The Role of TKS5 in Chromosome Stability and Bladder Cancer Progression
Abstract
1. Introduction
2. Results
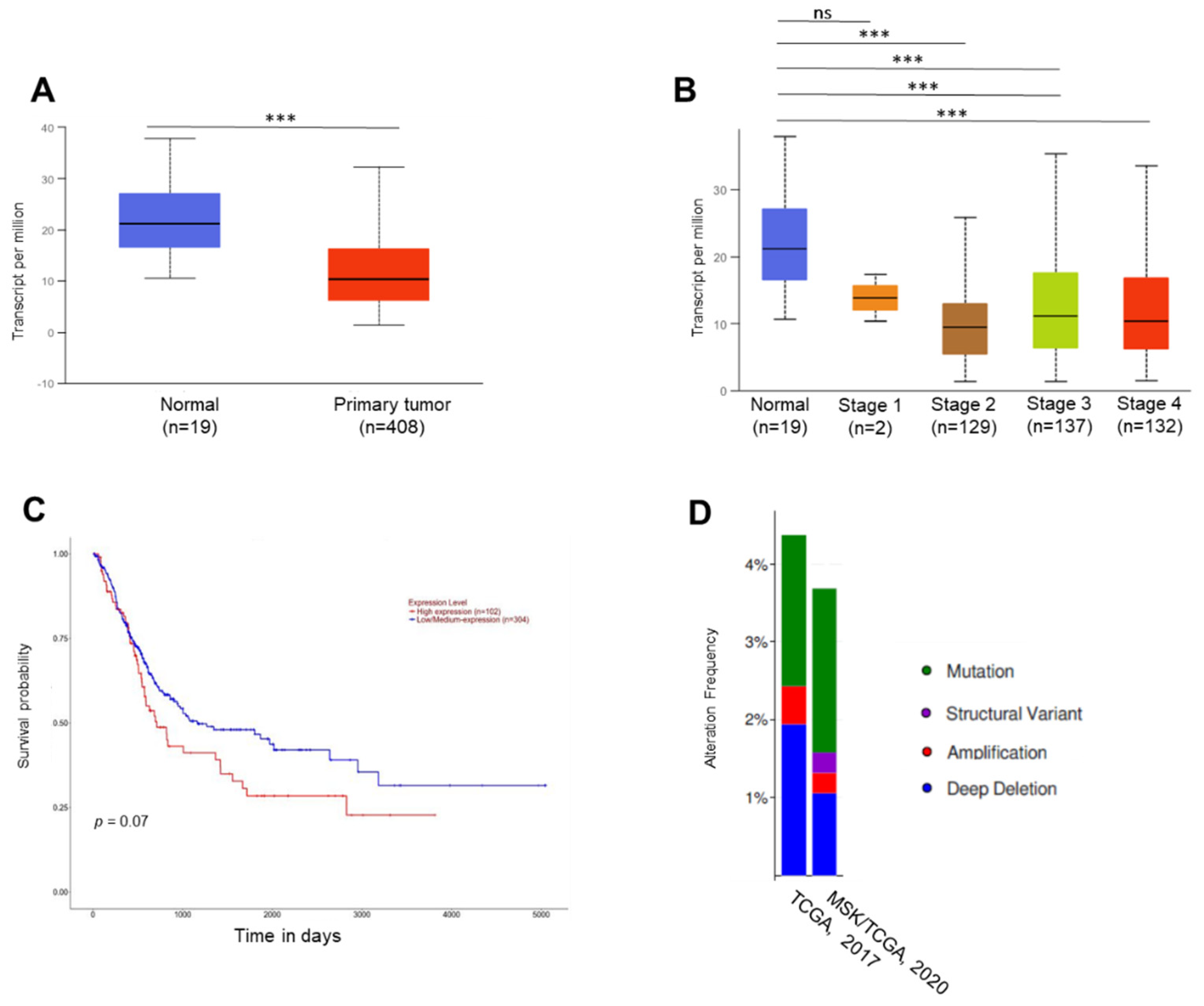
2.1. In Silico Analyses
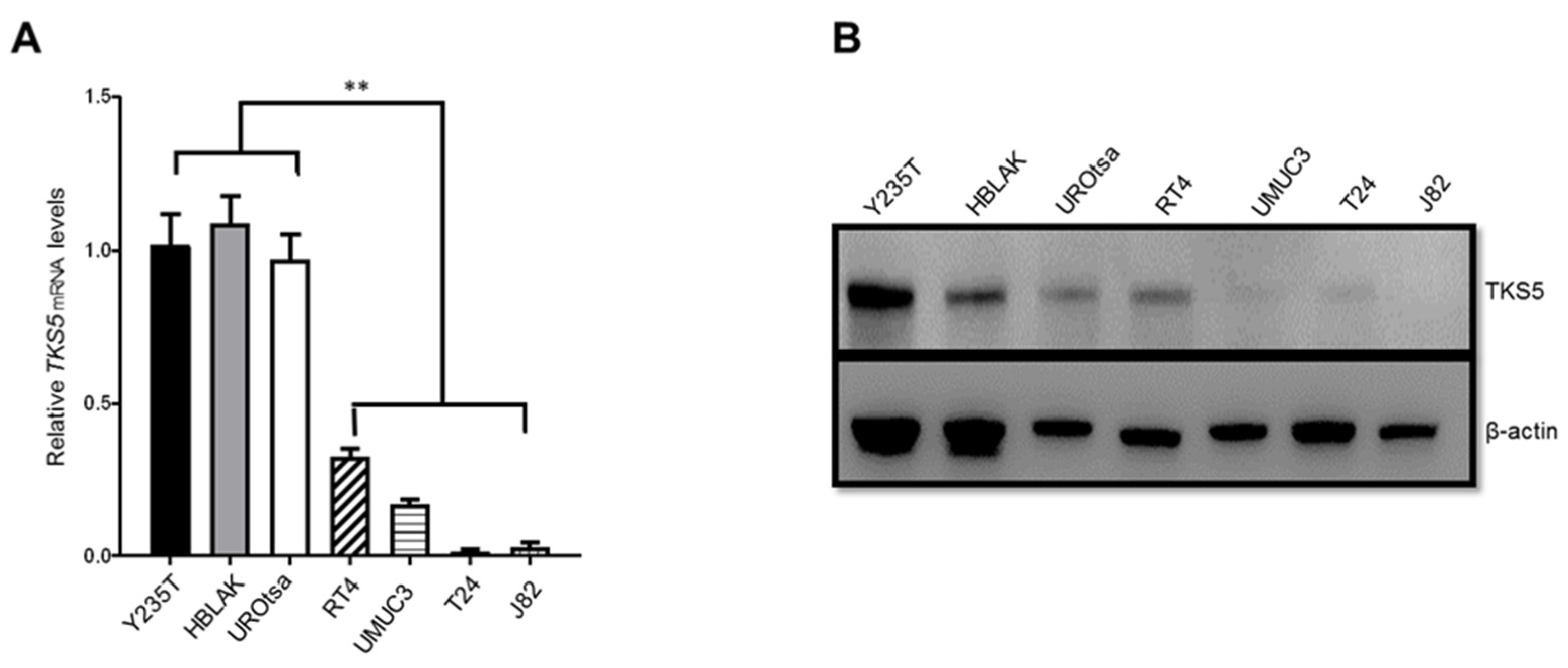
2.2. TKS5 Expression Is Higher in Urothelial Cells
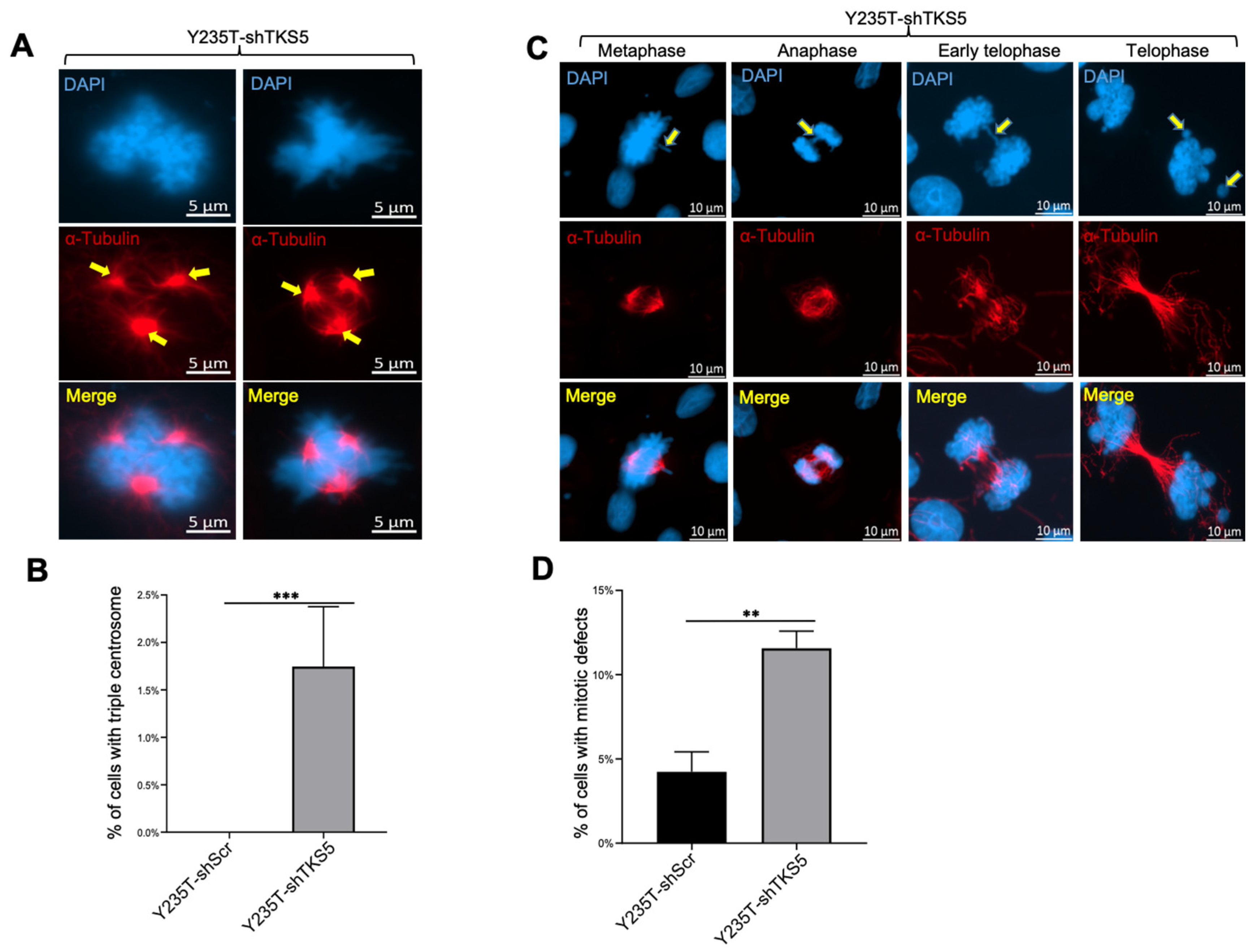
2.3. TKS5 Downregulation Leads to Aneuploidy and Genome Instability in Urothelial Cells
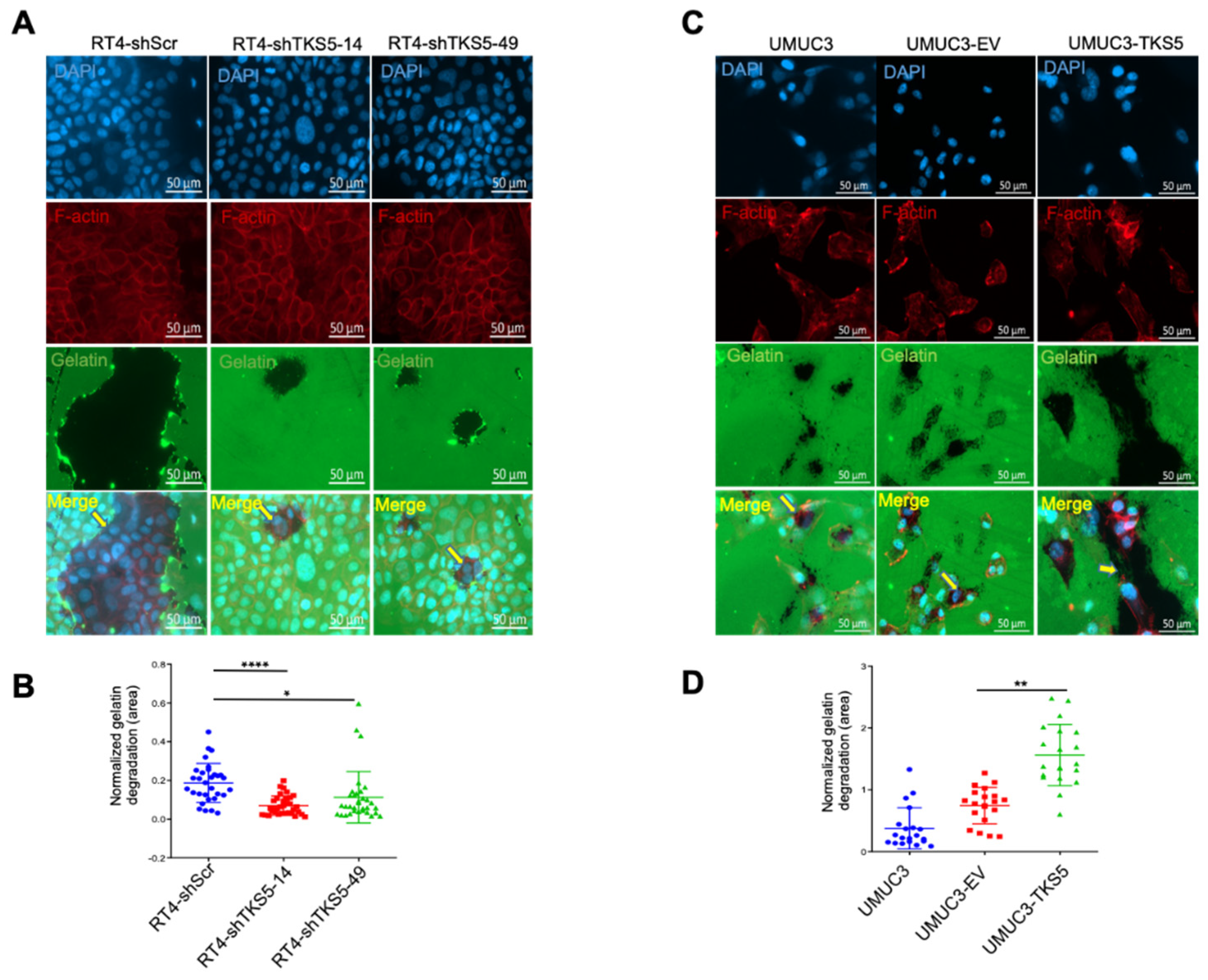
2.4. TKS5 Influences Colocalisation of Cortactin with F-Actin in Nontumour and Tumour Cells
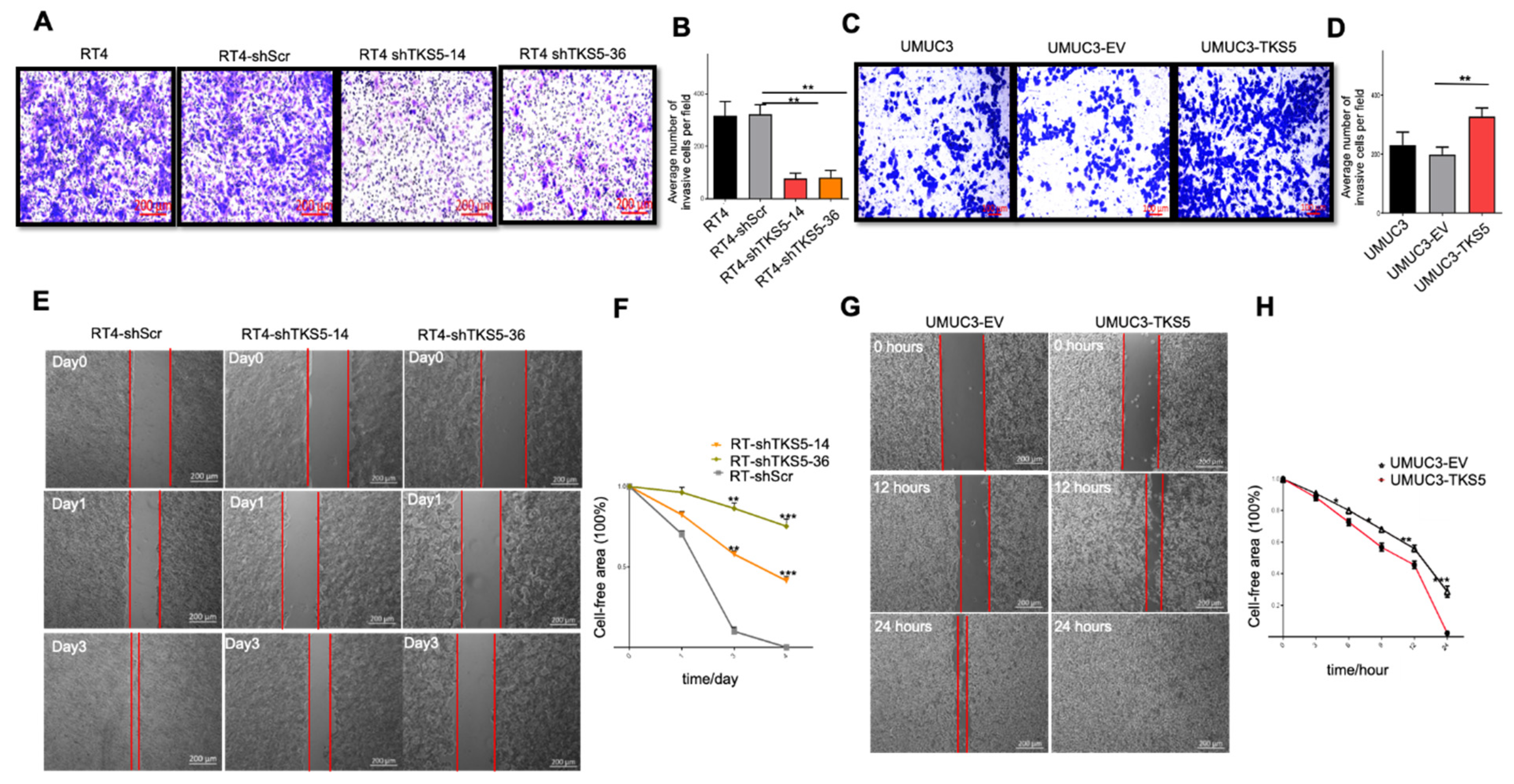
2.5. TKS5 Promotes Invasion/Migration of Bladder Cancer Cells
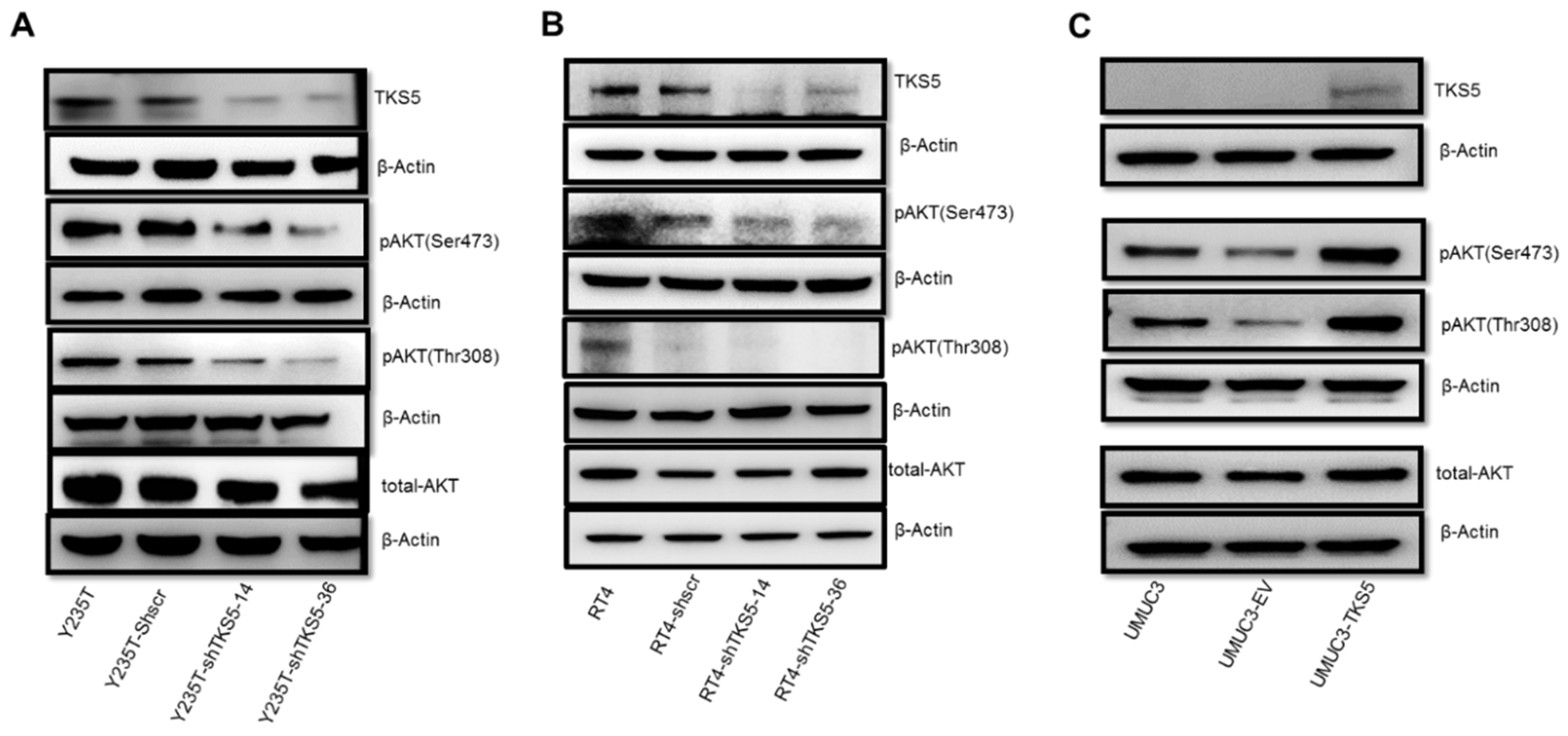
2.6. TKS5 Is Involved in Regulation of AKT Signalling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Antibodies
4.3. Transfection and Infection
4.4. Immunofluorescence
4.5. Metaphase Assay of Chromosome Counting
4.6. Microscopy
4.7. Gelatine Degradation Assay
4.8. Gelatine Zymography
4.9. Boyden Chamber Assay
4.10. Wound-Healing Assay
4.11. Real-Time Quantitative PCR
4.12. Western Blot
4.13. Porcine Bladder Invasion Model
4.14. In Silico Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, R.H.M.; Rueda, O.B.; Ibarz, L. Bladder cancer: Present and future. Med. Clin. 2017, 149, 449–455. [Google Scholar]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Van Batavia, J.; Yamany, T.; Molotkov, A.; Dan, H.; Mansukhani, M.; Batourina, E.; Schneider, K.; Oyon, D.; Dunlop, M.; Wu, X.R.; et al. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 2014, 16, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Florl, A.R.; Schulz, W.A. Chromosomal instability in bladder cancer. Arch. Toxicol. 