Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato
Abstract
:1. Introduction
2. Results
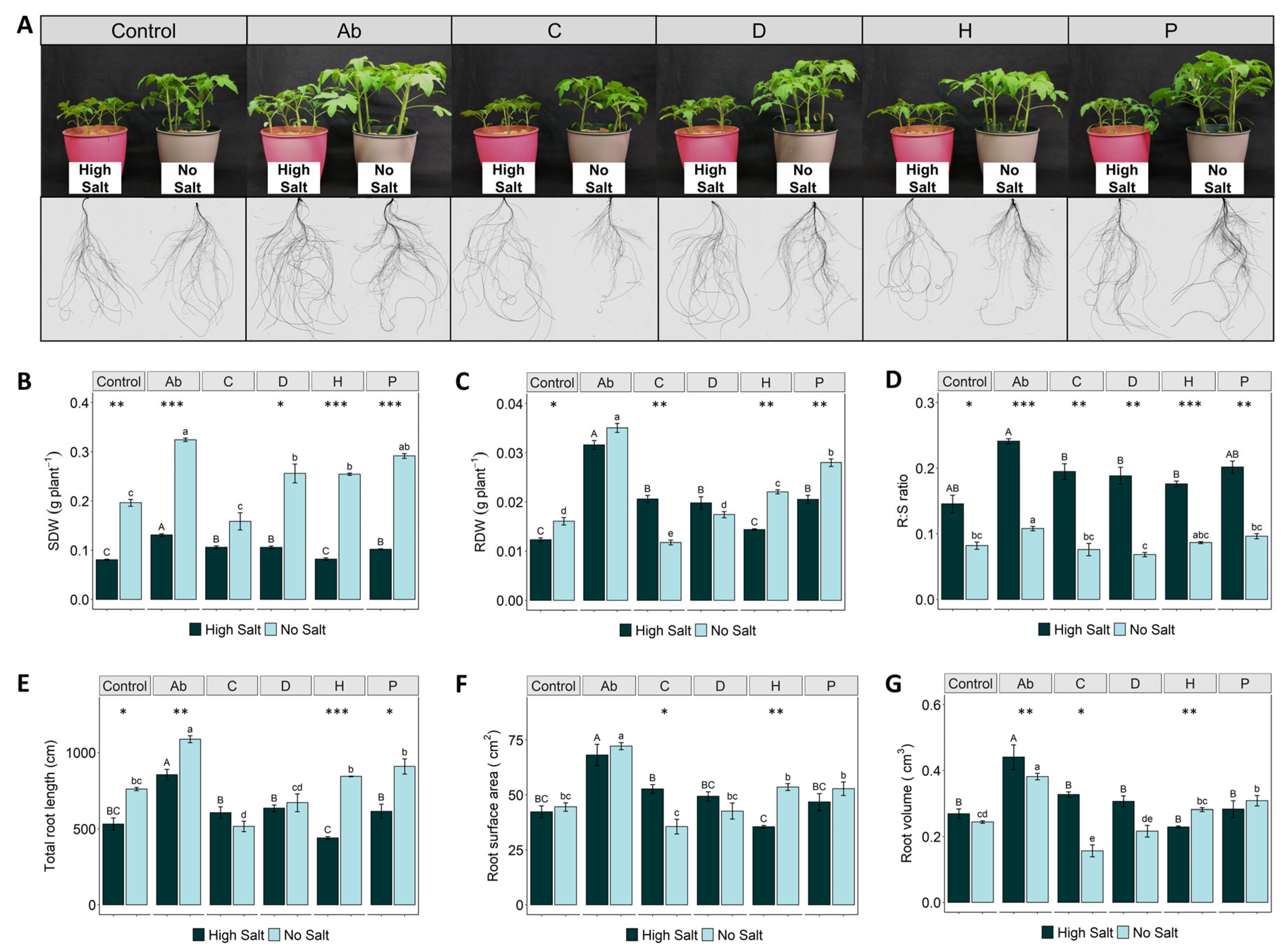
2.1. Plant Biomass Production and Root System Are Affected by Salinity Stress
2.2. Total Phenolics and Flavonoids in Leaves
2.3. Proline Accumulation and Oxidative Stress Protection
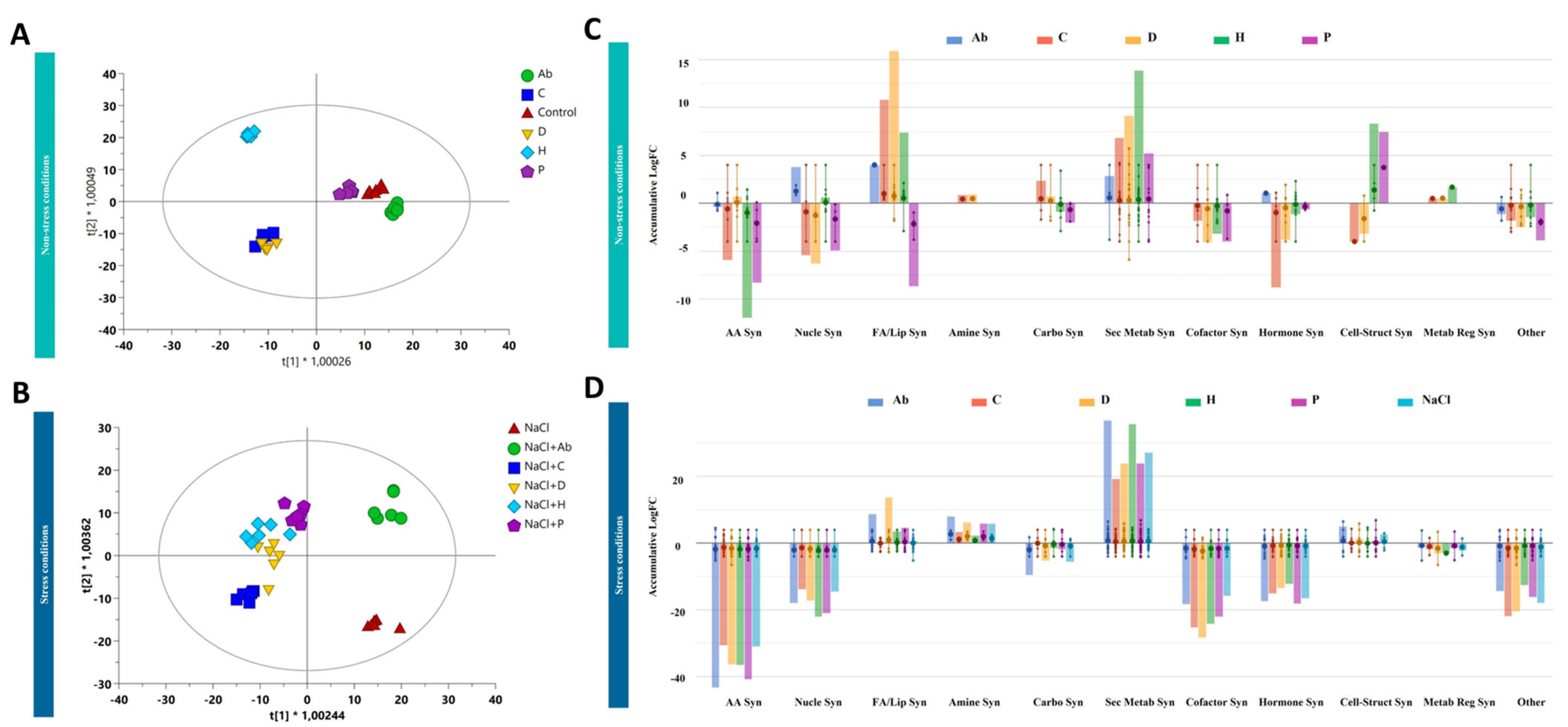
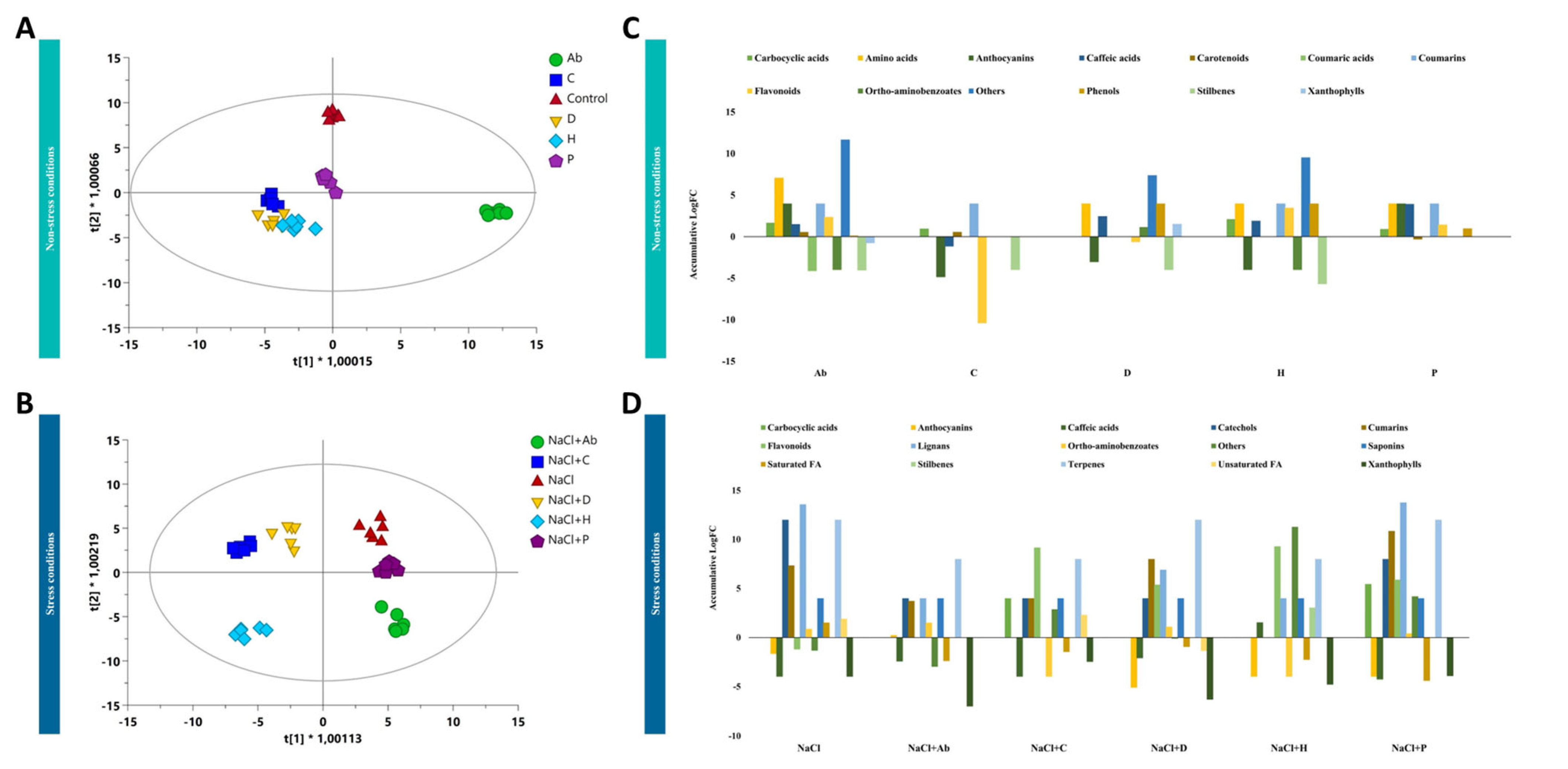
2.4. Metabolomic Profiling of Tomato Leaves and Root Exudates
2.5. PAL Gene Expression in Leaves Is Differentially Affected by Biostimulants Treatments
3. Discussion
4. Materials and Methods
4.1. Plant-Derived Biostimulants
4.2. Microbial Biostimulant (Ab)
4.3. Plant Material and Growing Conditions
4.4. Collection of Root Exudates
4.5. Root Morphology and Plant Biomass
4.6. Leaf Extraction and Estimation of Total Phenolics and Flavonoids Compounds
4.7. Untargeted Profiling of Root Exudates and Leaf Extracts by UHPLC-QTOF Mass Spectrometry
4.8. Leaf Proline Determination
4.9. Antioxidant Enzymes Assays
4.10. Gene Expression Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, A.; Sharma, V. Microbes mediated plant stress tolerance in saline agricultural ecosystem. Plant Soil 2019, 442, 1–22. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, M.R.; Islam, M.T.; Hasan, A.; Robin, K. Salinity stress alters root morphology and root hair. J. Plants 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Sang, L.; Xie, H.; Chai, M.; Wang, Z.Y. Comparative transcriptome analysis of salt stress-induced leaf senescence in Medicago truncatula. Front. Plant Sci. 2021, 12, 666660. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Cardarelli, M.; Rouphael, Y. Plant biostimulants: New tool for enhancing agronomic performance and fruit quality of cucurbits. Acta Hortic. 2020, 1294, 245–252. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal- versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Hoagland, L.; Giordano, M.; El-Nakhel, C.; Cardarelli, M. Vegetal-protein hydrolysates based microgranule enhances growth, mineral content, and quality traits of vegetable transplants. Sci. Hortic. 