The Role of PEMFs on Bone Healing: An In Vitro Study
Abstract
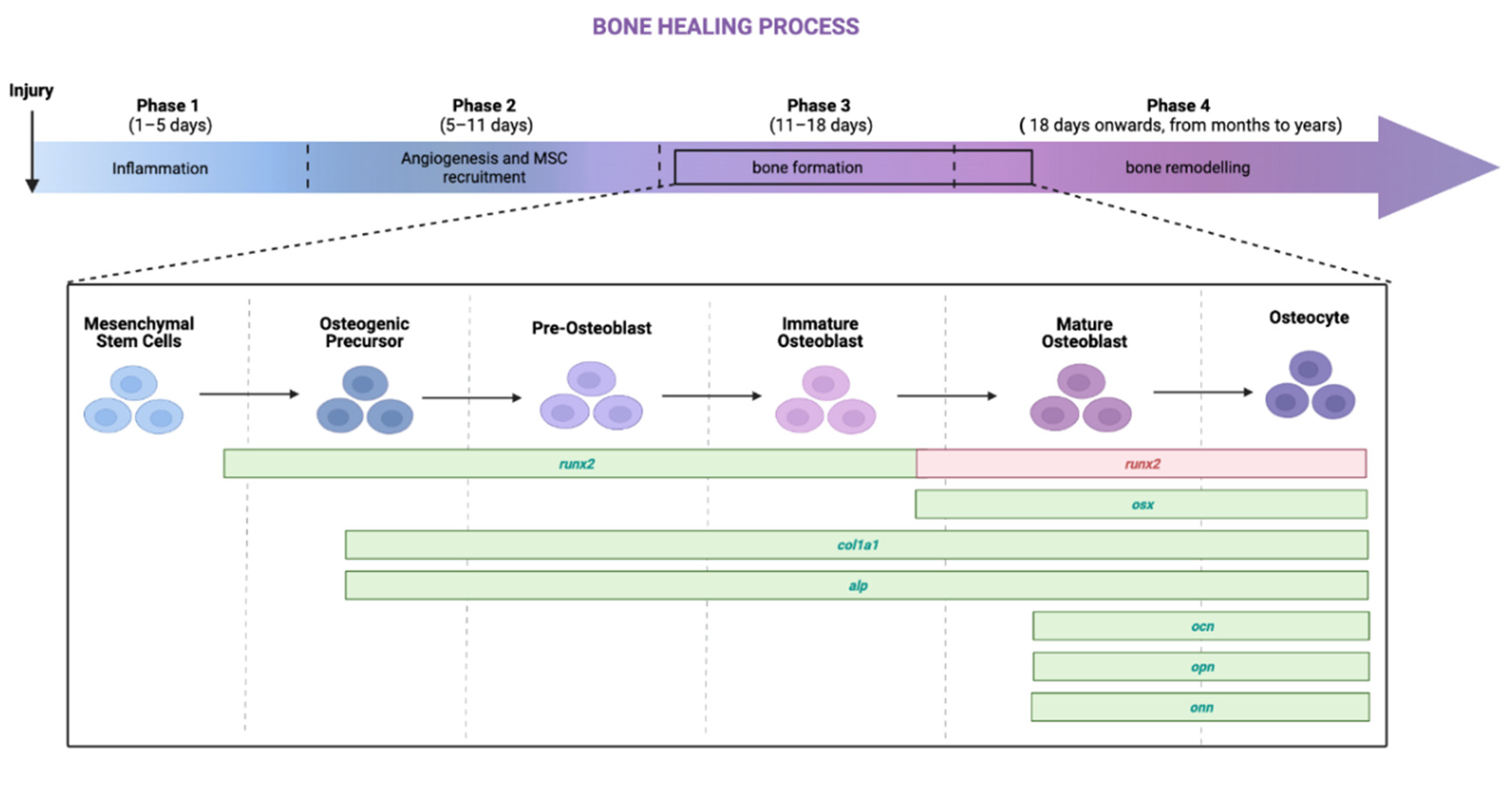
:1. Introduction
- Intensity: ranging from 0.1 mT to 2 mT;
- Frequency: ranging from 15 Hz to 75 Hz;
- Duration: ranging from 8 min to 24 h for many days (from 1 to 28 days).
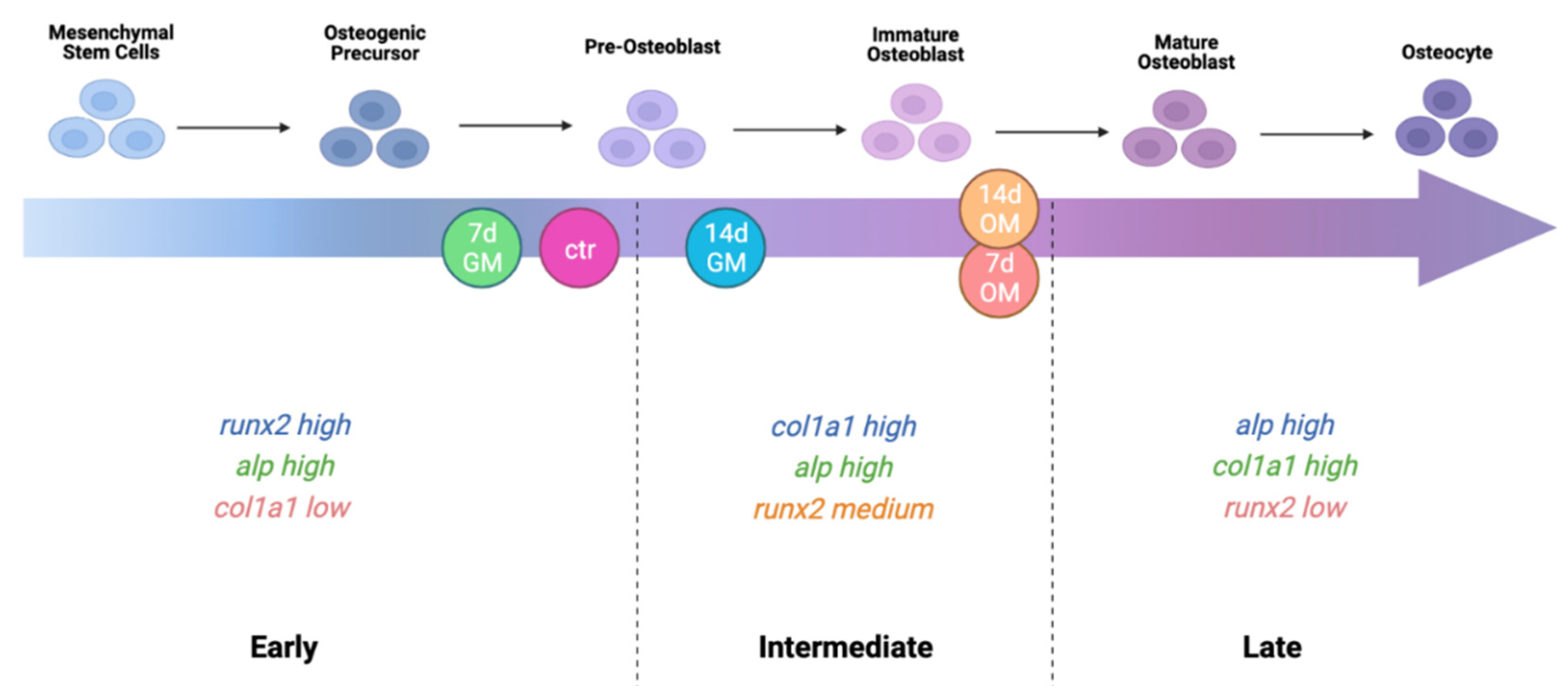
2. Results