2008, 82, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, A.; Chaker, A.; Burdelski, C.; Koop, C.; Tsourlakis, M.C.; Steurer, S.; Rink, M.; Eichenauer, T.S.; Wilczak, W.; Wittmer, C.; et al. βIII-tubulin overexpression is linked to aggressive tumour features and genetic instability in urinary bladder cancer. Hum. Pathol. 2017, 61, 210–220. [Google Scholar] [CrossRef] [PubMed]
- David-Pfeuty, T.; Singer, S.J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 1980, 77, 6687–6691. [Google Scholar] [CrossRef]
- Burns, S.; Thrasher, A.J.; Blundell, M.; Machesky, L.; E Jones, G.E. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 2001, 98, 1142–1149. [Google Scholar] [CrossRef]
- Cejudo-Martin, P.; Yuen, A.; Vlahovich, N.; Lock, P.; Courtneidge, S.A.; Díaz, B. Genetic Disruption of the Sh3pxd2a Gene Reveals an Essential Role in Mouse Development and the Existence of a Novel Isoform of Tks5. PLoS ONE 2014, 9, e107674. [Google Scholar] [CrossRef]
- Gaidano, G.; Bergui, L.; Schena, M.; Gaboli, M.; Cremona, O.; Marchisio, P.C.; Caligaris-Cappio, F. Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia 1990, 4, 682–687. [Google Scholar]
- Hai, C.M.; Hahne, P.; Harrington, E.O.; Gimona, M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp. Cell Res. 2002, 280, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, J.; Yamanaka, T.; Doi, S.; Turksen, K.; Heersche, J.; Aubin, J.E.; Takeuchi, H. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone 1990, 11, 287–293. [Google Scholar] [CrossRef]
