In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus
Abstract
1. Introduction
2. Results
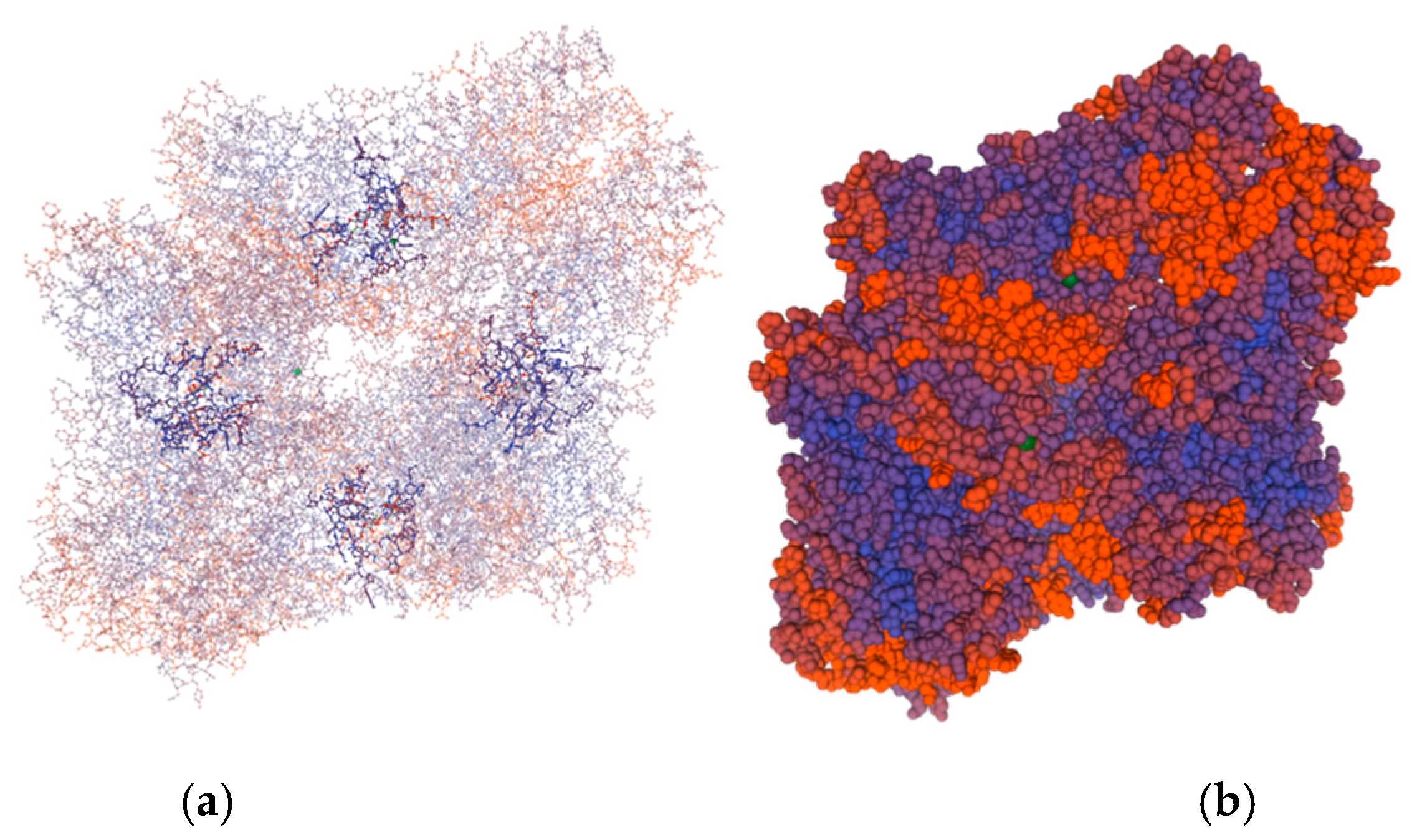
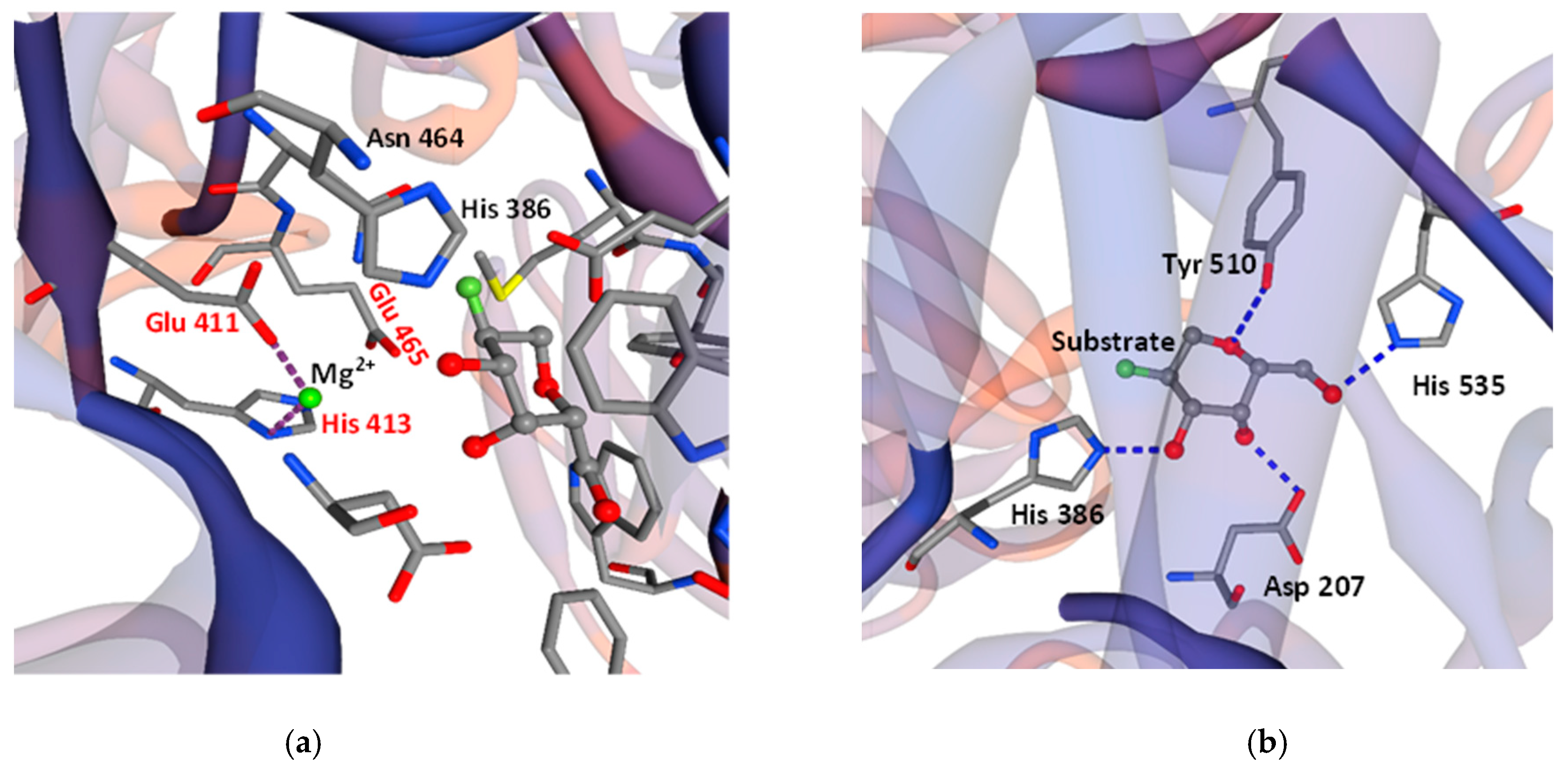
2.1. Gene Sequencing and Molecular Structure of the New β-Galactosidase of L. bulgaricus 43
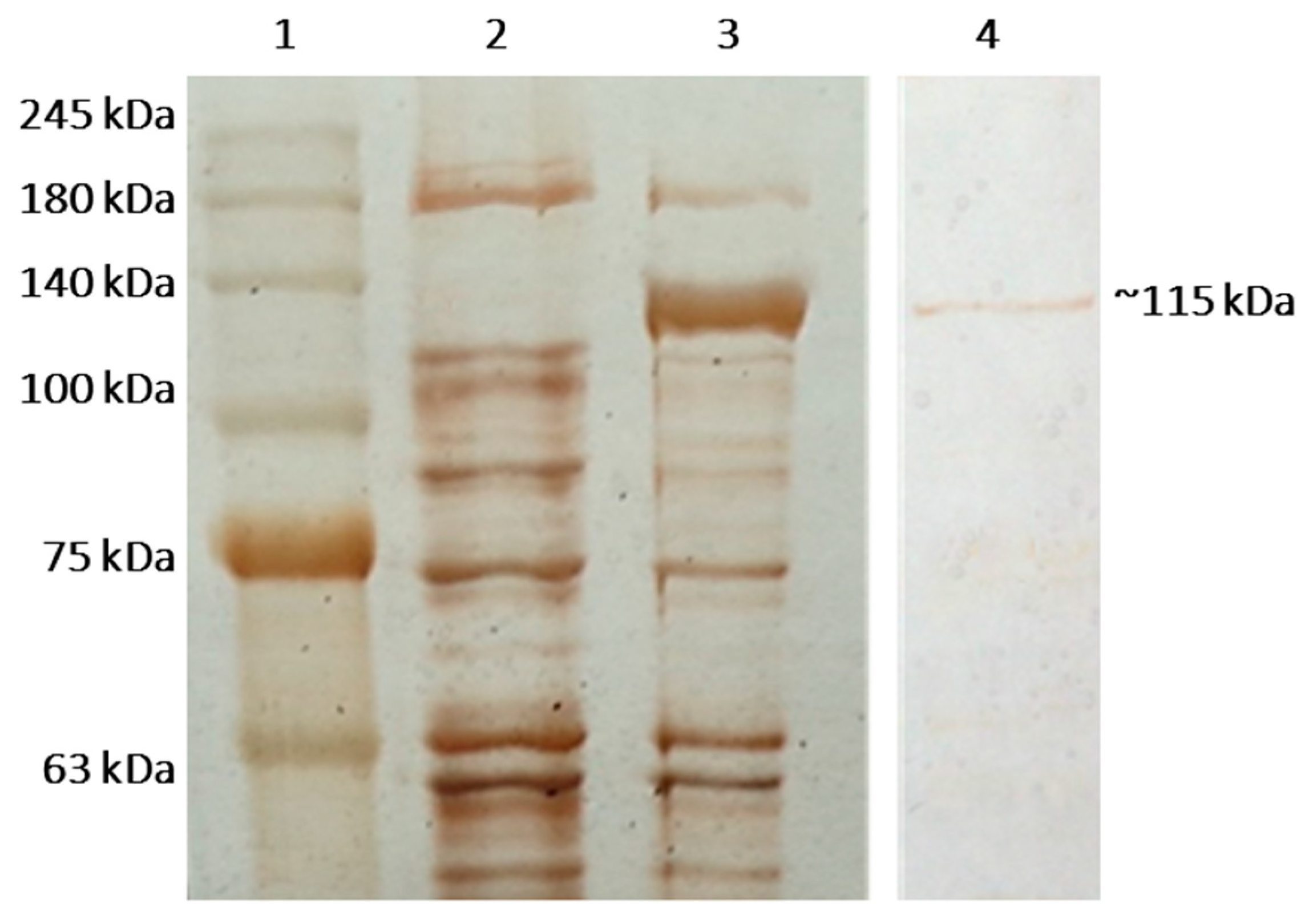
2.2. Cloning, Heterologous Expression in E. coli Strain BL21(DE3), and Purification of β-Galactosidase of L. bulgaricus 43
2.3. Optimal Activity of the Recombinant β-Galactosidase
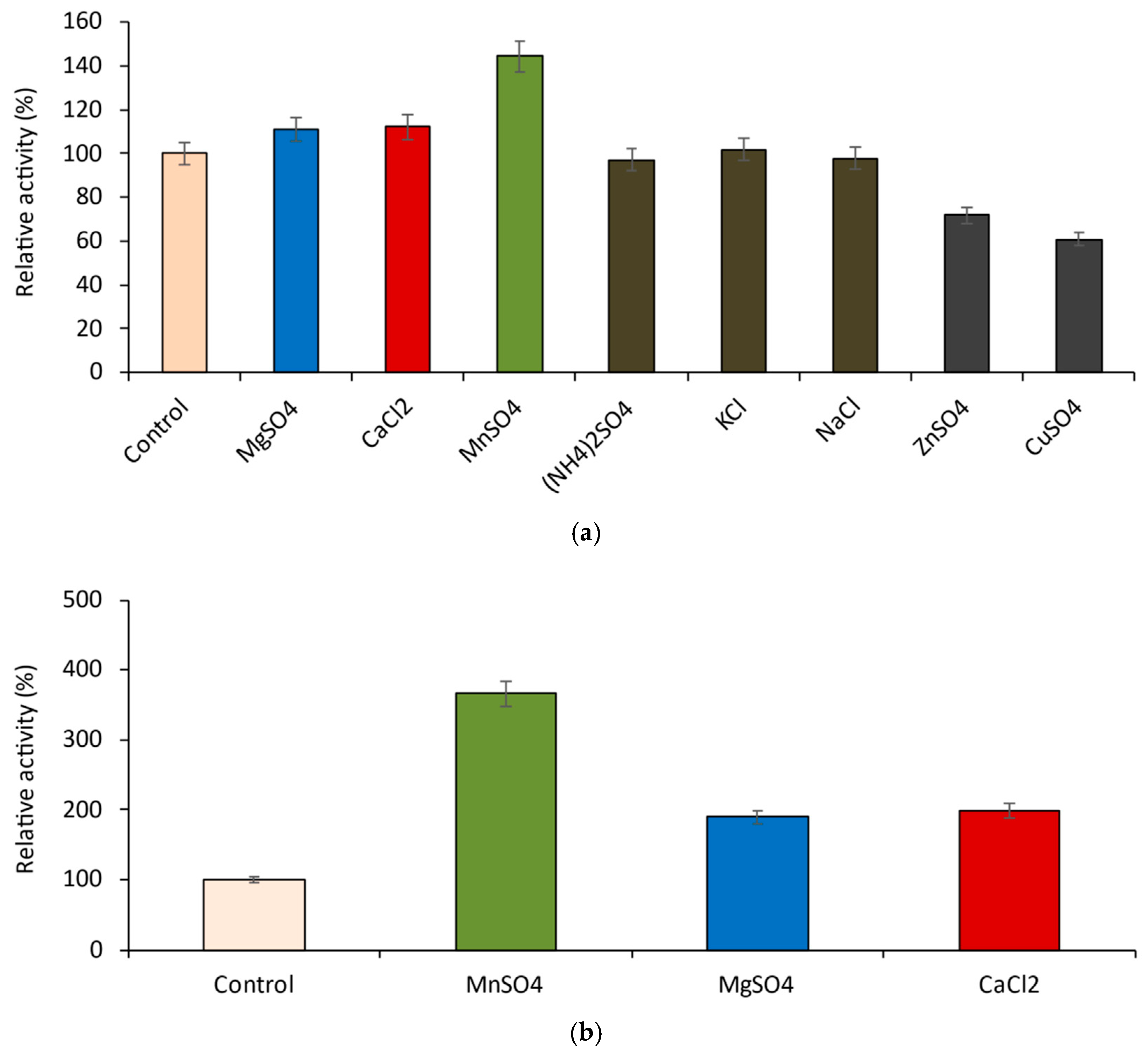
2.4. Influence of the Cations on the Enzyme Activity of the Recombinant β-Galactosidase
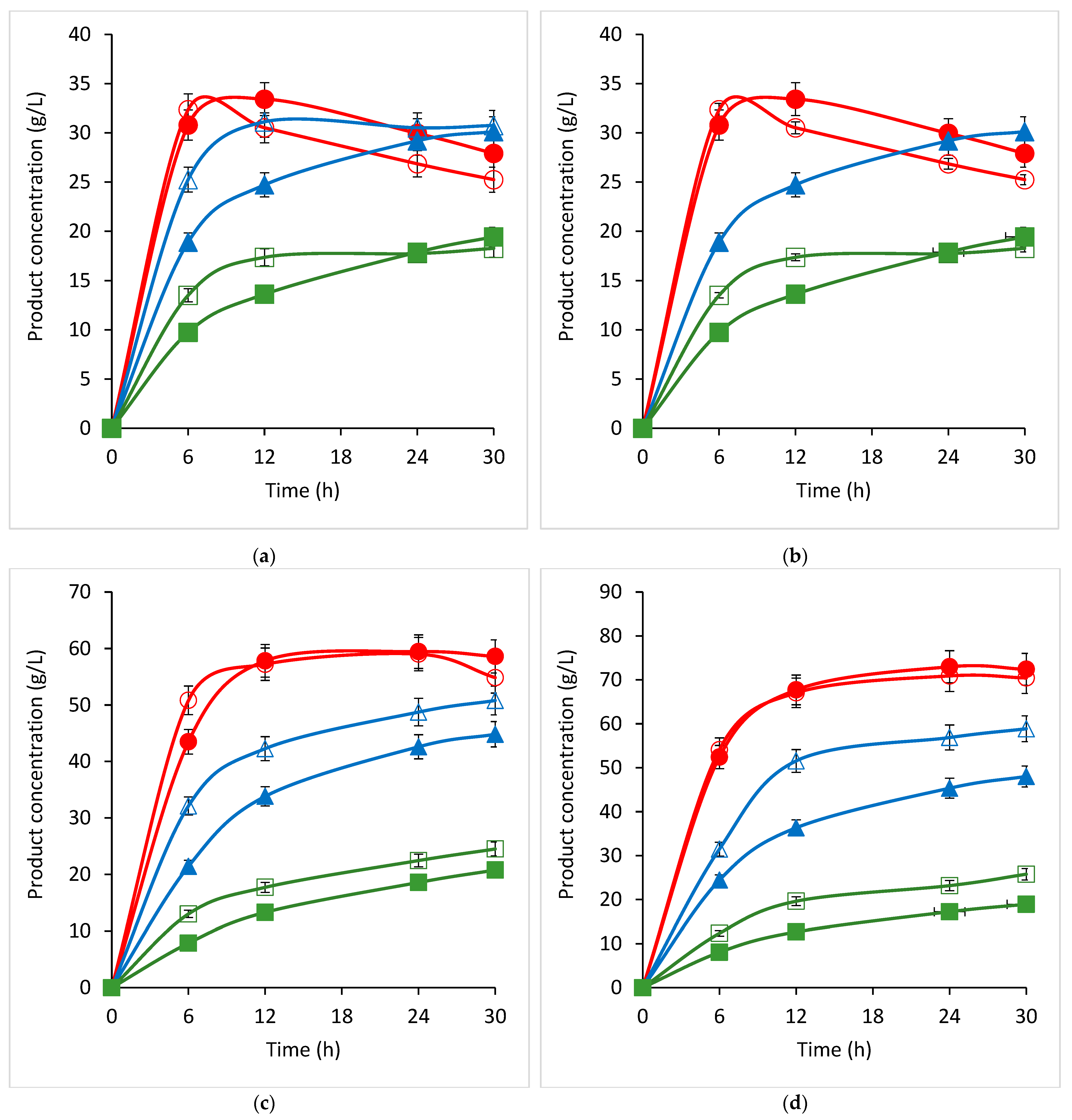
2.5. In Vitro GOS Production by the Recombinant β-Galactosidase
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Maintenance
4.2. Isolation of DNA, PCR, and Sequence Analysis of β-Galactosidase Gene of L. bulgaricus 43
