Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Analysis of the Synthesized Polymers
2.2. Preparation of Usnic Acid-Loaded Nanoparticles
2.3. Kinetics of Usnic Acid Release from Nanoparticles
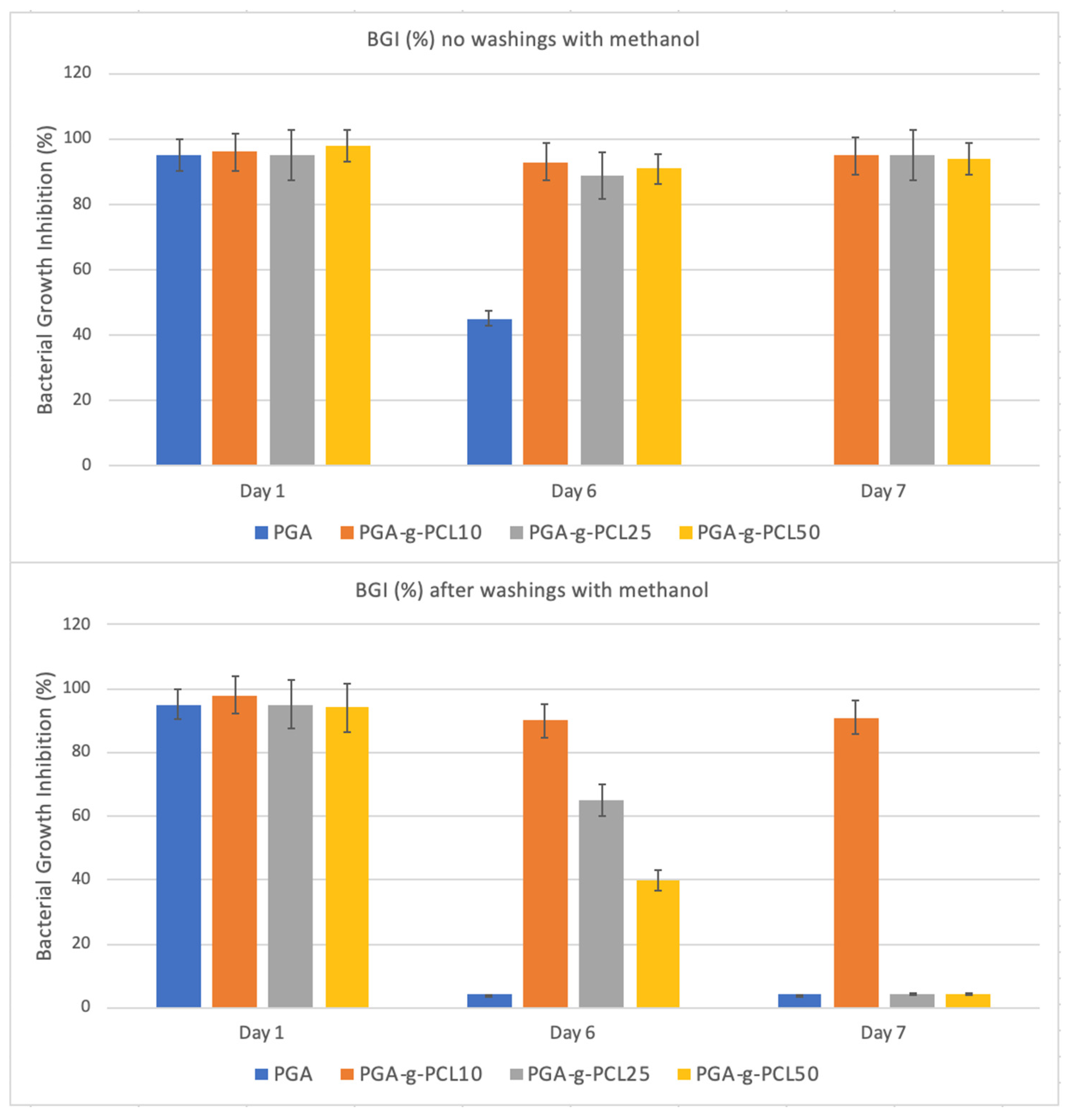
2.4. Antimicrobial Activity of UA-Loaded Nanoparticles
3. Materials and Methods
3.1. Materials
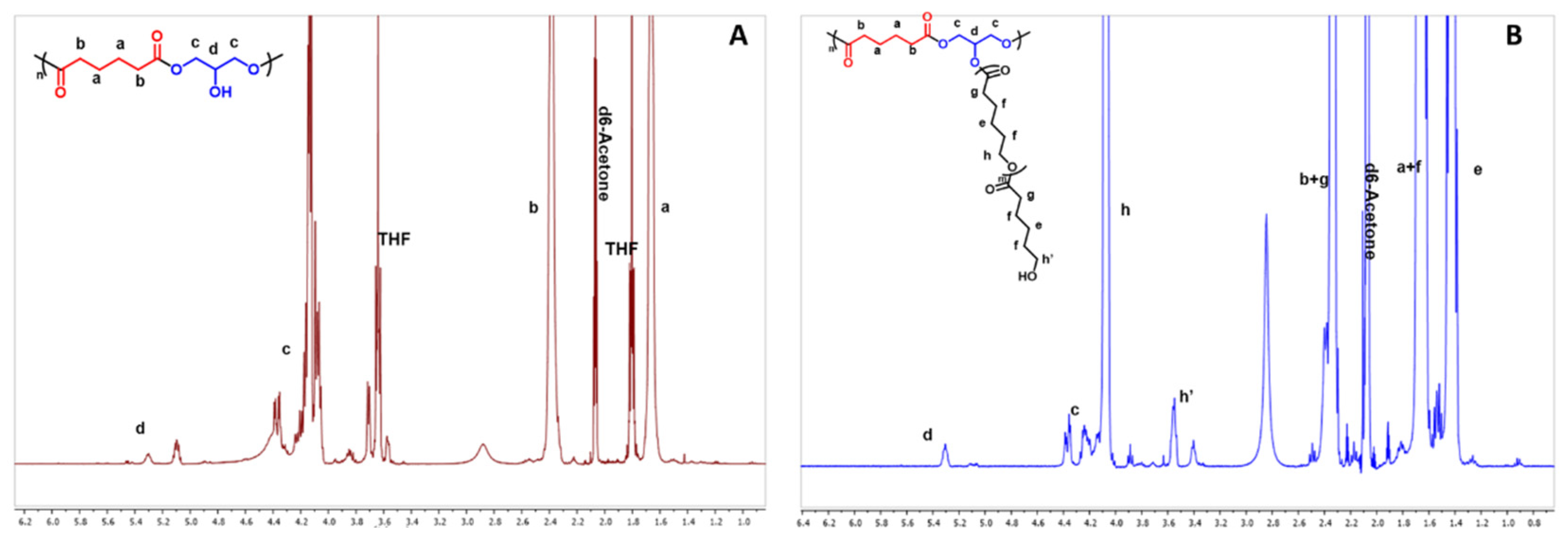
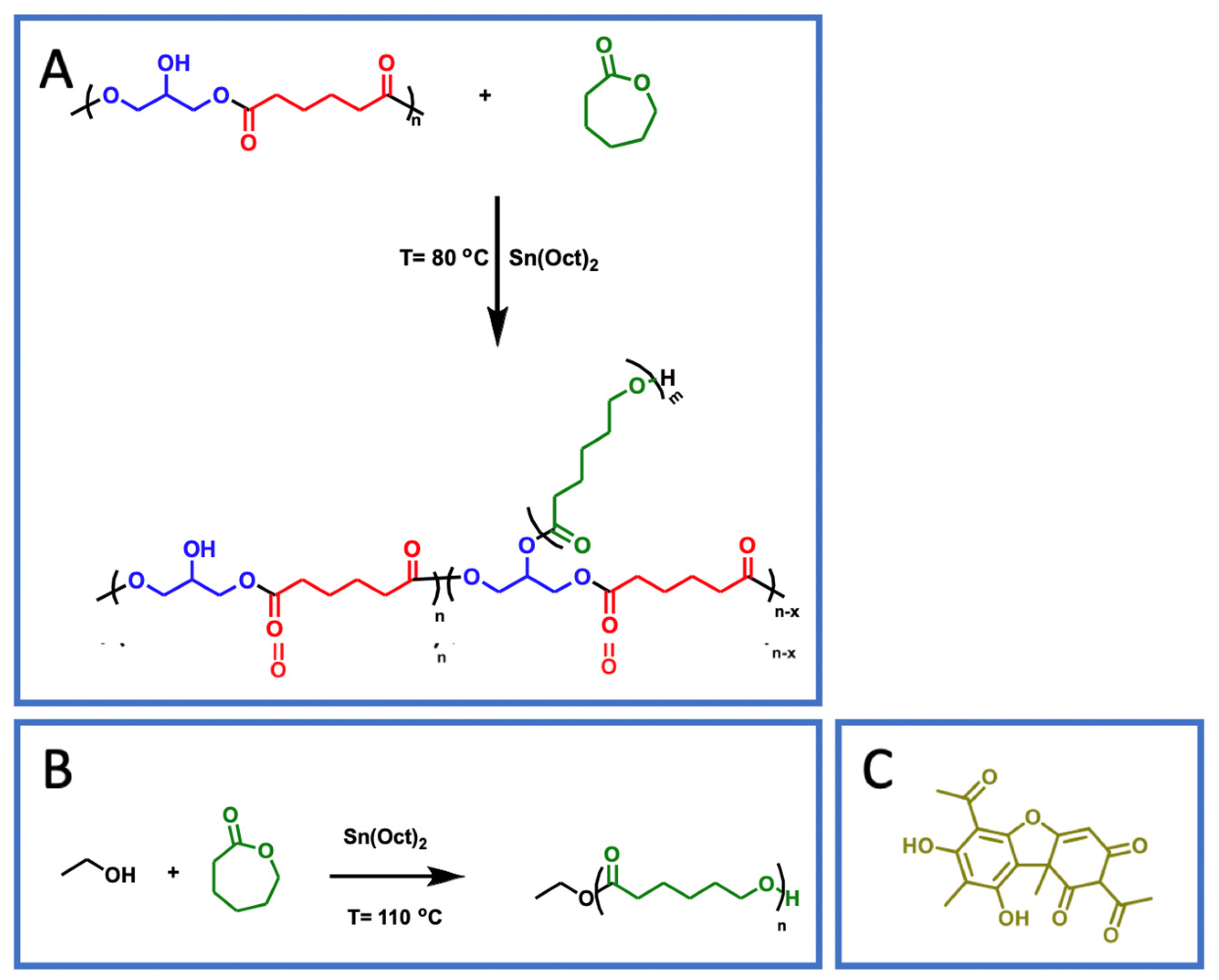
3.2. Polymer Synthesis
3.3. Polymer Characterization
3.4. Preparation and Characterization of Polymer Nanoparticles
3.5. Drug Release
3.6. Antimicrobial Activity
3.7. Statistics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francolini, I.; Taresco, V.; Crisante, F.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Water soluble usnic acid-polyacrylamide complexes with enhanced antimicrobial activity against Staphylococcus epidermidis. Int. J. Mol. Sci. 2013, 14, 7356–7369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taresco, V.; Gontrani, L.; Crisante, F.; Francolini, I.; Martinelli, A.; D’Ilario, L.; Bordi, F.; Piozzi, A. Self-Assembly of catecholic moiety-containing cationic random acrylic copolymers. J. Phys. Chem. B 2015, 119, 8369–8379. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Belić, D.; Del Giudice, A.; Alfredsson, V.; Carnerup, A.M.; Zhu, K.; Nyström, B.; Wang, Y.; Galantini, L.; Schillén, K. Condensed supramolecular helices: The twisted sisters of DNA. Angew. Chem. 2022, 134, e202113279. [Google Scholar] [CrossRef]
- Di Bonito, P.; Petrone, L.; Casini, G.; Francolini, I.; Ammendolia, M.G.; Accardi, L.; Piozzi, A.; D’Ilario, L.; Martinelli, A. Amino-functionalized poly(L-lactide) lamellar single crystals as a valuable substrate for delivery of HPV16-E7 tumor antigen in vaccine development. Int. J. Nanomed. 2015, 10, 3447–3458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively biodegradable polyesters: Nature-inspired construction materials for future biomedical applications. Polymers 2019, 11, 1061. [Google Scholar] [CrossRef] [Green Version]