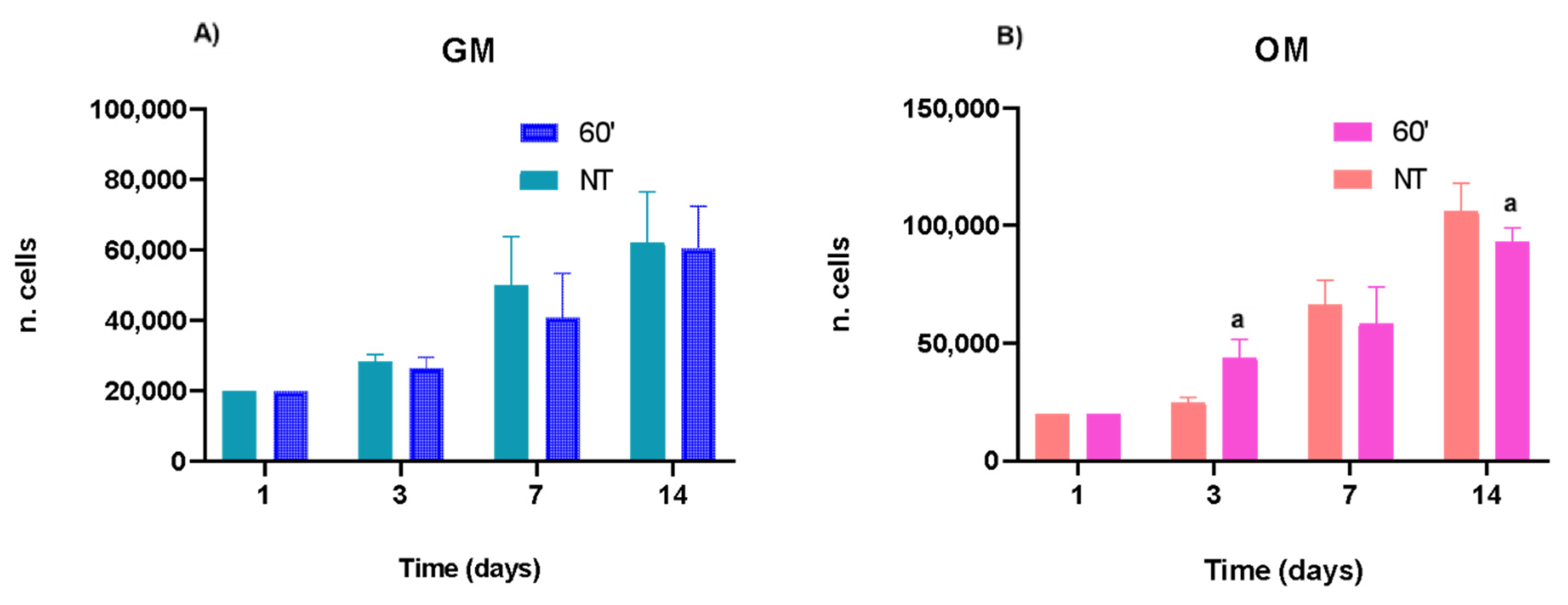
2.1. Proliferation of hASCs
2.2. hASCs’ Osteogenic Differentiation—Bone Formation Phase
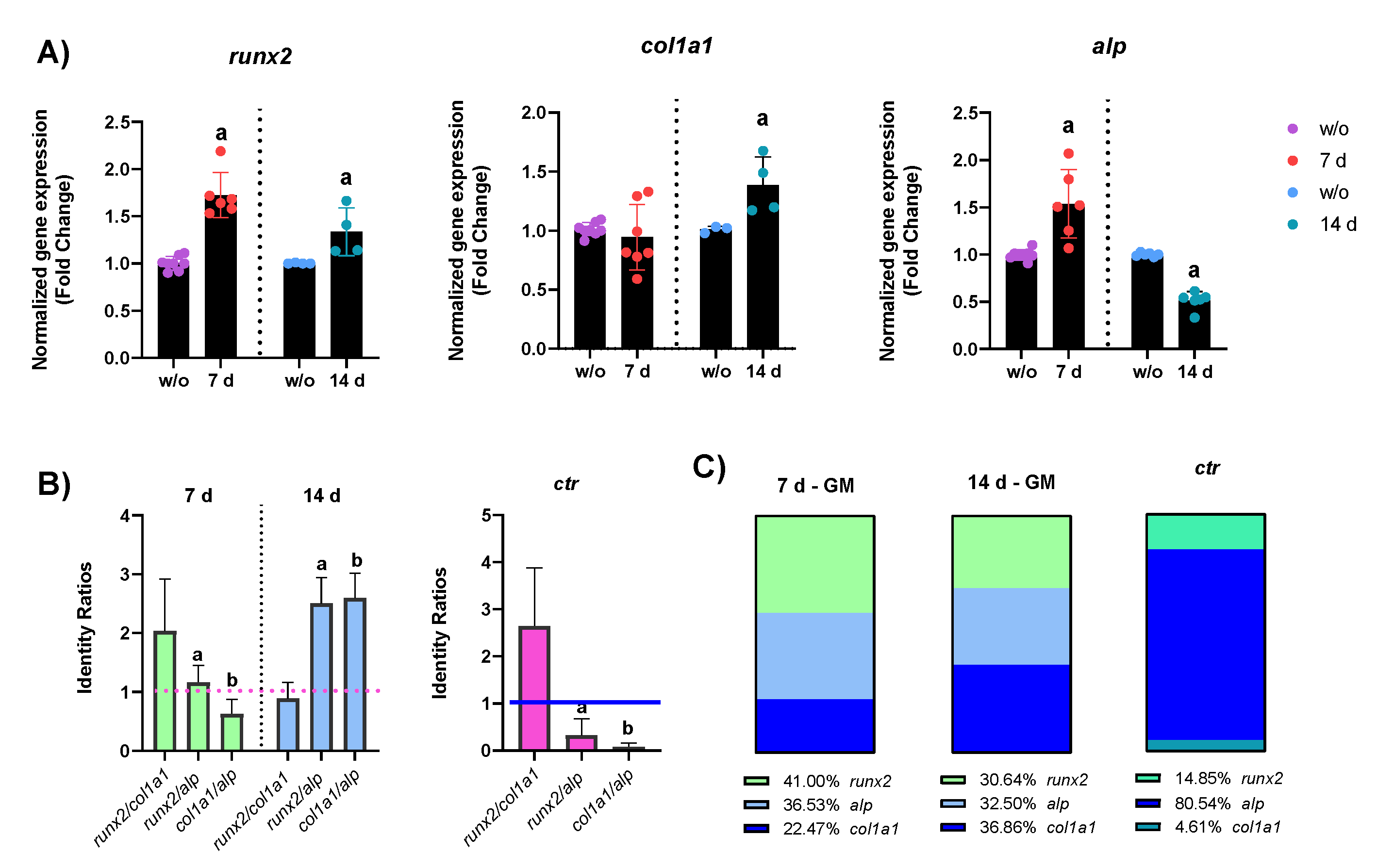
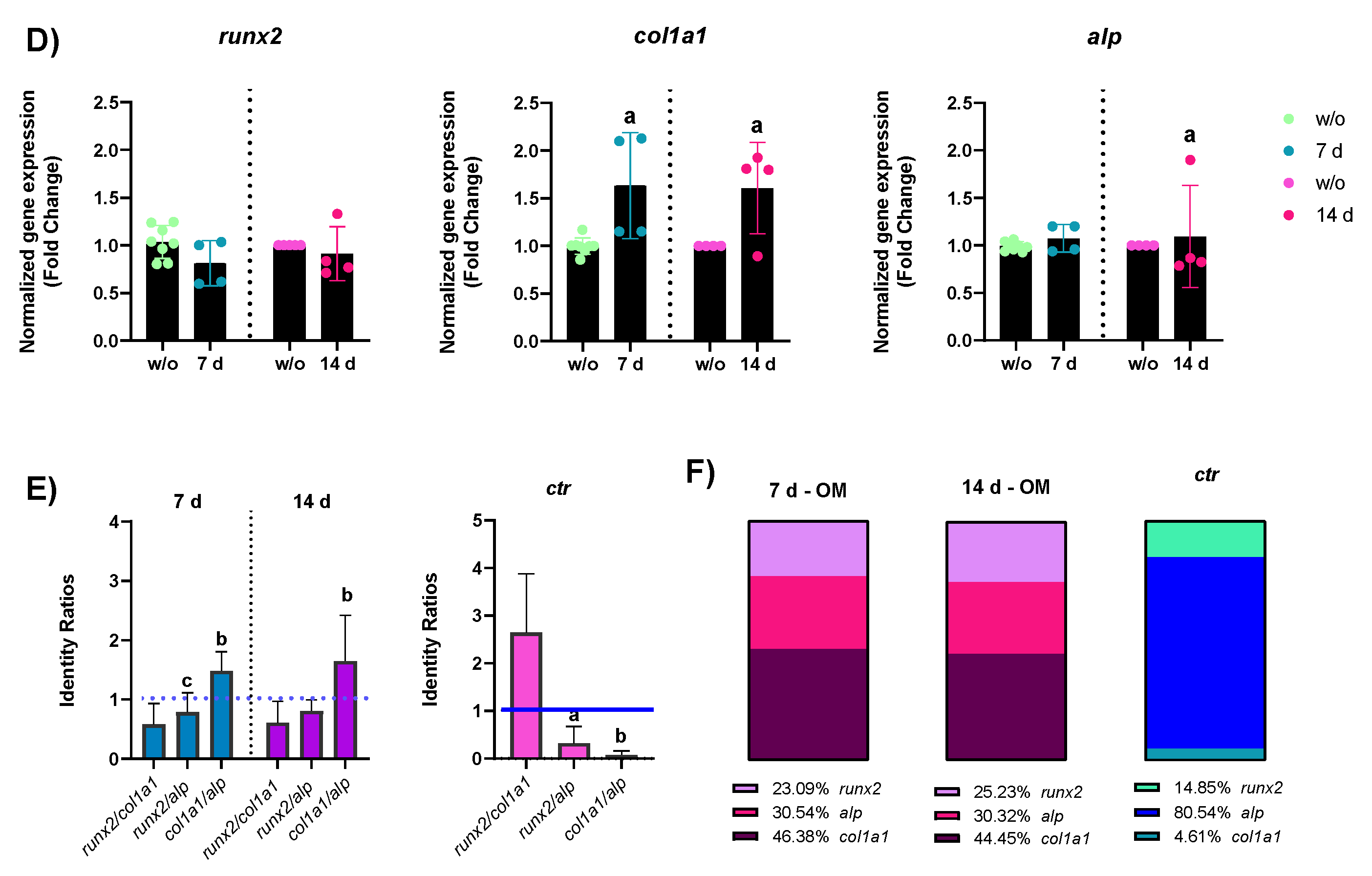
2.2.1. mRNA Expression
2.2.2. Protein Expression
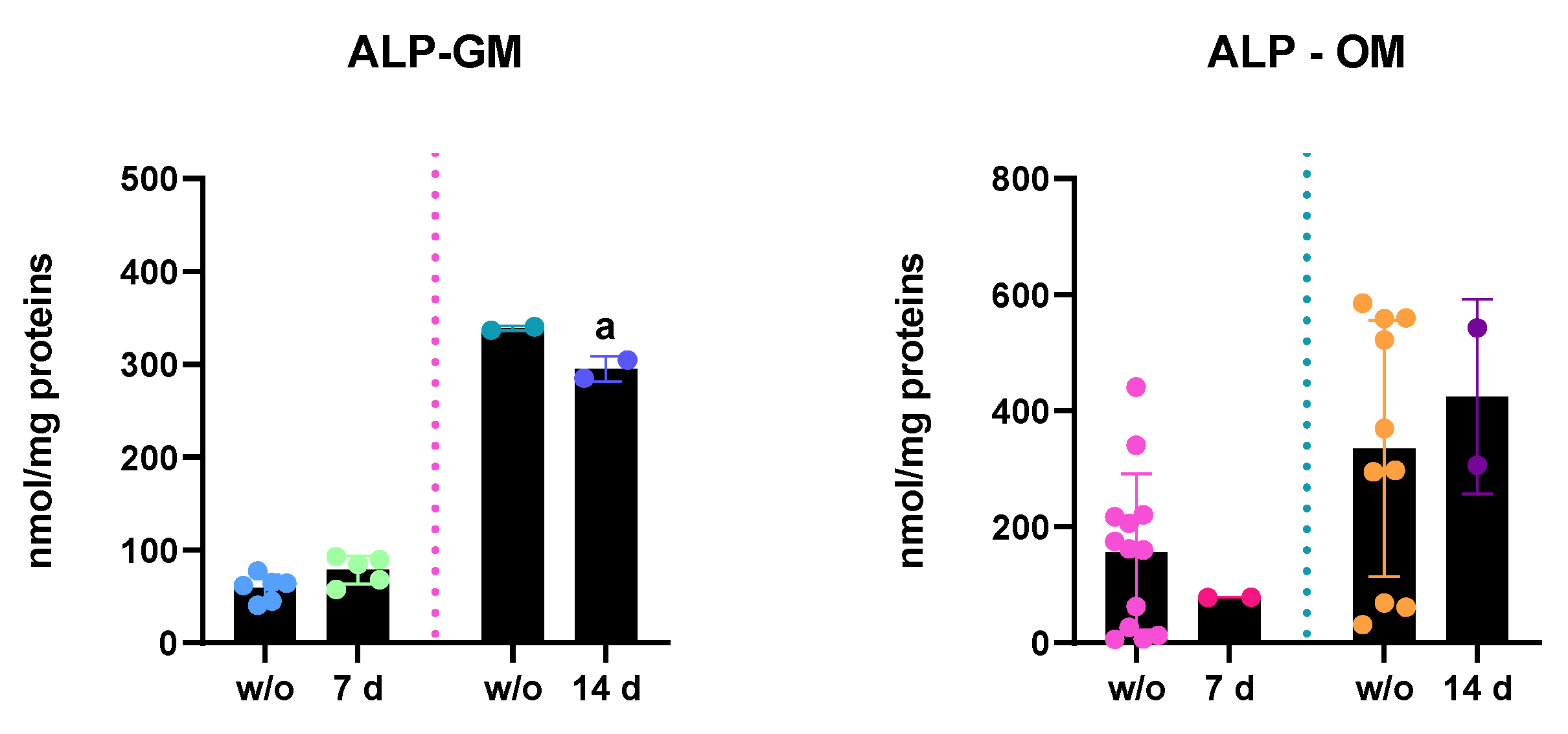
- ALP enzymatic activity: ALP activity of hASC lysates was measured after 7 and 14 days of PEMF treatments and results are reported in Figure 4. In accordance with increased ALP mRNA expression, in PEMF+ cells, kept in GM, ALP activity increased after 7 days of treatment, whereas at 14 days of treatment, there was a statistically significant decrease in ALP content versus the w/o cells, in accordance with the reduced gene expression. In OM, instead, after 7 days of PEMF treatment there was a slight decrease in the ALP activity, even if not statistically significant. However, at 14 days ALP activity seemed to increase a little, probably due to the progression of osteogenic differentiation.