4.3. Bioinformatics Analysis
4.4. Molecular Cloning of the β-Galactosidase Gene of L. bulgaricus 43
4.5. Preparation of the Crude β-Galactosidase
4.6. Purification and Visualization of β-Galactosidase of L. bulgaricus 43
4.7. Enzyme Activity Assay
4.8. Influence of Temperature, pH, and Cations on the Activity of β-Galactosidase
4.9. In Vitro GOS Production
4.10. Analytical Techniques
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Maraz, A.M.; Kovacs, Z.; Benjamins, E.; Pazmandi, M. Recent developments in microbial production of high-purity galacto-oligosaccharides. World J. Microbiol. Biotechnol. 2022, 38, 95. [Google Scholar] [CrossRef] [PubMed]
- Sijbers, A.M.; Schoemaker, R.J.W.; Nauta, A.; Alkema, W. Revealing new leads for the impact of galacto-oligosaccharides on gut commensals and gut health benefits through text mining. Benef. Microbes 2020, 11, 283–302. [Google Scholar] [CrossRef]
- Marín-Manzano, M.C.; Abecia, L.; Hernández-Hernández, O.; Sanz, M.L.; Montilla, A.; Olano, A.; Rubio, L.A.; Moreno, F.J.; Clemente, A. Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of Bifidobacterium animalis in the large intestine of growing rats. J. Agric. Food Chem. 2013, 61, 7560–7567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Mei, Z.; Yuan, J.; Li, D. Biological activity of galacto-oligosaccharides: A review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabinska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 2005, 82, 471–476. [Google Scholar] [CrossRef]
- Fedorak, R.N.; Madsen, K.L. Probiotics and prebiotics in gastrointestinal disorders. Curr. Opin. Gastroenterol. 2004, 20, 146–155. [Google Scholar] [CrossRef]