- Simonutti, R.; Bertani, D.; Marotta, R.; Ferrario, S.; Manzone, D.; Mauri, M.; Gregori, M.; Orlando, A.; Masserini, M. Morphogenic effect of common solvent in the self-assembly behavior of amphiphilic PEO-b-PLA. Polymer 2021, 218, 123511. [Google Scholar] [CrossRef]
- Shehata, S.; Serpell, C.J.; Biagini, S.C.G. Architecture-controlled release of ibuprofen from polymeric nanoparticles. Mater. Today Commun. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Jeong, J.-C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly(ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef]
- Vollrath, A.; Kretzer, C.; Beringer-Siemers, B.; Shkodra, B.; Czaplewska, J.A.; Bandelli, D.; Stumpf, S.; Hoeppener, S.; Weber, C.; Werz, O.; et al. Effect of crystallinity on the properties of polycaprolactone nanoparticles containing the dual FLAP/mPEGS-1 Inhibitor BRP-187. Polymers 2021, 13, 2557. [Google Scholar] [CrossRef]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond poly(ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef]
- Swainson, S.M.E.; Styliari, I.D.; Taresco, V.; Garnett, M.C. Poly (glycerol adipate) (PGA), an enzymatically synthesized functionalizable polyester and versatile drug delivery carrier: A literature update. Polymers 2019, 11, 1561. [Google Scholar] [CrossRef] [Green Version]
- Jacob, P.L.; Ruiz Cantu, L.A.; Pearce, A.K.; He, Y.; Lentz, J.C.; Moore, J.C.; Machado, F.; Rivers, G.; Apebende, E.; Fernandez, M.R.; et al. Poly (glycerol adipate) (PGA) backbone modifications with a library of functional diols: Chemical and physical effects. Polymer 2021, 228, 123912. [Google Scholar] [CrossRef]
- Animasawun, R.K.; Taresco, V.; Swainson, S.M.E.; Suksiriworapong, J.; Walker, D.A.; Garnett, M.C. Screening and matching polymers with drugs to improve drug incorporation and retention in nanoparticles. Mol. Pharm. 2020, 17, 2083–2098. [Google Scholar] [CrossRef]
- Weiss, V.M.; Lucas, H.; Mueller, T.; Chytil, P.; Etrych, T.; Naolou, T.; Kressler, J.; Mader, K. Intended and unintended targeting of polymeric nanocarriers: The case of modified poly(glycerol adipate) nanoparticles. Macromol. Biosci. 2018, 18, 1700240. [Google Scholar] [CrossRef] [Green Version]