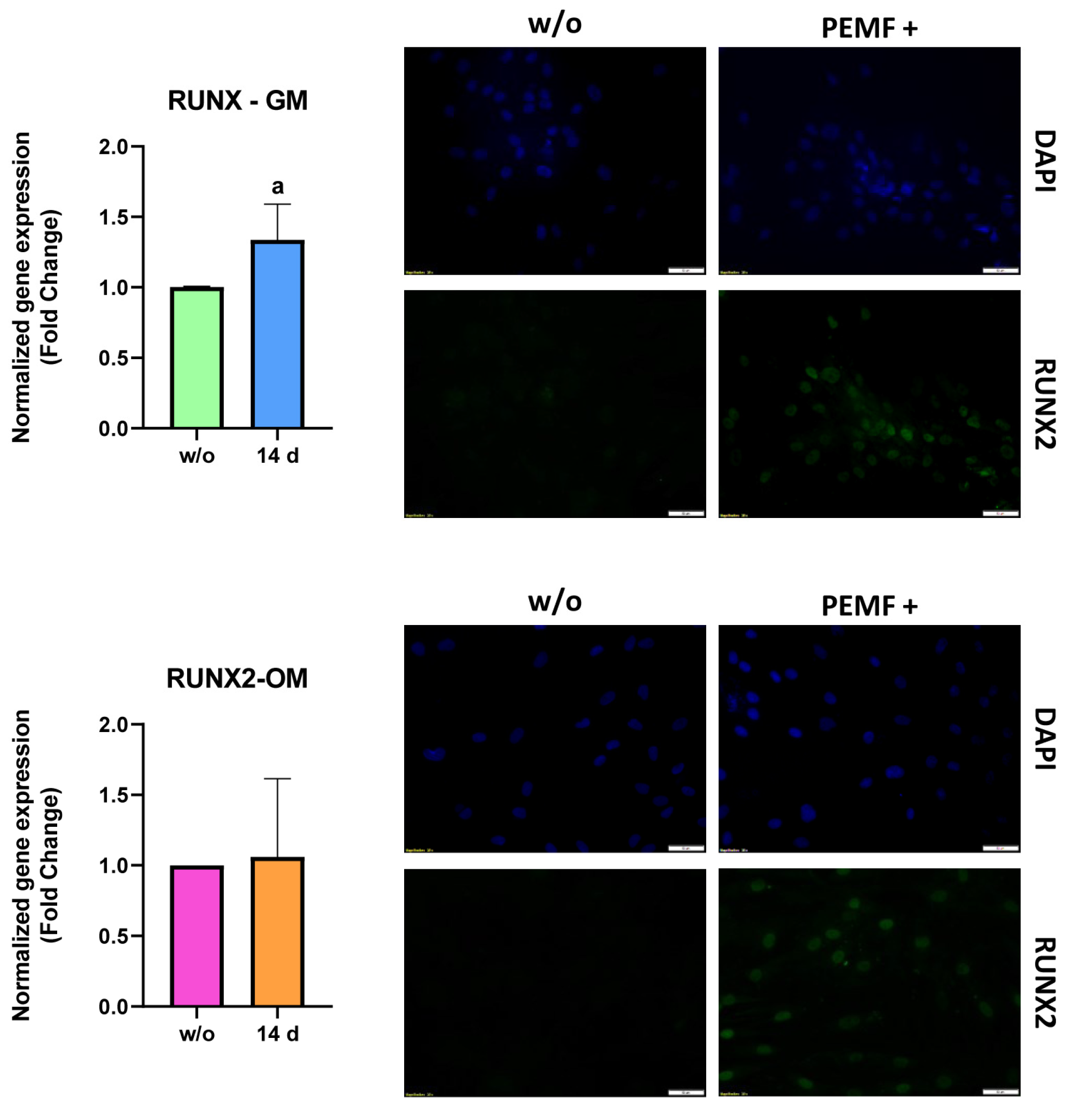
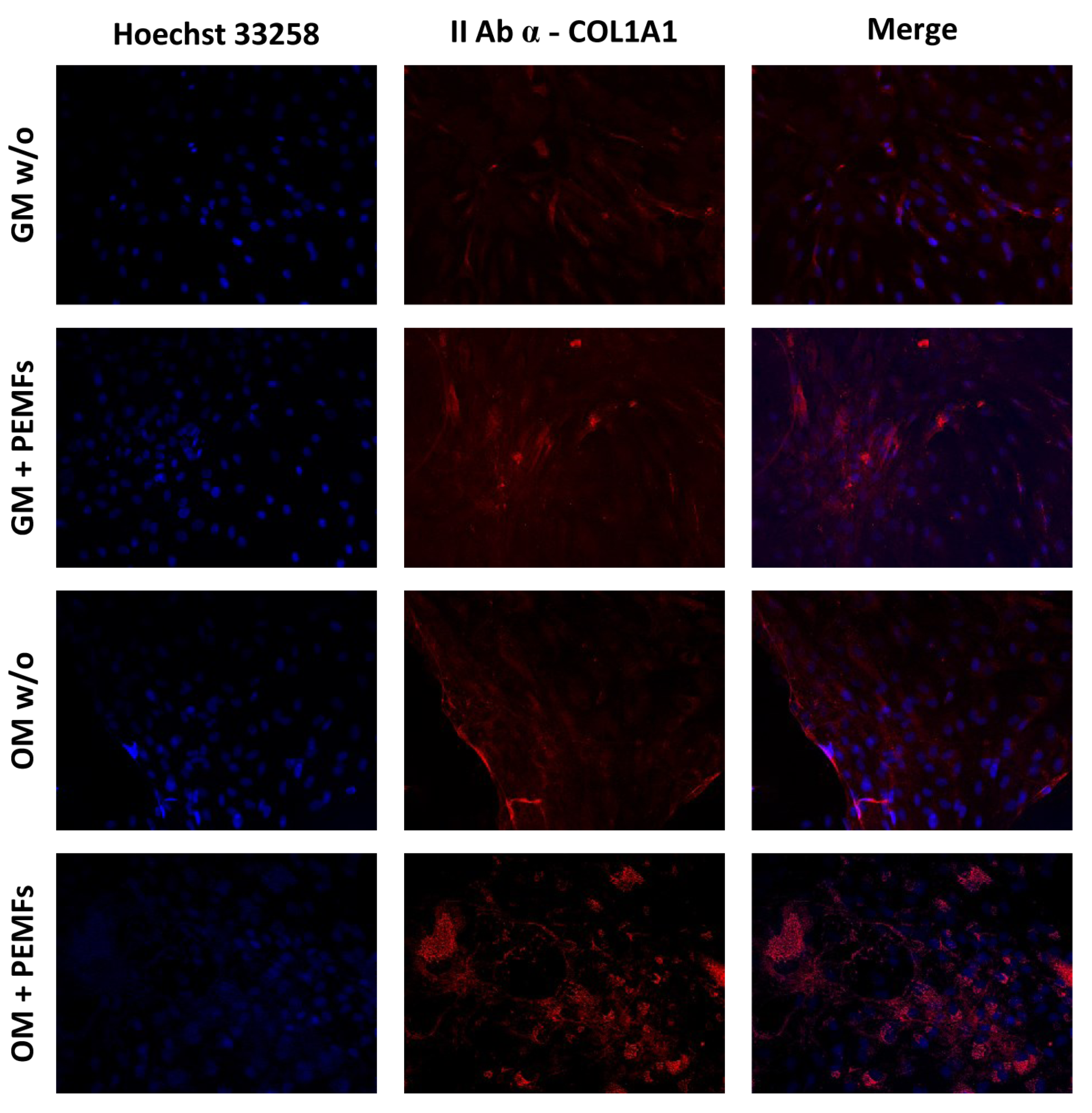
- Immunofluorescence staining: Immunofluorescence staining was used to analyze the synthesis of both RUNX2 and COL1A1 proteins.