- Marchisio, P.C.; Cirillo, D.; Teti, A.; Zambonin-Zallone, A.; Tarone, G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 1987, 169, 202–214. [Google Scholar] [CrossRef]
- Miyauchi, A.; Hruska, K.A.; Greenfield, E.M.; Duncan, R.; Alvarez, J.; Barattolo, R.; Colucci, S.; Zambonin-Zallone, A.; Teitelbaum, S.; Teti, A.M. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell Biol. 1990, 111, 2543–2552. [Google Scholar] [CrossRef]
- Moreau, V.; Tatin, F.; Varon, C.; Génot, E. Actin Can Reorganize into Podosomes in Aortic Endothelial Cells, a Process Controlled by Cdc42 and RhoA. Mol. Cell. Biol. 2003, 23, 6809–6822. [Google Scholar] [CrossRef]
- Xiao, H.; Bai, X.-H.; Wang, Y.; Kim, H.; Mak, A.S.; Liu, M. MEK/ERK pathway mediates PKC activation-induced recruitment of PKCζ and MMP-9 to podosomes. J. Cell. Physiol. 2013, 228, 416–427. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Géminard, J.-C.; Jurdic, P.; Bard, F. Podosomes Display Actin Turnover and Dynamic Self-Organization in Osteoclasts Expressing Actin-Green Fluorescent Protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Courtneidge, S.A. The 'ins' and 'outs' of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Sanes, J.R.; Yamagata, M. Formation of lamina-specific synaptic connections. Curr. Opin. Neurobiol. 1999, 9, 79–87. [Google Scholar] [CrossRef]
- Gawden-Bone, C.; Zhou, Z.; King, E.; Prescott, A.; Watts, C.; Lucocq, J. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J. Cell Sci. 2010, 123, 1427–1437. [Google Scholar] [CrossRef]
