The Role of Type VI Collagen in Alveolar Bone
Abstract
:1. Introduction
2. Results
2.1. Type VI Collagen Is Expressed in Oral Tissues
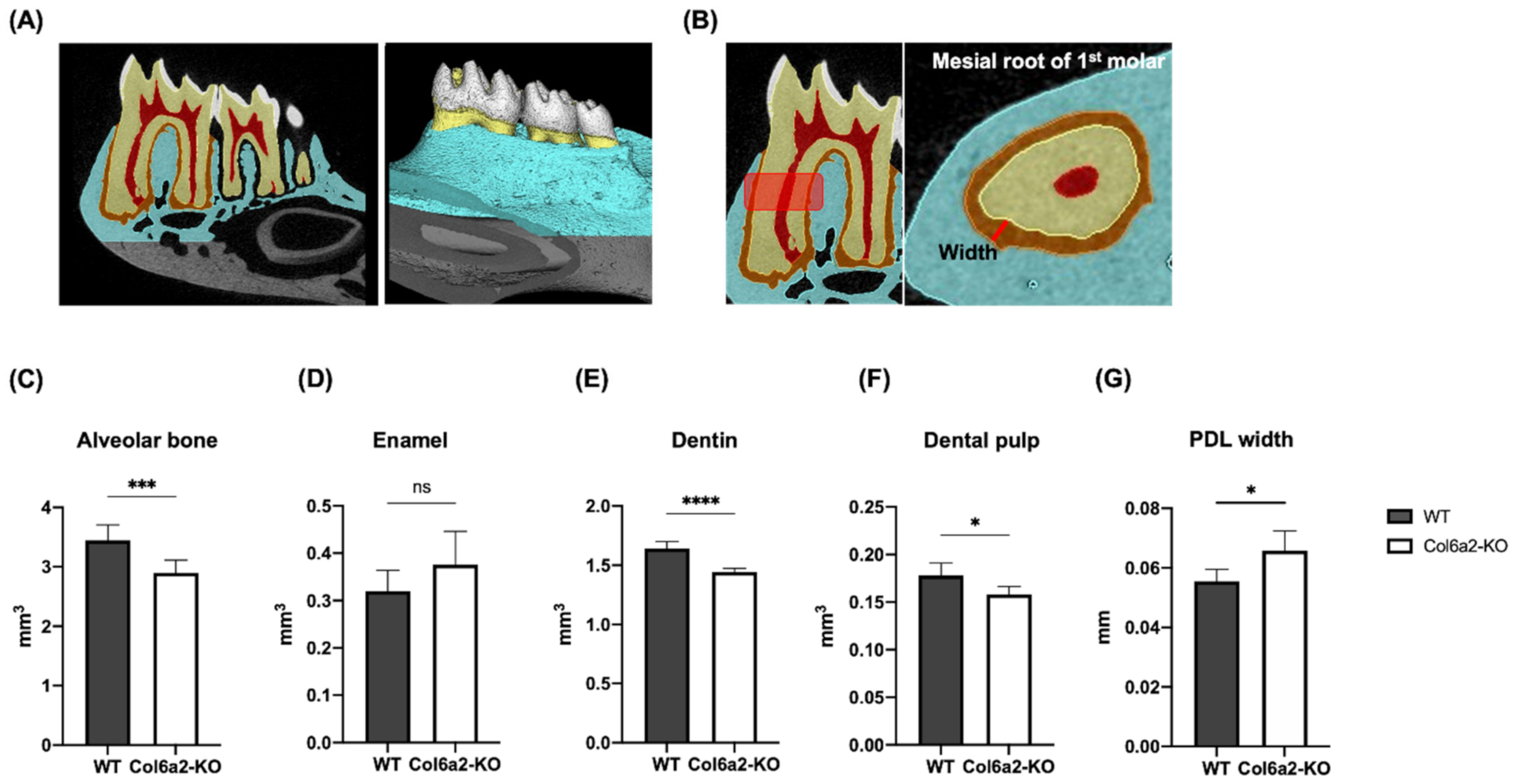
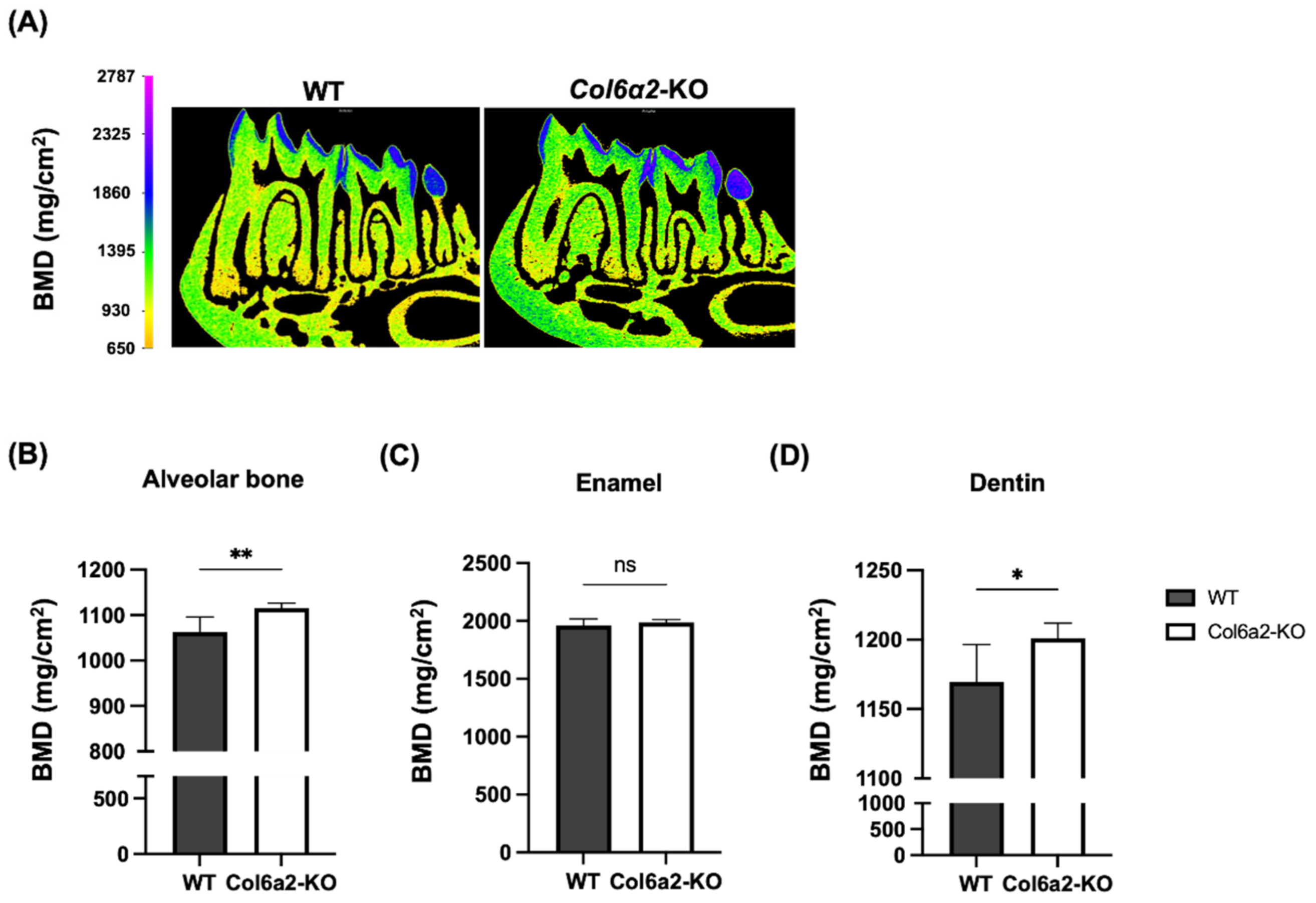
2.2. Col6α2-KO Mice Have Compromised Oral Tissues
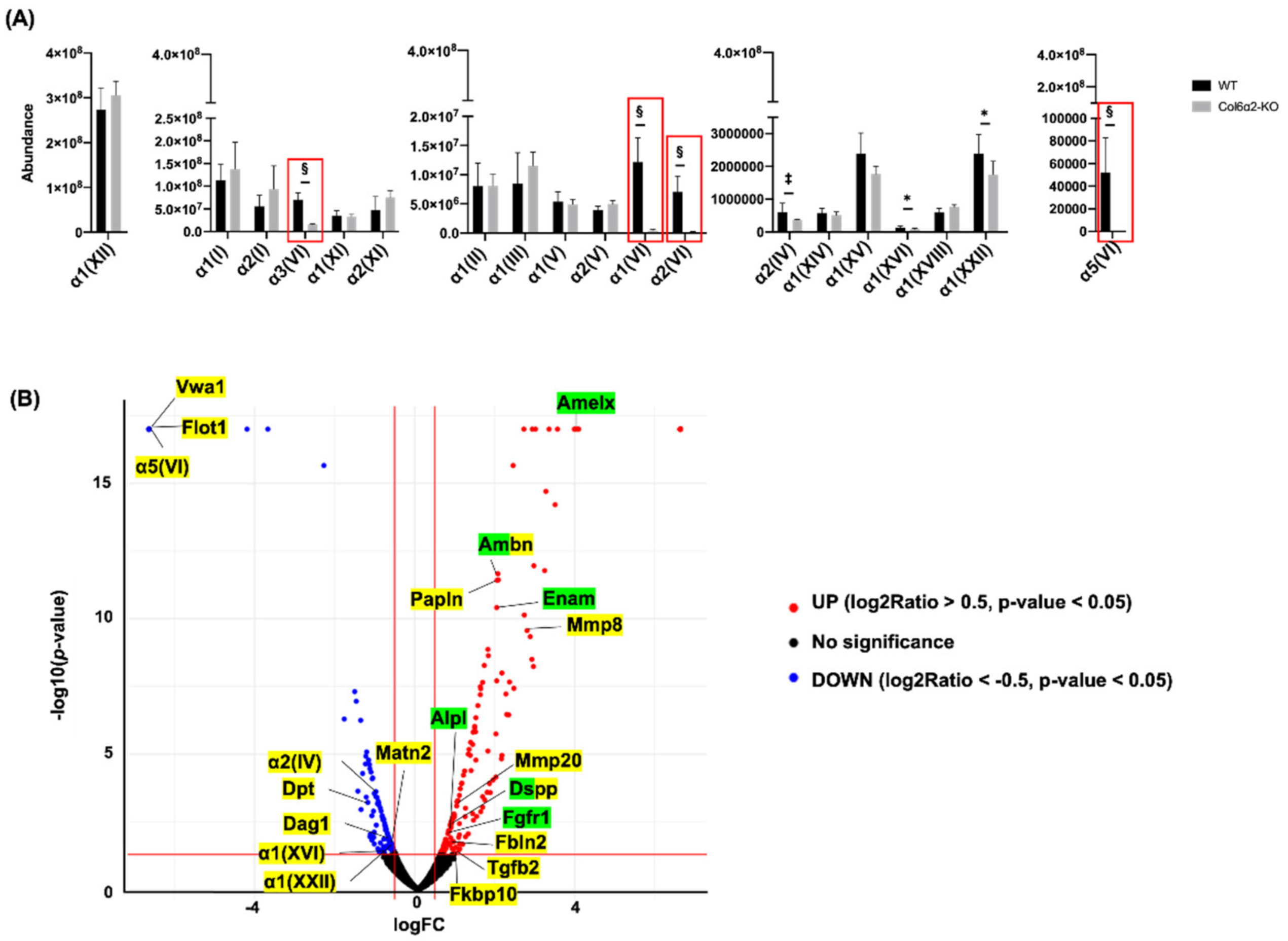
2.3. Type VI Collagen Affects ECM Molecules in the Alveolar Bone
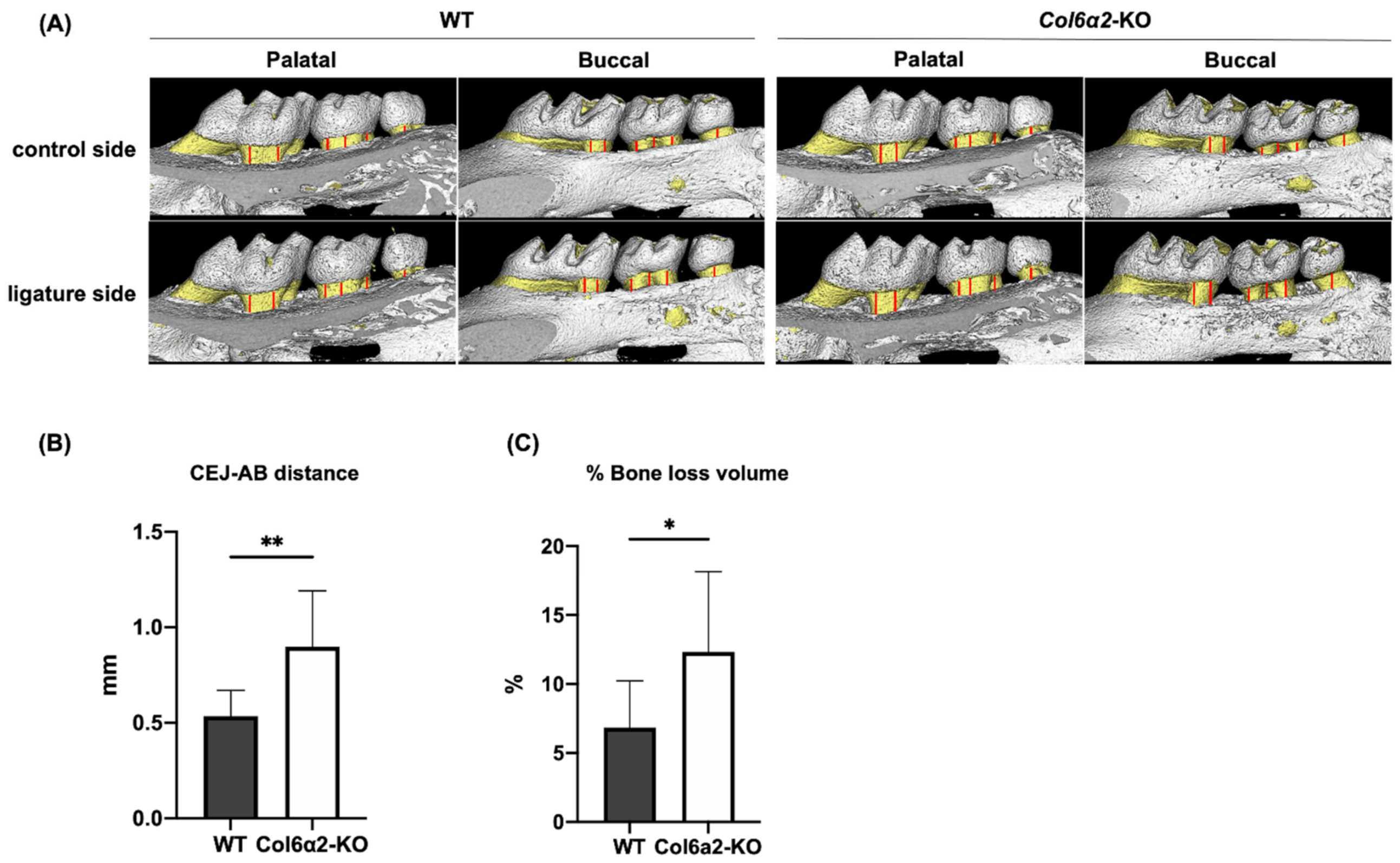
2.4. Col6α2-KO Mice Have More Bone Resorption in an Induced Model of Periodontitis Compared with WT
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Micro-Computed Tomography (µCT)
4.3. Immunohistochemistry
4.4. Protein Extraction
4.5. Proteomics
4.6. Ligature-Induced Periodontitis Model
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keene, D.R.; Engvall, E.; Glanville, R.W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J. Cell Biol. 1988, 107, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Bönnemann, C.G. The collagen VI-related myopathies: Muscle meets its matrix. Nat. Rev. Neurol. 2011, 7, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.J.; Eyre, D.R.; Slayter, H.S. Type VI collagen of the intervertebral disc. Biochemical and electron-microscopic characterization of the native protein. Biochem. J. 1987, 248, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.-L.; Mann, K.; Deutzmann, R.; Pribula-Conway, D.; Hsu-Chen, C.-C.; Bernard, M.P.; Timpl, R. Characterization of three constituent chains of collagen type VI by peptide sequences and cDNA clones. Eur. J. Biochem. 