2021, 290, 110554. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, M.B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; de Oliveira, A.L.M. The adaptive metabolomic profile and functional activity of tomato rhizosphere are revealed upon PGPB inoculation under saline stress. Environ. Exp. Bot. 2021, 189, 104552. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Tsukanova, K.A.; Chеbоtаr, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Gayoso, C.; Pomar, F.; Novo-Uzal, E.; Merino, F.; Martínez de Ilárduya, Ó. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PAL gene expression. BMC Plant Biol. 2010, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, J.; Berni, R.; Sutera, F.; Gutsch, A.; Hausman, J.; Saffie-siebert, S.; Guerriero, G. The effects of salinity on the anatomy and gene expression patterns in leaflets of tomato cv. Micro-Tom. Genes 2021, 12, 1165. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Sholi, N. Effect of salt stress on seed germination, plant growth, photosynthesis and ion accumulation of four tomato cultivars. Am. J. Plant Physiol. 2012, 7, 269–275. [Google Scholar] [CrossRef]
- Stavridou, E.; Hastings, A.; Webster, R.J.; Robson, P.R.H. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB Bioenergy 2017, 9, 92–104. [Google Scholar] [CrossRef] [Green Version]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Mallahi, T.; Saharkhiz, M.J.; Javanmardi, J. Salicylic acid changes morpho-physiological attributes of feverfew (Tanacetum parthenium L.) under salinity stress. Acta Ecol. Sin. 2018, 38, 351–355. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Long-term effect of salinity on plant quality, water relations, photosynthetic parameters and ion distribution in Callistemon citrinus. Plant Biol. 2014, 16, 757–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-based protein hydrolysate improves salinity tolerance in hemp: Agronomical and physiological aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and root applications of vegetal-derived protein hydrolysates differentially enhance the yield and qualitative attributes of two lettuce cultivars grown in floating system. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bilal, M.; Hassani, D.; Iqbal, H.M.N.; Wang, H.; Huang, D. Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Bot. Stud. 2017, 58, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pii, Y.; Marastoni, L.; Springeth, C.; Chiara, M.; Maria, G.; Cesco, S.; Mimmo, T. Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA biosynthesis in Azospirillum brasilense under environmental stress conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Rivera, D.; Mora, V.; Lopez, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassan, F. New insights into indole-3-acetic acid metabolism in Azospirillum brasilense. J. Appl. Microbiol. 2018, 125, 1774–1785. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, Y.; Lee, S.; Oh, B.; Chae, J.; Lee, K. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sen, S.; Mohapatra, S. Modulation of proline metabolic gene expression in Arabidopsis thaliana under water-stressed conditions by a drought-mitigating Pseudomonas putida strain. Ann. Microbiol. 2017, 67, 655–668. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.H.; Verslues, P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef] [Green Version]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Checchio, M.V.; Alves, C.R.; de Oliveira, K.R.; Moro, G.V.; Santos, D.M.M.; Gratão, P.L. Enhancement of salt tolerance in corn using Azospirillum brasilense: An approach on antioxidant systems. J. Plant Res. 2021, 134, 1279–1289. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Janda, M.; Ruelland, E. Magical mystery tour: Salicylic acid signalling. Environ. Exp. Bot. 2015, 114, 117–128. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Zaid, A.; Latef, A.A.H.A. Salicylic acid spraying-induced resilience strategies against the damaging impacts of drought and/or salinity stress in two varieties of Vicia faba L. seedlings. J. Plant Growth Regul. 2021, 40, 1919–1942. [Google Scholar] [CrossRef]