- Oikawa, T.; Oyama, M.; Kozuka-Hata, H.; Uehara, S.; Udagawa, N.; Saya, H.; Matsuo, K. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J. Cell Biol. 2012, 197, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Ertych, N.; Stolz, A.; Stenzinger, A.; Weichert, W.; Kaulfuß, S.; Burfeind, P.; Aigner, A.; Wordeman, L.; Bastians, H. Increased microtubule assembly rates influence chromosomal instability in colorectal cancer cells. Nat. Cell Biol. 2014, 16, 779–791. [Google Scholar] [CrossRef]
- Revach, O.Y.; Sandler, O.; Samuels, Y.; Geiger, B. Cross-Talk between Receptor Tyrosine Kinases AXL and ERBB3 Regulates Invadopodia Formation in Melanoma Cells. Cancer Res. 2019, 79, 2634–2648. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Yoshida, S.; Muroi, E.; Yoshida, N.; Kawamura, M.; Kouchi, Z.; Nakamura, Y.; Sakai, R.; Fukami, K. Phosphoinositide 3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J. Cell Biol. 2011, 193, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Bromann, P.A.; Korkaya, H.; Courtneidge, S.A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 2004, 23, 7957–7968. [Google Scholar] [CrossRef]
- Kelley, L.C.; Ammer, A.G.; Hayes, K.E.; Martin, K.; Machida, K.; Jia, L.; Mayer, B.J.; Weed, S.A. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J. Cell Sci. 2010, 123, 3923–3932. [Google Scholar] [CrossRef]
- Imanishi, K.; Yoneyama, M.; Hatakeyama, S.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Nakamura, T.; Ohyama, C.; et al. Invadopodia are essential in transurothelial invasion during the muscle invasion of bladder cancer cells. Mol. Med. Rep. 2014, 9, 2159–2165. [Google Scholar] [CrossRef]