1987, 168, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Rich, C.; Zhou, F.H.; Hansen, U. Three Novel Collagen VI Chains, α4(VI), α5(VI), and α6(VI). J. Biol. Chem. 2008, 283, 20170–20180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampe, A.K. Collagen VI related muscle disorders. J. Med. Genet. 2005, 42, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamande, S.R.; Bateman, J.F. Collagen VI disorders: Insights on form and function in the extracellular matrix and beyond. Matrix Biol. 2018, 71–72, 348–367. [Google Scholar] [CrossRef]
- Bonaldo, P.; Russo, V.; Bucciotti, F.; Doliana, R.; Colombatti, A. Structural and functional features of the .alpha.3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry 1990, 29, 1245–1254. [Google Scholar] [CrossRef]
- Wiberg, C.; Klatt, A.R.; Wagener, R.; Paulsson, M.; Bateman, J.F.; Heinegard, D.; Morgelin, M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 2003, 278, 37698–37704. [Google Scholar] [CrossRef]
- Kuo, H.-J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI Collagen Anchors Endothelial Basement Membranes by Interacting with Type IV Collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillet, E.; Wiedemann, H.; Golbik, R.; Pan, T.-C.; Zhang, R.-Z.; Mann, K.; Chu, M.-L.; Timpl, R. Recombinant expression and structural and binding properties of alpha1(VI) and alpha2(VI) chains of human collagen type VI. Eur. J. Biochem. 1994, 221, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bidanset, D.J.; Guidry, C.; Rosenberg, L.C.; Choi, H.U.; Timpl, R.; Hook, M. Binding of the proteoglycan decorin to collagen type VI. J. Biol. Chem. 1992, 267, 5250–5256. [Google Scholar] [CrossRef]