- Iula, G.; Miras-Moreno, B.; Rouphael, Y.; Lucini, L.; Trevisan, M. The complex metabolomics crosstalk triggered by four molecular elicitors in tomato. Plants 2022, 11, 678. [Google Scholar] [CrossRef]
- Fedina, E.; Yarin, A.; Mukhitova, F.; Blufard, A.; Chechetkin, I. Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 2017, 117, 25–28. [Google Scholar] [CrossRef]
- Badri, D.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2019, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Huang, Q.; Zhang, R.; Li, J.; Luo, K.; Chen, Y. Molecular characterisation of PAL gene family reveals their role in abiotic stress response in lucerne (Medicago sativa). Crop. Pasture Sci. 2022, 73, 300–311. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Niazi, A.; Moghadam, A. Phenylalanine ammonia lyase isolation and functional analysis of phenylpropanoid pathway under salinity stress in Salvia species. Aust. J. Crop. Sci. 2015, 9, 656–665. [Google Scholar]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed priming with protein hydrolysates improves Arabidopsis growth and stress tolerance to abiotic stresses. Front. Plant Sci. 2021, 12, 626301. [Google Scholar] [CrossRef]
- Cesco, S.; Lucini, L.; Miras-Moreno, B.; Borruso, L.; Mimmo, T.; Pii, Y.; Puglisi, E.; Spini, G.; Taskin, E.; Tiziani, R.; et al. The hidden effects of agrochemicals on plant metabolism and root-associated microorganisms. Plant Sci. 2021, 311, 111012. [Google Scholar] [CrossRef]
- Sorrentino, M.; Panzarová, K.; Spyroglou, I.; Spíchal, L.; Buffagni, V.; Ganugi, P.; Rouphael, Y.; Colla, G.; Lucini, L.; De Diego, N. Integration of phenomics and metabolomics datasets reveals different mode of action of biostimulants based on protein hydrolysates in Lactuca sativa L. and Solanum lycopersicum L. under salinity. Front. Plant Sci. 2022, 12, 808711. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Martinez de Oliveira, A.L.; Valentinuzzi, F.; Tiziani, R.; Pii, Y.; Mimmo, T.; Cesco, S. Can inoculation with the bacterial biostimulant Enterobacter sp. strain 15S be an approach for the smarter P fertilization of maize and cucumber plants? Front. Plant Sci. 2021, 12, 719873. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. -Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Hawkins, C.; Ginzburg, D.; Zhao, K.; Dwyer, W.; Xue, B.; Xu, A.; Rice, S.; Cole, B.; Paley, S.; Karp, P.; et al. Plant Metabolic Network 15: A resource of genome-wide metabolism databases for 126 plants and algae. J. Integr. Plant Biol. 2021, 63, 1888–1905. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating integrated metabolomics data access. Metabolomics 2015, 11, 1587–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- García-Pérez, P.; Miras-Moreno, B.; Lucini, L.; Gallego, P.P. The metabolomics reveals intraspecies variability of bioactive compounds in elicited suspension cell cultures of three Bryophyllum species. Ind. Crops Prod. 2021, 163, 113322. [Google Scholar] [CrossRef]
- Caspi, R.; Dreher, K.; Karp, P. The challenge of constructing, classifying and representing metabolic pathways. FEMS Microbiol. Lett. 2013, 345, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Barupal, D.K.; Fan, S.; Fiehn, O. Integrating bioinformatics approaches for a comprehensive interpretation of metabolomics datasets. Curr. Opin. Biotechnol. 2018, 54, 1–9. [Google Scholar] [CrossRef]
| Parameters a | Salt Level b | Biostimulant Treatments c | |||||
|---|---|---|---|---|---|---|---|
| Control | Ab | C | D | H | P | ||
| Phenolics | High salt | 0.42 ± 0.00 Aa | 0.45 ± 0.00 a | 0.37 ± 0.00 Ab | 0.34 ± 0.00 Bc | 0.43 ± 0.02 a | 0.39 ± 0.02 Bb |
| No salt | 0.35 ± 0.00 Bc | 0.46 ± 0.00 a | 0.31 ± 0.00 Bd | 0.44 ± 0.01 Aa | 0.41 ± 0.00 b | 0.45 ± 0.02 Aa | |
| Flavonoids | High salt | 1.90 ± 0.04 Abc | 2.61 ± 0.06 Ba | 1.78 ± 0.02 cd | 1.66 ± 0.09 Bd | 2.05 ± 0.03 Ab | 1.78 ± 0.07 cd |
| No salt | 1.32 ± 0.06 Bd | 3.29 ± 0.16 Aa | 1.75 ± 0.02 c | 2.67 ± 0.02 Ab | 1.95 ± 0.05 Bc | 1.77 ± 0.03 c | |
| Proline | High salt | 64.74 ± 4.66 Ab | 97.73 ± 4.52 Aa | 27.28 ± 3.34 Ad | 38.74 ± 2.19 Ac | 21.32 ± 2.05 Ad | 65.60 ± 3.50 Ab |
| No salt | 1.65 ± 0.148 Ba | 1.36 ± 0.06 Bb | 0.79 ± 0.07 Bcd | 0.67 ± 0.03 Bd | 0.97 ± 0.11 Bc | 1.00 ± 0.14 Bc | |
| CAT | High salt | 3.49 ± 0.11 d | 5.37 ± 0.16 Aa | 3.81 ± 0.12 Acd | 4.53 ± 0.27 Ab | 4.09 ± 0.14 Abc | 4.11 ± 0.23 Abc |