- Kline, B.J.; Beckman, E.J.; Russell, A.J. One-step biocatalytic synthesis of linear polyesters with pendant hydroxyl groups. J. Am. Chem. Soc. 1998, 120, 9475–9480. [Google Scholar] [CrossRef]
- Swainson, S.M.E.; Taresco, V.; Pearce, A.K.; Clapp, L.H.; Ager, B.; McAllister, M.; Bosquillon, C.; Garnett, M.C. Exploring the enzymatic degradation of poly(glycerol adipate). Eur. J. Pharm. Biopharm. 2019, 142, 377–386. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K. Molecules of interest. Usnic acid. Phytochemistry 2002, 140, 729–736. [Google Scholar] [CrossRef]
- Francolini, I.; Piozzi, A.; Donelli, G. Usnic Acid: Potential role in management of wound infections. Adv. Exp. Med. Biol. 2019, 1214, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lauterwein, M.; Oethinger, M.; Belsner, K.; Peters, T.; Marre, R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 1995, 39, 2541–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial resistance to antibiotics and effective antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Rauschenbach, M.; Lawrenson, S.B.; Taresco, V.; Pearce, A.K.; O’Reilly, R.K. Antimicrobial hyperbranched polymer–usnic acid complexes through a combined ROP-RAFT strategy. Macromol. Rapid Commun. 2020, 41, 2000190. [Google Scholar] [CrossRef]
- Da Silva Santos, N.P.; Nascimento, S.C.; Wanderley, M.S.; Pontes-Filho, N.T.; da Silva, J.F.; de Castro, C.M.; Pereira, E.C.; da Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. Eur. J. Pharm. Biopharm. 2006, 64, 154–160. [Google Scholar] [CrossRef]
- Lira, M.C.; Siqueira-Moura, M.P.; Rolim-Santos, H.M.; Galetti, F.C.; Simioni, A.R.; Santos, N.P.; Tabosa Do Egito, E.S.; Silva, C.L.; Tedesco, A.C.; Santos-Magalhães, N.S. In vitro uptake and antimycobacterial activity of liposomal usnic acid formulation. J. Liposome Res. 2009, 19, 49–58. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cotar, A.I.; An-dronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@ usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1766–1776. [Google Scholar] [CrossRef]