- Wiberg, C.; Hedbom, E.; Khairullina, A.; Lamande, S.R.; Oldberg, A.; Timpl, R.; Morgelin, M.; Heinegard, D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J. Biol. Chem. 2001, 276, 18947–18952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatelli, P.; Bonaldo, P.; Lattanzi, G.; Braghetta, P.; Bergamin, N.; Capanni, C.; Mattioli, E.; Columbaro, M.; Ognibene, A.; Pepe, G.; et al. Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 2001, 20, 475–486. [Google Scholar] [CrossRef]
- Loeser, R.F.; Sadiev, S.; Tan, L.; Goldring, M.B. Integrin expression by primary and immortalized human chondrocytes: Evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthr. Cartil. 2000, 8, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Stallcup, W.B.; Dahlin, K.; Healy, P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J. Cell Biol. 1990, 111, 3177–3188. [Google Scholar] [CrossRef] [Green Version]
- Izu, Y.; Ansorge, H.L.; Zhang, G.; Soslowsky, L.J.; Bonaldo, P.; Chu, M.-L.; Birk, D.E. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 2011, 30, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Leiphart, R.J.; Pham, H.; Harvey, T.; Komori, T.; Kilts, T.M.; Shetye, S.S.; Weiss, S.N.; Adams, S.M.; Birk, D.E.; Soslowsky, L.J.; et al. Coordinate roles for collagen VI and biglycan in regulating tendon collagen fibril structure and function. Matrix Biol. Plus 2022, 13, 100099. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef]