2.3. Osteoblast Biosynthetic Activities—Bone Remodeling Phase
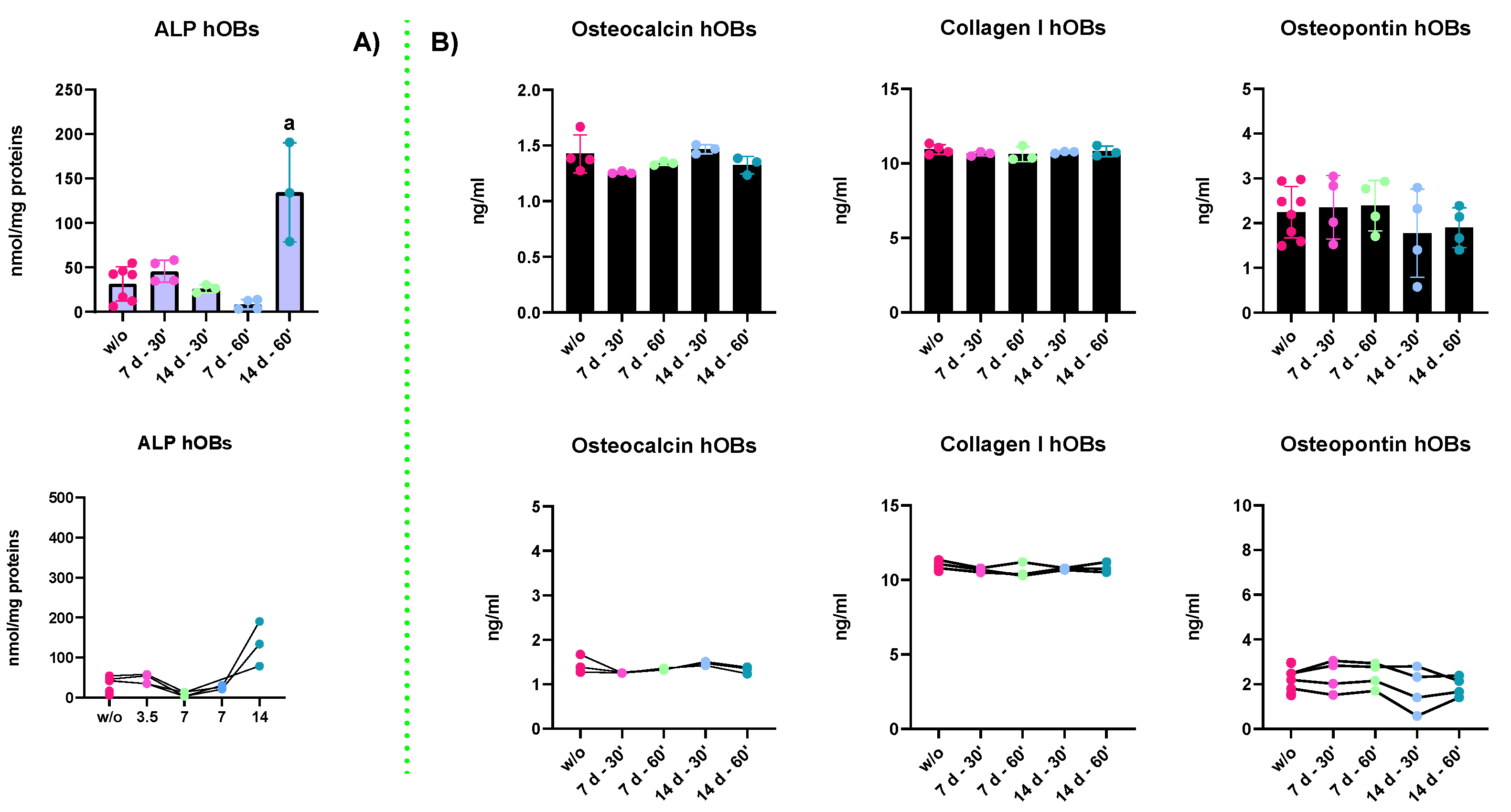
- ALP enzymatic activity: The results in Figure 7A report the ALP enzymatic activity of hOBs kept in GM for 7 and 14 days and treated for 30 and 60 min. The results indicated that a strong increase in the ALP synthesis and activity was detected upon 14 days of treatment. Indeed, all the other experimental groups displayed a lower ALP content. These results may suggest that hOBs need long-lasting treatments to cause a physiological response. The ALP activity trends depending on the treatment duration are represented in the plot right below. In fact, the relationship between the cellular response and the duration of the stimuli becomes evident. Indeed, the only statistical differences (p-value < 0.0001) were found between the 14 d—60′ and all the other groups.
- ELISA assay: ELISA assays were performed to evaluate the secretion of bony ECM proteins. The proteins investigated were collagen type I (COL1A1), osteopontin (OPN), and osteocalcin (OCN). However, the results reported in Figure 7B indicate that irrespective of PEMF treatments, there were no changes in the secretory activities of osteoblasts, an important feature of bone remodeling. Moreover, as shown by the trends in the last line of the figure, there were no changes in the proportion of the secreted proteins in the medium.