- Battista, S.; Köber, M.; Bellio, P.; Celenza, G.; Galantini, L.; Vargas-Nadal, G.; Fagnani, L.; Veciana, J.; Ventosa, N.; Giansanti, L. Quatsomes formulated with l-prolinol-derived surfactants as antibacterial nanocarriers of (+)-usnic acid with antioxidant activity. ACS Appl. Nano Mater. 2022, 5, 6140–6148. [Google Scholar] [CrossRef]
- Martinelli, A.; Bakry, A.; D’Ilario, L.; Francolini, I.; Piozzi, A.; Taresco, V. Release behavior and antibiofilm activity of usnic acid-loaded carboxylated poly(L-lactide) microparticles. Eur. J. Pharm. Biopharm. 2014, 88, 415–423. [Google Scholar] [CrossRef]
- Taresco, V.; Francolini, I.; Padella, F.; Bellusci, M.; Boni, A.; Innocenti, C.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Design and characterization of antimicrobial usnic acid loaded-core/shell magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 72–81. [Google Scholar] [CrossRef]
- Francolini, I.; Giansanti, L.; Piozzi, A.; Altieri, B.; Mauceri, A.; Mancini, G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf. B Biointerfaces 2019, 181, 632–638. [Google Scholar] [CrossRef]
- Taresco, V.; Creasey, R.G.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Naolou, T.; Meister, A.; Schops, R.; Pietzsch, M.; Kressler, J. Synthesis and characterization of graft copolymers able to form polymersomes and worm-like aggregates. Soft Matter 2013, 9, 10364–10372. [Google Scholar] [CrossRef]
- Alaneed, R.; Golitsyn, Y.; Hauenschild, T.; Pietzsch, M.; Reichert, D.; Kressler, J. Network formation by aza-michael addition of primary amines to vinyl end groups of enzymatically synthesized poly(glycerol adipate). Polym. Int. 2021, 70, 135–144. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St Pourçain, C.B.; Garnett, M.C. Novel functionalized biodegradable polymers for nanoparticle drug delivery systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of acyl modified poly(glycerol-adipate) comb-like polymers and their self-assembly into nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.M.; Evans, A.R.; Iftikhar, A.; Mohammed, A.R.; Shabir, A.