- Buschman, M.D.; Bromann, P.A.; Cejudo-Martin, P.; Wen, F.; Pass, I.; Courtneidge, S.A. The Novel Adaptor Protein Tks4 (SH3PXD2B) Is Required for Functional Podosome Formation. Mol. Biol. Cell 2009, 20, 1302–1311. [Google Scholar] [CrossRef]
- Seals, D.F.; Azucena Jr, E.F.; Pass, I.; Tesfay, L.; Gordon, R.; Woodrow, M.; Resau, J.H.; Courtneidge, S.A. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 2005, 7, 155–165. [Google Scholar] [CrossRef]
- Ádám, C.; Fekete, A.; Bőgel, G.; Németh, Z.; Tőkési, N.; Ovádi, J.; Liliom, K.; Pesti, S.; Geiszt, M.; Buday, L. Accumulation of the PX domain mutant Frank-ter Haar syndrome protein Tks4 in aggresomes. Cell Commun. Signal. 2015, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Lock, P.; Abram, C.L.; Gibson, T.J.; Courtneidge, S.A. A new method for isolating tyrosine kinase substrates used to identify Fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998, 17, 4346–4357. [Google Scholar] [CrossRef]
- Szeder, B.; Tárnoki-Zách, J.; Lakatos, D.; Vas, V.; Kudlik, G.; Merő, B.; Koprivanacz, K.; Bányai, L.; Hámori, L.; Róna, G.; et al. Absence of the Tks4 Scaffold Protein Induces Epithelial-Mesenchymal Transition-Like Changes in Human Colon Cancer Cells. Cells 2019, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Itoh, T.; Takenawa, T. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 2008, 182, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.; Bőgel, G.; Pesti, S.; Péterfi, Z.; Geiszt, M.; Buday, L. EGF regulates tyrosine phosphorylation and membrane-translocation of the scaffold protein Tks5. J. Mol. Signal. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bögel, G.; Gujdár, A.; Geiszt, M.; Lányi, Á.; Fekete, A.; Sipeki, S.; Downward, J.; Buday, L. Frank-ter Haar Syndrome Protein Tks4 Regulates Epidermal Growth Factor-dependent Cell Migration. J. Biol. Chem. 2012, 287, 31321–31329. [Google Scholar] [CrossRef] [PubMed]
