ATP: An unexpected role as a signaler in plants. Trends Plant Sci. 2007, 12, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Matthus, E.; Sun, J.; Wang, L.M.; Bhat, M.G.; Mohammad-Sidik, A.B.; Wilkins, K.A.; Leblanc-Fournier, N.; Legue, V.; Moulia, B.; Stacey, G.; et al. DORN1/P2K1 and purino-calcium signalling in plants: Making waves with extracellular ATP. Ann. Bot. 2019, 124, 1227–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.R.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wilkins, K.A.; Davies, J.M. Arabidopsis DORN1 extracellular ATP receptor; activation of plasma membrane K+ -and Ca2+-permeable conductances. New Phytol. 2018, 218, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Sidik, A.; Sun, J.; Shin, R.; Song, Z.Z.; Ning, Y.Z.; Matthus, E.; Wilkins, K.A.; Davies, J.M. Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots. Int. J. Mol. Sci. 2021, 22, 494. [Google Scholar] [CrossRef]
- Moeder, W.; Phan, V.; Yoshioka, K. Ca2+ to the rescue—Ca2+ channels and signaling in plant immunity. Plant Sci. 2019, 279, 19–26. [Google Scholar] [CrossRef]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef]
- Du, L.Q.; Poovaiah, B.W. Ca2+/calmodulin is critical for brassinosteroid biosynthesis and plant growth. Nature 2005, 437, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Du, L.; Poovaiah, B.W. Ca2+/Calmodulin-Dependent AtSR1/CAMTA3 Plays Critical Roles in Balancing Plant Growth and Immunity. Int. J. Mol. Sci. 2018, 19, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiøtt, M.; Romanowsky, S.M.; Bækgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Zhang, T.; Koo, A.J.; Stacey, G.; Tanaka, K. Extracellular ATP Acts on Jasmonate Signaling to Reinforce Plant Defense. Plant Physiol. 2018, 176, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tripathi, D.; Okubara, P.A.; Tanaka, K. Purinoceptor P2K1/DORN1 Enhances Plant Resistance Against a Soilborne Fungal Pathogen, Rhizoctonia solani. Front. Plant Sci. 2020, 11, 572920. [Google Scholar] [CrossRef]
- Tanaka, K.; Choi, J.M.; Cao, Y.R.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5, 446. [Google Scholar] [CrossRef] [Green Version]
- Nizam, S.; Qiang, X.Y.; Wawra, S.; Nostadt, R.; Getzke, F.; Schwanke, F.; Dreyer, I.; Langen, G.; Zuccaro, A. Serendipita indica E5’NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep. 2019, 20, e47430. [Google Scholar] [CrossRef]
- Hou, Q.Z.; Sun, K.; Zhang, H.; Su, X.; Fan, B.Q.; Feng, H.Q. The responses of photosystem II and intracellular ATP production of Arabidopsis leaves to salt stress are affected by extracellular ATP. J. Plant Res. 2018, 131, 331–339. [Google Scholar] [CrossRef]
- Shan, Y.X.; Li, F.J.; Lian, Q.Q.; Xie, L.H.; Zhu, H.; Li, T.T.; Zhang, J.; Duan, X.W.; Jiang, Y.M. Role of apyrase-mediated eATP signal in chilling injury of postharvest banana fruit during storage. Postharvest Biol. Technol. 2022, 187, 111874. [Google Scholar] [CrossRef]
- Jiao, Q.S.; Feng, H.Q.; Tian, W.Y.; Bai, J.Y.; Sun, K.; Jia, L.Y.; Zhang, J.P. Extracellular ATP functions in alleviating the decrease of PSII photochemistry caused by the infection of Xanthomonas campestris pv. phaseoli. Plant Pathol. 2016, 65, 819–825. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Baba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, R.; Abbasi, A.; Kalaji, H.M.; Eskandari, I.; Sedghieh, V.; Khorsandi, H.; Sadeghian, N.; Yadav, S.; Rastogi, A. The role of potassium on drought resistance of winter wheat cultivars under cold dryland conditions: Probed by chlorophyll a fluorescence. Plant Physiol. Biochem. 2022, 182, 45–54. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Holzwarth, A.R.; Garab, G.J.P.R. Chlorophyll a fluorescence: Beyond the limits of the QA model. Photosynth. Res. 2014, 120, 43–58. [Google Scholar] [CrossRef]