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Dry powder inhalation of macromolecules using novel PEG-co-polyester microparticle carriers. Int. J. Pharm. 2013, 441, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Damrongrak, K.; Kloysawat, K.; Bunsupa, S.; Sakchasri, K.; Wongrakpanich, A.; Taresco, V.; Cuzzucoli Crucitti, V.; Garnett, M.C.; Suksiriworapong, J. Delivery of acetogenin-enriched Annona muricata Linn leaf extract by folic acid-conjugated and triphenylphosphonium-conjugated poly(glycerol adipate) nanoparticles to enhance toxicity against ovarian cancer cells. Int. J. Pharm. 2022, 618, 121636. [Google Scholar] [CrossRef]
- Gordhan, D.; Swainson, S.M.E.; Pearce, A.K.; Styliari, I.D.; Lovato, T.; Burley, J.C.; Garnett, M.C.; Taresco, V. Poly (Glycerol Adipate): From a functionalized nanocarrier to a polymeric-prodrug matrix to create amorphous solid dispersions. J. Pharm. Sci. 2020, 109, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkon, D.; Pulst, M.; Naolou, T.; Busse, K.; Balko, J.; Kressler, J. Crystallization and melting of Poly(glycerol adipate)-based graft copolymers with single and double crystallizable side chains. J. Polym. Sci. 2013, 51, 1581–1591. [Google Scholar] [CrossRef]
- Naolou, T.; Busse, K.; Lechner, B.D.; Kressler, J. The behavior of poly(ε-caprolactone) and poly(ethylene oxide)-b-poly(ε-caprolactone) grafted to a poly(glycerol adipate) backbone at the air/water interface. Colloid Polym. Sci. 2014, 292, 1199–1208. [Google Scholar] [CrossRef]
- Wahab, A.; Favretto, M.E.; Onyeagor, N.D.; Khan, G.M.; Douroumis, D.; Casely-Hayford, M.A.; Kallinteri, P. Development of poly(glycerol adipate) nanoparticles loaded with non-steroidal anti-inflammatory drugs. J. Microencapsul. 2012, 29, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kitagawa, R.; Tsuboi, R.; Hirose, N.; Thongthai, P.; Sakai, H.; Ueda, M.; Ono, S.; Sasaki, J.I.; Ooya, T.; et al. Development of endodontic sealers containing antimicrobial-loaded polymer particles with long-term antibacterial effects. Dent. Mater. 2021, 37, 1248–1259. [Google Scholar] [CrossRef]