- Bisson, N.; James, D.A.; Ivosev, G.; Tate, S.; Bonner, R.; Taylor, L.; Pawson, T. Selected reaction monitoring mass spectrometry reveals the dynamics of signaling through the GRB2 adaptor. Nat. Biotechnol. 2011, 29, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Crimaldi, L.; Courtneidge, S.A.; Gimona, M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp. Cell Res. 2009, 315, 2581–2592. [Google Scholar] [CrossRef]
- Lányi, Á.; Baráth, M.; Péterfi, Z.; Bőgel, G.; Orient, A.; Simon, T.; Petrovszki, E.; Kis-Tóth, K.; Sirokmány, G.; Rajnavölgyi, É.; et al. The Homolog of the Five SH3-Domain Protein (HOFI/SH3PXD2B) Regulates Lamellipodia Formation and Cell Spreading. PLoS ONE 2011, 6, e23653. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Wezel, F.; Lustig, J.; Azoitei, A.; Liu, J.; Meessen, S.; Najjar, G.; Zehe, V.; Faustmann, P.; Zengerling, F.; John, A.; et al. Grainyhead-Like 3 Influences Migration and Invasion of Urothelial Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 2959. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, S.; Abdullah, C.; Buschman, M.D.; Diaz, B.; Courtneidge, S.A. The role of Tks adaptor proteins in invadopodia formation, growth and metastasis of melanoma. Oncotarget 2016, 7, 78473–78486. [Google Scholar] [CrossRef]