- Jiao, Q.S.; Niu, G.T.; Wang, F.; Dong, J.Y.; Hong, Z. N-glycosylation regulates photosynthetic efficiency of Arabidopsis thaliana. Photosynthetica 2019, 58, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Lazar, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Stirbet, A. Excitonic connectivity between photosystem II units: What is it, and how to measure it? Photosynth. Res. 2013, 116, 189–214. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of Q A Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nose, T.; Jikumaru, Y.; Kamiya, Y. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 2013, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, R.P.; Raissig, M.T. Morphology made for movement: Formation of diverse stomatal guard cells. Curr. Opin. Plant Biol. 2021, 63, 102090. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.Q.; Cho, S.H.; Nguyen, C.T.; Stacey, G. Arabidopsis Lectin Receptor Kinase P2K2 Is a Second Plant Receptor for Extracellular ATP and Contributes to Innate Immunity. Plant Physiol. 2020, 183, 1364–1375. [Google Scholar] [CrossRef]
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 2017, 8, 2265. [Google Scholar] [CrossRef] [Green Version]
- Matthus, E.; Wilkins, K.A.; Mohammad-Sidik, A.; Ning, Y.; Davies, J.M. Spatial origin of the extracellular ATP-induced cytosolic calcium signature in Arabidopsis thaliana roots: Wave formation and variation with phosphate nutrition. Plant Biol. 2022, 24, 863–873. [Google Scholar] [CrossRef]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-Assisted Calcium Wave Dependent on the AtRBOHD NADPH Oxidase and TPC1 Cation Channel Propagates the Systemic Response to Salt Stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [Green Version]
- Rhaman, M.S.; Nakamura, T.; Nakamura, Y.; Munemasa, S.; Murata, Y. The Myrosinases TGG1 and TGG2 Function Redundantly in Reactive Carbonyl Species Signaling in Arabidopsis Guard Cells. Plant Cell Physiol. 2020, 61, 967–977. [Google Scholar] [CrossRef]
- Tan, Y.-Q.; Yang, Y.; Shen, X.; Zhu, M.; Shen, J.; Zhang, W.; Hu, H.; Wang, Y.-F. Multiple cyclic nucleotide-gated channels function as ABA-activated Ca2+ channels required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2022. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Ni, Y.; Meng, Z.; Lu, T.; Li, T. Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves. PLoS ONE 2014, 9, e97322. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X.H. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams Iii, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Jiao, Q.; Chen, T.; Niu, G.; Zhang, H.; Zhou, C.; Hong, Z. N-glycosylation is involved in stomatal development by modulating the release of active abscisic acid and auxin in Arabidopsis. J. Exp. Bot. 2020, 71, 5865–5879. [Google Scholar] [CrossRef]
- Zhang, J.Y.; He, S.B.; Li, L.; Yang, H.Q. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc. Natl. Acad. Sci. USA 2014, 111, E3015–E3023. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Bai, H.; Zada, A.; Jiao, Q. DORN1 Is Involved in Drought Stress Tolerance through a Ca2+-Dependent Pathway. Int. J. Mol. Sci. 2022, 23, 14213. https://doi.org/10.3390/ijms232214213
Wang Q, Bai H, Zada A, Jiao Q. DORN1 Is Involved in Drought Stress Tolerance through a Ca2+-Dependent Pathway. International Journal of Molecular Sciences. 2022; 23(22):14213. https://doi.org/10.3390/ijms232214213
Chicago/Turabian StyleWang, Qingwen, Hongbao Bai, Ahmad Zada, and Qingsong Jiao. 2022. "DORN1 Is Involved in Drought Stress Tolerance through a Ca2+-Dependent Pathway" International Journal of Molecular Sciences 23, no. 22: 14213. https://doi.org/10.3390/ijms232214213
APA StyleWang, Q., Bai, H., Zada, A., & Jiao, Q. (2022). DORN1 Is Involved in Drought Stress Tolerance through a Ca2+-Dependent Pathway. International Journal of Molecular Sciences, 23(22), 14213. https://doi.org/10.3390/ijms232214213