- Takenaka-Ninagawa, N.; Kim, J.; Zhao, M.; Sato, M.; Jonouchi, T.; Goto, M.; Yoshioka, C.K.B.; Ikeda, R.; Harada, A.; Sato, T.; et al. Collagen-VI supplementation by cell transplantation improves muscle regeneration in Ullrich congenital muscular dystrophy model mice. Stem Cell Res. Ther. 2021, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose Tissue Collagen VI in Obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Cescon, M.; Zuccolotto, G.; Nobbio, L.; Colombelli, C.; Filaferro, M.; Vitale, G.; Feltri, M.L.; Bonaldo, P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015, 129, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Kram, V.; Dar, Q.-A.; Komori, T.; Ji, Y.; Mohassel, P.; Rooney, J.; Li, L.; Kilts, T.M.; Bonnemann, C.; et al. Collagen VIα2 chain deficiency causes trabecular bone loss by potentially promoting osteoclast differentiation through enhanced TNFα signaling. Sci. Rep. 2020, 10, 13749. [Google Scholar] [CrossRef]
- Foster, B.L.; Ao, M.; Salmon, C.R.; Chavez, M.B.; Kolli, T.N.; Tran, A.B.; Chu, E.Y.; Kantovitz, K.R.; Yadav, M.; Narisawa, S.; et al. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 2018, 107, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chavez, M.B.; Kolli, T.N.; Tan, M.H.; Fong, H.; Chu, E.Y.; Li, Y.; Ren, X.; Watanabe, K.; Kim, D.G.; et al. Dentoalveolar Defects in the Hyp Mouse Model of X-linked Hypophosphatemia. J. Dent. Res. 2020, 99, 419–428. [Google Scholar] [CrossRef]
- Xu, H.; Lenhart, S.A.; Chu, E.Y.; Chavez, M.B.; Wimer, H.F.; Dimori, M.; Somerman, M.J.; Morello, R.; Foster, B.L.; Hatch, N.E. Dental and craniofacial defects in the Crtap−/− mouse model of osteogenesis imperfecta type VII. Dev. Dyn. 2020, 249, 884–897. [Google Scholar] [CrossRef]