- Tanabe, S.; Hochi, S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. J. Mol. Med. 2010, 25, 331–336. [Google Scholar] [CrossRef]
- Hashmi, A.; Naeem, N.; Farooq, Z.; Masood, S.; Iqbal, S.; Naseer, R. Effect of Prebiotic Galacto-Oligosaccharides on Serum Lipid Profile of Hypercholesterolemics. Probiotics Antimicrob. Prot. 2016, 8, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Daliri, E.B.-M.; Baltriukiene, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Moreno, F.J.; Olano, A.; Clemente, A.; Villar, C.J.; Lombó, F. A Galacto-Oligosaccharides Preparation Derived from Lactulose Protects against Colorectal Cancer Development in an Animal Model. Front. Microbiol. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Alander, M.; Matto, J.; Kneifel, W.; Johansson, M.; Kogler, B.; Crittenden, R.; Mattila-Sandholm, T.; Saarela, M. Effect of galacto-oligosaccharide supplementation on human faecal microfora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. Int. Dairy J. 2001, 11, 817–825. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Prebiotic–Probiotic Relationship: The Genetic Fundamentals of Polysaccharides Conversion by Bifidobacterium and Lactobacillus Genera. In Handbook of Food Bioengineering: Food Bioconversion, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2017; Volume 2, pp. 237–278. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli, a review. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Torres, D.P.M.; do Pilar, G.; Gonçalves, M.; Teixeira, J.A.; Rodrigues, L.R. Galactooligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Kobata, A. Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. Ser. 2010, 86, 731–747. [Google Scholar] [CrossRef]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef]
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; van Sinderen, D. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 3, 1–14. [Google Scholar] [CrossRef]
- Rabiu, B.A.; Jay, A.J.; Gibson, G.R.; Rastall, R.A. Synthesis and fermentation properties of novel galacto-oligosaccharides by β-galactosidases from Bifidobacterium species. Appl. Environ. Microbiol. 2001, 67, 2526–2530. [Google Scholar] [CrossRef] [PubMed]
- Arreola, S.L.; Intanon, M.; Suljic, J.; Kittl, R.; Pham, N.H.; Kosma, P.; Haltrich, D.; Nguyen, T.-H. Two β-Galactosidases from the Human Isolate Bifidobacterium breve DSM 20213: Molecular Cloning and Expression, Biochemical Characterization and Synthesis of Galacto-Oligosaccharides. PLoS ONE 2014, 9, e104056. [Google Scholar] [CrossRef]
- Carbohydrate Active Enzymes Database (CAZy). Available online: http://www.cazy.org (accessed on 10 October 2022).