- Donelli, G.; Francolini, I.; Ruggeri, V.; Guaglianone, E.; D’Ilario, L.; Piozzi, A. Pore formers promoted release of an antifungal drug from functionalized polyurethanes to inhibit Candida colonization. J. Appl. Microbiol. 2006, 100, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A. Analysis of Fickian and non-fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Tavares, J.K.; Ulson de Souza, A.A.; José Vladimir de Oliveira, J.; Priamo, W.L.; Guelli Ulson de Souza, S.M.A. Modeling of the controlled release of betacarotene into anhydrous ethanol from microcapsules. OpenNano 2016, 1, 25–35. [Google Scholar] [CrossRef]




| Polymer | Tm (°C) | ΔH (J/g) |
|---|---|---|
| PCL10 | 62 | 98 |
| PCL25 | 65 | 100 |
| PCL50 | 66 | 90 |
| PGA-g-PCL10 | 54 | 66 |
| PGA-g-PCL25 | 61 | 79 |
| PGA-g-PCL50 | 62 | 88 |
| Polymer | Size (nm) without UA | Size (nm) Loaded with UA |
|---|---|---|
| PGA | 180 ± 20 | 110 ± 10 |
| PGA-g-PCL10 | 90 ± 4 | 108 ± 9 |
| PGA-g-PCL25 | 95 ± 3 | 102 ± 8 |
| PGA-g-PCL50 | 89 ± 4 | 109 ± 8 |
| Polymer | n | R |
|---|---|---|
| PGA | 0.37 ± 0.06 | 0.97 |
| PGA-g-PCL10 | 0.38 ± 0.04 | 0.97 |
| PGA-g-PCL25 | 0.68 ± 0.04 | 0.99 |
| PGA-g-PCL50 | 0.75 ± 0.03 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taresco, V.; Tulini, I.; Francolini, I.; Piozzi, A. Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid. Int. J. Mol. Sci. 2022, 23, 14339. https://doi.org/10.3390/ijms232214339
Taresco V, Tulini I, Francolini I, Piozzi A. Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid. International Journal of Molecular Sciences. 2022; 23(22):14339. https://doi.org/10.3390/ijms232214339
Chicago/Turabian StyleTaresco, Vincenzo, Isotta Tulini, Iolanda Francolini, and Antonella Piozzi. 2022. "Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid" International Journal of Molecular Sciences 23, no. 22: 14339. https://doi.org/10.3390/ijms232214339
APA StyleTaresco, V., Tulini, I., Francolini, I., & Piozzi, A. (2022). Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid. International Journal of Molecular Sciences, 23(22), 14339. https://doi.org/10.3390/ijms232214339