- Goldberg, M.; Marchadier, A.; Vidal, C.; Harichane, Y.; Kamoun-Goldrat, A.; Kellermann, O.; Kilts, T.; Young, M. Differential Effects of Fibromodulin Deficiency on Mouse Mandibular Bones and Teeth: A Micro-CT Time Course Study. Cells Tissues Organs 2011, 194, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Tamburstuen, M.V.; Reseland, J.E.; Spahr, A.; Brookes, S.J.; Kvalheim, G.; Slaby, I.; Snead, M.L.; Lyngstadaas, S.P. Ameloblastin expression and putative autoregulation in mesenchymal cells suggest a role in early bone formation and repair. Bone 2011, 48, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Papagerakis, P.; MacDougall, M.; Hotton, D.; Bailleul-Forestier, I.; Oboeuf, M.; Berdal, A. Expression of amelogenin in odontoblasts. Bone 2003, 32, 228–240. [Google Scholar] [CrossRef]
- Fuchs, H.; Sabrautzki, S.; Seedorf, H.; Rathkolb, B.; Rozman, J.; Hans, W.; Schneider, R.; Klaften, M.; Hölter, S.M.; Becker, L.; et al. Does enamelin have pleiotropic effects on organs other than the teeth? Lessons from a phenotyping screen of two enamelin-mutant mouse lines. Eur. J. Oral Sci. 2012, 120, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Chaweewannakorn, W.; Ariyoshi, W.; Okinaga, T.; Morikawa, K.; Saeki, K.; Maki, K.; Nishihara, T. Ameloblastin and enamelin prevent osteoclast formation by suppressing RANKL expression via MAPK signaling pathway. Biochem. Bio. Res. Commun. 2017, 485, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Hotton, D.; De La Dure-Molla, M.; Petit, S.; Asselin, A.; Kulkarni, A.B.; Gibson, C.W.; Brookes, S.J.; Berdal, A.; Isaac, J. Tracking Endogenous Amelogenin and Ameloblastin In Vivo. PLoS ONE 2014, 9, e99626. [Google Scholar] [CrossRef] [Green Version]