- Andersen, J.M.; Barrangou, R.; Hachem, M.A.; Lahtinen, S.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional and functional analysis of galactooligosaccharide uptake by lacS in Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2011, 108, 17785–17790. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Barrangou, R.; Hachem, M.A.; Lahtinen, S.J.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional analysis of prebiotic uptake and catabolism by Lactobacillus acidophilus NCFM. PLoS ONE 2012, 7, e44409. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, H.A.; Arreola, S.L.; Mlynek, G.; Djinović-Carugo, K.; Mathiesen, G.; Nguyen, T.H.; Haltrich, D. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: Expression in Lactobacillus plantarum and biochemical characterization. J. Agric. Food Chem. 2012, 7, 1713–1721. [Google Scholar] [CrossRef]
- Lu, L.; Xu, S.; Jin, L.; Zhang, D.; Li, Y.; Xiao, M. Synthesis of galactosyl sucralose by β-galactosidase from Lactobacillus bulgaricus L3. Food Chem. 2012, 134, 269–275. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Huang, C.Y.; Yu, Q.; Liu, H.C.; Zhang, C.W.; Pei, X.F.; Xu, X.; Wang, G.Q. Analysis of β-galactosidase production and their genes of two strains of Lactobacillus bulgaricus. Biotechnol Lett. 2012, 34, 1067–1071. [Google Scholar] [CrossRef]
- Weber, M.; Schneider, D. Six amino acids define a minimal dimerization sequence and stabilize a transmembrane helix dimer by close packing and hydrogen bonding. FEBS Lett. 2013, 587, 1592–1596. [Google Scholar] [CrossRef]
- Matthews, B.W. The structure of E. coli β-galactosidase. Comptes Rendus Biol. 2005, 328, 549–556. [Google Scholar] [CrossRef]
- Bultema, J.B.; Kuipers, B.L.H.; Dijkhuizen, L. Biochemical characterization of mutants in the active site residues of the β-galactosidase enzyme of Bacillus circulans ATCC 31382. FEBS Open Bio 2014, 4, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colinas, B.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Galacto-oligosaccharide Synthesis from Lactose Solution or Skim Milk Using the β-Galactosidase from Bacillus circulans. J. Agric. Food Chem. 2012, 60, 6391–6398. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Oey, I.; Agyei, D. Purification, characterization and thermal inactivation kinetics of β-galactosidase from Lactobacillus leichmannii 313. LWT 2019, 116, 108545. [Google Scholar] [CrossRef]
- Fara, A.; Sabater, C.; Palacios, J.; Requena, T.; Montilla, A.; Zárate, G. Prebiotic galactooligosaccharides production from lactose and lactulose by Lactobacillus delbrueckii subsp. bulgaricus CRL450. Food Funct. 2020, 11, 5875–5886. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Splechtna, B.; Krasteva, S.; Kneifel, W.; Kulbe, K.D.; Divne, C.; Haltrich, D. Characterization and molecular cloning of a heterodimeric beta-galactosidase from the probiotic strain Lactobacillus acidophilus R22. FEMS Microbiol. Lett. 2007, 269, 136–144. [Google Scholar] [CrossRef]
- Liu, G.X.; Kong, J.; Lu, W.W.; Kong, W.T.; Tian, H.; Tian, X.Y.; Huo, G.C. β-Galactosidase with transgalactosylation activity from Lactobacillus fermentum K4. J. Dairy Sci. 2011, 94, 5811–5820. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Pham, M.-L.; Tran, A.-M.; Nguyen, T.-H. β-Galactosidase from Lactobacillus helveticus DSM 20075: Biochemical Characterization and Recombinant Expression for Applications in Dairy Industry. Int. J. Mol. Sci. 2019, 20, 947. [Google Scholar] [CrossRef]