3. Discussion
4. Materials and Methods
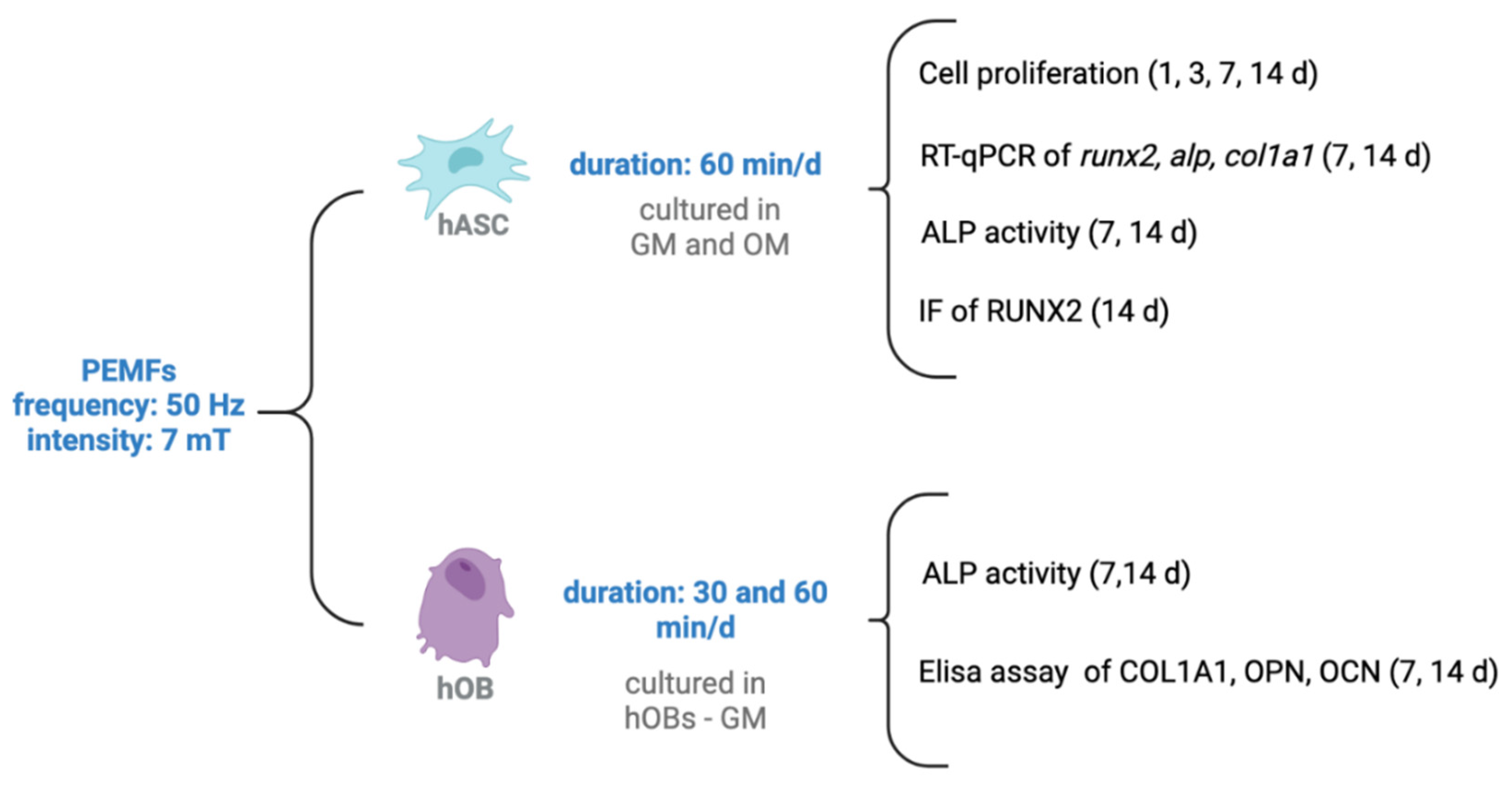
4.1. Types of Cells
- Human adipose-derived stem cells (hASCs)
- Human Osteoblasts (hOBs)
4.2. Cell Seeding and PEMF Treatment
4.3. Proliferation Assay (WST)
4.4. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR (qRT-PCR)
4.5. Alkaline Phosphatase (ALP) Activity
4.6. Immunofluorescence (IF)
4.7. ELISA Assays
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP/alp | alkaline phosphatase protein/gene |
| ATF4/atf4 | activating transcription factor 4 protein/gene |
| B2M/β2m | beta-2-microglobulin protein/gene |
| COL1A1/col1a1 | collagen α-1 type I protein/gene |
| ctr | control |
| dmp1 | dentin matrix acidic phosphoprotein 1 |
| ECM | extracellular matrix |
| FBS | fetal bovine serum |
| GM | growth medium |
| hASCs | human adipose mesenchymal stem cells |
| hOBs | primary human osteoblasts |
| BSPII/ibsp | bone sialoprotein II protein/gene |
| IF | immunofluorescence |
| MSCs | mesenchymal stem cells |
| OBs | osteoblasts |
| OCN/ocn | osteocalcin protein/gene |
| OCs | osteoclasts |
| OM | osteogenic medium |
| ONN/onn | osteonectin protein/gene |
| OPN/opn | osteopontin protein/gene |
| OSX/osx | osterix protein/gene |
| PBS | phosphate buffered saline |
| PEMFs | pulsed electromagnetic fields |
| PFA | paraformaldehyde |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RT | room temperature |
| RUNX2/runx2 | runt-related transcription factor 2 protein/gene |
| SOX9/sox9 | transcription factor 9 protein/gene |
| WST-1 | water-soluble tetrazolium 1 |
References
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Kim, J.H.; Hutton, W.C.; Yoon, S.T. Upregulation of Intervertebral Disc-Cell Matrix Synthesis by Pulsed Electromagnetic Field Is Mediated by Bone Morphogenetic Proteins. Clin. Spine Surg. 2013, 26, 167–173. [Google Scholar] [CrossRef]
- Peng, L.; Wu, F.; Cao, M.; Li, M.; Cui, J.; Liu, L.; Zhao, Y.; Yang, J. Effects of different physical factors on osteogenic differentiation. Biochimie 2022. [Google Scholar] [CrossRef]
- Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.M.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef]
- Huang, X.; Das, R.; Patel, A.; Duc Nguyen, T. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell. Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Mizokami, A.; Kawakubo-Yasukochi, T.; Hirata, M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef]
- Basal, O.; Atay, T.; Ciris, İ.M.; Baykal, Y.B. Epidermal growth factor (EGF) promotes bone healing in surgically induced osteonecrosis of the femoral head (ONFH). Bosn. J. Basic Med. Sci. 2018, 18, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Correlo, V.M.; Oliveira, J.M.; Mano, J.F.; Neves, N.M.; Reis, R.L. Natural Origin Materials for Bone Tissue Engineering–Properties, Processing, and Performance. In Principles of Regenerative Medicine, 2nd ed.; Atala, A., Lanza, R., Thomson, J.A., Nerem, R., Eds.; Academic Press: San Diego, CA, USA, 2011; Chapter 32; pp. 557–586. ISBN 978-0-12-381422-7. [Google Scholar]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Bodle, J.C.; Hanson, A.D.; Loboa, E.G. Adipose-Derived Stem Cells in Functional Bone Tissue Engineering: Lessons from Bone Mechanobiology. Tissue Eng. Part B Rev. 2011, 17, 195–211. [Google Scholar] [CrossRef]
- Palanisamy, P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y.-P. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing. J. Ultrasound Med. 2022, 41, 547–563. [Google Scholar] [CrossRef]