| No salt | 3.74 ± 0.41 b | 4.27 ± 0.02 Ba | 3.39 ± 0.13 Bb | 3.31 ± 0.02 Bb | 3.53 ± 0.13 Bb | 3.32 ± 0.14 Bb | |
| APX | High salt | 281.55 ± 20.54 Ade | 494.80 ± 50.56 Ab | 414.95 ± 6.18 Ac | 217.71 ± 10.25 e | 345.73 ± 7.72 Bd | 600.02 ± 18.09 Aa |
| No salt | 212.34 ± 15.84 Be | 347.37 ± 5.14 Bc | 279.21 ± 25.37 Bd | 225.71 ± 1.43 e | 391.86 ± 2.99 Ab | 456.86 ± 22.34 Ba | |
| GPX | High salt | 63.61 ± 13.24 d | 209.65 ± 5.92 Aa | 119.25 ± 15.41 Ac | 155.29 ± 2.39 Ab | 152.34 ± 15.40 Ab | 99.81 ± 2.73 Ac |
| No salt | 52.49 ± 1.25 b | 81.06 ± 5.28 Ba | 47.22 ± 12.68 Bb | 40.28 ± 1.01 Bb | 47.72 ± 2.22 Bb | 53.17 ± 3.08 Bb | |
| SOD | High salt | 2.28 ± 0.17 Ac | 3.78 ± 0.08 Aa | 3.01 ± 0.02 Ab | 3.39 ± 0.31 Aab | 2.57 ± 0.02 Ac | 1.63 ± 0.13 d |
| No salt | 1.57 ± 0.08 Bc | 2.52 ± 0.16 Ba | 1.76 ± 0.19 Bbc | 2.04 ± 0.05 Bb | 2.52 ± 0.00 Ba | 1.54 ± 0.12 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzate Zuluaga, M.Y.; Miras-Moreno, B.; Monterisi, S.; Rouphael, Y.; Colla, G.; Lucini, L.; Cesco, S.; Pii, Y. Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato. Int. J. Mol. Sci. 2022, 23, 14216. https://doi.org/10.3390/ijms232214216
Alzate Zuluaga MY, Miras-Moreno B, Monterisi S, Rouphael Y, Colla G, Lucini L, Cesco S, Pii Y. Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato. International Journal of Molecular Sciences. 2022; 23(22):14216. https://doi.org/10.3390/ijms232214216
Chicago/Turabian StyleAlzate Zuluaga, Mónica Yorlady, Begoña Miras-Moreno, Sonia Monterisi, Youssef Rouphael, Giuseppe Colla, Luigi Lucini, Stefano Cesco, and Youry Pii. 2022. "Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato" International Journal of Molecular Sciences 23, no. 22: 14216. https://doi.org/10.3390/ijms232214216
APA StyleAlzate Zuluaga, M. Y., Miras-Moreno, B., Monterisi, S., Rouphael, Y., Colla, G., Lucini, L., Cesco, S., & Pii, Y. (2022). Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato. International Journal of Molecular Sciences, 23(22), 14216. https://doi.org/10.3390/ijms232214216