- Du, M.; Yang, S.; Jiang, T.; Liang, T.; Li, Y.; Cai, S.; Wu, Q.; Zhang, J.; Chen, W.; Xie, X. Cloning, Expression, Purification, and Characterization of β-Galactosidase from Bifidobacterium longum and Bifidobacterium pseudocatenulatum. Molecules 2022, 27, 4497. [Google Scholar] [CrossRef]
- Daabrowski, S.; Sobiewska, G.; Maciuńska, J.; Synowiecki, J.; Kur, J. Cloning, expression, and purification of the His6-tagged thermostable beta-galactosidase from Pyrococcus woesei in Escherichia coli and some properties of the isolated enzyme. Protein Expr. Purif. 2000, 19, 107–112. [Google Scholar] [CrossRef]
- Urrutia, P.; Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Wilson, L.; Illanes, A.; Plou, F.J. Detailed analysis of galactooligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. J. Agric. Food Chem. 2013, 61, 1081–1087. [Google Scholar] [CrossRef]





| Species, Strain | Type of Enzyme 1 | Lactose (g/L) | GOS (g/L) | GOS (%) 2 | SEA 3 (U/mg) | Metal Ions 4 | Ref. |
|---|---|---|---|---|---|---|---|
| L. bulgaricus 43 | Crude | 200 | 70.91 (DP3) | 34 (DP3) | 2011 (O) | Mn2+, Mg2+, Ca2+ Zn2+, Cu2+ | This study |
| L. bulgaricus CRL450 | Cell-free extract | 300 | n/a | 41.3 | 2.06 (O) | n/a | [36] |
| L. bulgaricus DSM 20081 | Purified, non-His-tag | 205 | 102 | 50 | 317 (O) 123 (L) | Na+, K+ Mg2+, Ca2+ | [27] |
| L. bulgaricus wch9901 | Crude | n/a | n/a | n/a | 6.2 (O) | n/a | [35] |
| L. acidophilus R22 | Purified natural | 205 | n/a | 38.5 | 361 (O) 28.8 (L) | Mg2+ Mn2+, Cu2+, Zn2+ | [37] |
| Lim. fermentum K4 | Purified | 200-400 | n/a | 37 | 184 (O) 41 (L) | Na+, K+, Mg2+ | [38] |
| L. helveticus DSM 20075 | Purified | 205 | n/a | n/a | 476 (O) 11.1 (L) | K+, Na+ Mn2+, Mg2+, Ca2+, Zn2+ | [39] |
| L. leichmannii 313 | Purified | n/a | n/a | n/a | 31.28 (O) | Na+ Ca2+, Mn2+ | [35] |
| Bifidobacterium breve DSM 20213 | Purified (2 enzymes) | 200 | n/a | 33-44 | 489 (O) 59 (L) | n/a | [23] |
| Bif. longum Bif. pseudocatenulatum | Purified (2 enzymes) | n/a | n/a | n/a | 2200 (O) 0.58 (O) | Zn2+, Na+, Ca2+, Mn2+ Al3+ | [40] |
| B. circulans | commercial | 400 | 198 | 41 | n/a | n/a | [34] |
| Pyrococcus woesei | Purified | n/a | n/a | n/a | 5400 (O) | n/a | [41] |
| Aspergillus oryzae | commercial | 400 | 107 | 26.8 | n/a | n/a | [42] |
| Primer | Sequence (5′-3′) 1 | Positions in β-gal 2 | Purpose |
|---|---|---|---|
| LacZ_F | ATGAGCAATAAGTTAGTAAAAGAAAAAAG | 1–29 | Sequencing |
| LacZ_R | TTATTTTAGTAAAAGGGGCTGAATCAC | 3000–3027 | Sequencing |
| LacZ_FF | GTGAAGGTGACTTGGTTGCTGAAAA | 803–828 | Sequencing |
| LacZ_RR | CCAGAAGGTAAATTCCGGCAGCCGCTTC | 2285–2313 | Sequencing |
| LacZ_Nde | CAGTCCATATGATGAGCAATAAGTTAGTAAAAGAAAAAAG | 1–29 | Cloning |
| LacZ_Xho | CTAGTCTCGAGTTTTAGTAAAAGGGGCTGAATCAC | 3000–3024 | Cloning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K.; Petrova, P. In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus. Int. J. Mol. Sci. 2022, 23, 14308. https://doi.org/10.3390/ijms232214308
Arsov A, Ivanov I, Tsigoriyna L, Petrov K, Petrova P. In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus. International Journal of Molecular Sciences. 2022; 23(22):14308. https://doi.org/10.3390/ijms232214308
Chicago/Turabian StyleArsov, Alexander, Ivan Ivanov, Lidia Tsigoriyna, Kaloyan Petrov, and Penka Petrova. 2022. "In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus" International Journal of Molecular Sciences 23, no. 22: 14308. https://doi.org/10.3390/ijms232214308
APA StyleArsov, A., Ivanov, I., Tsigoriyna, L., Petrov, K., & Petrova, P. (2022). In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus. International Journal of Molecular Sciences, 23(22), 14308. https://doi.org/10.3390/ijms232214308