- Puts, R.; Vico, L.; Beilfuß, N.; Shaka, M.; Padilla, F.; Raum, K. Pulsed ultrasound for bone regeneration–outcomes and hurdles in the clinical application: A systematic review. Eur. Cells Mater. 2021, 42, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ning, C.; Zhang, Y.; Tan, G.; Lin, Z.; Liu, S.; Wang, X.; Yang, H.; Li, K.; Yi, X.; et al. Bone-Inspired Spatially Specific Piezoelectricity Induces Bone Regeneration. Theranostics 2017, 7, 3387–3397. [Google Scholar] [CrossRef] [Green Version]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Carter, A.; Popowski, K.; Cheng, K.; Greenbaum, A.; Ligler, F.S.; Moatti, A. Enhancement of Bone Regeneration Through the Converse Piezoelectric Effect, A Novel Approach for Applying Mechanical Stimulation. Bioelectricity 2021, 3, 255–271. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Ricci, C.; Milazzo, M.; Strangis, G.; Forli, F.; Buda, G.; Petrini, M.; Berrettini, S.; Uddin, M.J.; Danti, S.; et al. Piezoelectric Signals in Vascularized Bone Regeneration. Biomolecules 2021, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, W.; Wang, W.; Wang, J.; Ma, T.; Chen, J.; Wu, H.; Liu, C. Sinusoidal electromagnetic fields accelerate bone regeneration by boosting the multifunctionality of bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-L.; Shao, J.-S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 Promotes Osteogenesis and Suppresses Adipogenic Differentiation of Multipotent Mesenchymal Progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Kapuy, O.; Domingo-Sananes, M.R.; Tyson, J.J. Regulated protein kinases and phosphatases in cell cycle decisions. Curr. Opin. Cell Biol. 2010, 22, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment —Specific factors involved in the regulation of MSC plasticity. Genes Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Rosso, F.; Bonasia, D.E.; Marmotti, A.; Cottino, U.; Rossi, R. Mechanical Stimulation (Pulsed Electromagnetic Fields “PEMF” and Extracorporeal Shock Wave Therapy “ESWT”) and Tendon Regeneration: A Possible Alternative. Front. Aging Neurosci. 2015, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-C.; Cheung, K.-K.; Li, X.; Cheing, G.L.-Y. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound. Arch. Dermatol. Res. 2016, 308, 21–29. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.F.; Ventura, U.; Fontana, J.M.; Orsenigo, M.N.; Corazza, G.R. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 2010, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Bina, V.; Botta, L.; Benazzo, F.M.; Medetti, M.; Maestretti, G.; Mosconi, M.; Cofano, F.; Tartara, F.; Gastaldi, G. Osteogenic potential of human adipose derived stem cells (hASCs) seeded on titanium trabecular spinal cages. Sci. Rep. 2020, 10, 18284. [Google Scholar] [CrossRef] [PubMed]
- Benazzo, F.; Botta, L.; Scaffino, M.F.; Caliogna, L.; Marullo, M.; Fusi, S.; Gastaldi, G. Trabecular titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors: Effects of TT Scaffolds on hASCs. J. Biomed. Mater. Res. Part A 2014, 102, 2061–2071. [Google Scholar] [CrossRef]
- Pellavio, G.; Martinotti, S.; Patrone, M.; Ranzato, E.; Laforenza, U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells 2022, 11, 1892. [Google Scholar] [CrossRef]
- Wang, T.; Xie, W.; Ye, W.; He, C. Effects of electromagnetic fields on osteoarthritis. Biomed. Pharmacother. 2019, 118, 109282. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliogna, L.; Bina, V.; Brancato, A.M.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; Grassi, F.A.; Benazzo, F.; Pasta, G. The Role of PEMFs on Bone Healing: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 14298. https://doi.org/10.3390/ijms232214298
Caliogna L, Bina V, Brancato AM, Gastaldi G, Annunziata S, Mosconi M, Grassi FA, Benazzo F, Pasta G. The Role of PEMFs on Bone Healing: An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(22):14298. https://doi.org/10.3390/ijms232214298
Chicago/Turabian StyleCaliogna, Laura, Valentina Bina, Alice Maria Brancato, Giulia Gastaldi, Salvatore Annunziata, Mario Mosconi, Federico Alberto Grassi, Francesco Benazzo, and Gianluigi Pasta. 2022. "The Role of PEMFs on Bone Healing: An In Vitro Study" International Journal of Molecular Sciences 23, no. 22: 14298. https://doi.org/10.3390/ijms232214298