- Burger, K.L.; Learman, B.S.; Boucherle, A.K.; Sirintrapun, S.J.; Isom, S.; Díaz, B.; Courtneidge, S.A.; Seals, D.F. Src-dependent Tks5 phosphorylation regulates invadopodia-associated invasion in prostate cancer cells. Prostate 2014, 74, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Stylli, S.S.; Stacey, T.T.; Kaye, A.H.; Lock, P. Prognostic significance of Tks5 expression in gliomas. J. Clin. Neurosci. 2012, 19, 436–442. [Google Scholar] [CrossRef]
- Burger, K.L.; Davis, A.L.; Isom, S.; Mishra, N.; Seals, D.F. The podosome marker protein Tks5 regulates macrophage invasive behavior. Cytoskeleton 2011, 68, 694–711. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Meng, X.; Sun, R.; Wang, W.; Zhang, N.; Cao, S.; Liu, B.; Fang, P.; Deng, S.; Yang, S. ADFP promotes cell proliferation in lung adenocarcinoma via Akt phosphorylation. J. Cell Mol. Med. 2021, 25, 827–839. [Google Scholar] [CrossRef]
- Moodley, S.; Hui Bai, X.; Kapus, A.; Yang, B.; Liu, M. XB130/Tks5 scaffold protein interaction regulates Src-mediated cell proliferation and survival. Mol. Biol. Cell 2015, 26, 4492–4502. [Google Scholar] [CrossRef] [PubMed]
- Buttrick, G.J.; Beaumont, L.M.; Leitch, J.; Yau, C.; Hughes, J.R.; Wakefield, J.G. Akt regulates centrosome migration and spindle orientation in the early Drosophila melanogaster embryo. J. Cell Biol. 2008, 180, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Shtivelman, E.; Sussman, J.; Stokoe, D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr. Biol. 2002, 12, 919–924. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sato, E. Protein kinase B (PKB/Akt) is required for the completion of meiosis in mouse oocytes. Dev. Biol. 2008, 314, 215–223. [Google Scholar] [CrossRef]