- Gara, S.K.; Grumati, P.; Urciuolo, A.; Bonaldo, P.; Kobbe, B.; Koch, M.; Paulsson, M.; Wagener, R. Three Novel Collagen VI Chains with High Homology to the α3 Chain. J. Biol. Chem. 2008, 283, 10658–10670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Hajishengallis, G.; Divaris, K.; et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 2021, 184, 4090–4104.e15. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Kawamoto, K. Preparation of Thin Frozen Sections from Nonfixed and Undecalcified Hard Tissues Using Kawamot’s Film Method (2012). In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; pp. 149–164. [Google Scholar]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komori, T.; Pham, H.; Jani, P.; Perry, S.; Wang, Y.; Kilts, T.M.; Li, L.; Young, M.F. The Role of Type VI Collagen in Alveolar Bone. Int. J. Mol. Sci. 2022, 23, 14347. https://doi.org/10.3390/ijms232214347
Komori T, Pham H, Jani P, Perry S, Wang Y, Kilts TM, Li L, Young MF. The Role of Type VI Collagen in Alveolar Bone. International Journal of Molecular Sciences. 2022; 23(22):14347. https://doi.org/10.3390/ijms232214347
Chicago/Turabian StyleKomori, Taishi, Hai Pham, Priyam Jani, Sienna Perry, Yan Wang, Tina M. Kilts, Li Li, and Marian F. Young. 2022. "The Role of Type VI Collagen in Alveolar Bone" International Journal of Molecular Sciences 23, no. 22: 14347. https://doi.org/10.3390/ijms232214347
APA StyleKomori, T., Pham, H., Jani, P., Perry, S., Wang, Y., Kilts, T. M., Li, L., & Young, M. F. (2022). The Role of Type VI Collagen in Alveolar Bone. International Journal of Molecular Sciences, 23(22), 14347. https://doi.org/10.3390/ijms232214347