- Buttrick, G.J.; Wakefield, J.G. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle 2008, 7, 2621–2625. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B. Invadopodia Detection and Gelatin Degradation Assay. Bio-Protocol 2013, 3, e997. [Google Scholar] [CrossRef]
- Chhabra, A.; Rani, V. Gel-Based Gelatin Zymography to Examine Matrix Metalloproteinase Activity in Cell Culture. Methods Mol. Biol. 2018, 1731, 83–96. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zheng, X.; Azoitei, A.; John, A.; Zengerling, F.; Wezel, F.; Bolenz, C.; Günes, C. The Role of TKS5 in Chromosome Stability and Bladder Cancer Progression. Int. J. Mol. Sci. 2022, 23, 14283. https://doi.org/10.3390/ijms232214283
Wang W, Zheng X, Azoitei A, John A, Zengerling F, Wezel F, Bolenz C, Günes C. The Role of TKS5 in Chromosome Stability and Bladder Cancer Progression. International Journal of Molecular Sciences. 2022; 23(22):14283. https://doi.org/10.3390/ijms232214283
Chicago/Turabian StyleWang, Wenya, Xi Zheng, Anca Azoitei, Axel John, Friedemann Zengerling, Felix Wezel, Christian Bolenz, and Cagatay Günes. 2022. "The Role of TKS5 in Chromosome Stability and Bladder Cancer Progression" International Journal of Molecular Sciences 23, no. 22: 14283. https://doi.org/10.3390/ijms232214283
APA StyleWang, W., Zheng, X., Azoitei, A., John, A., Zengerling, F., Wezel, F., Bolenz, C., & Günes, C. (2022). The Role of TKS5 in Chromosome Stability and Bladder Cancer Progression. International Journal of Molecular Sciences, 23(22), 14283. https://doi.org/10.3390/ijms232214283







