A Compilation of Study Models for Dental Pulp Regeneration
Abstract
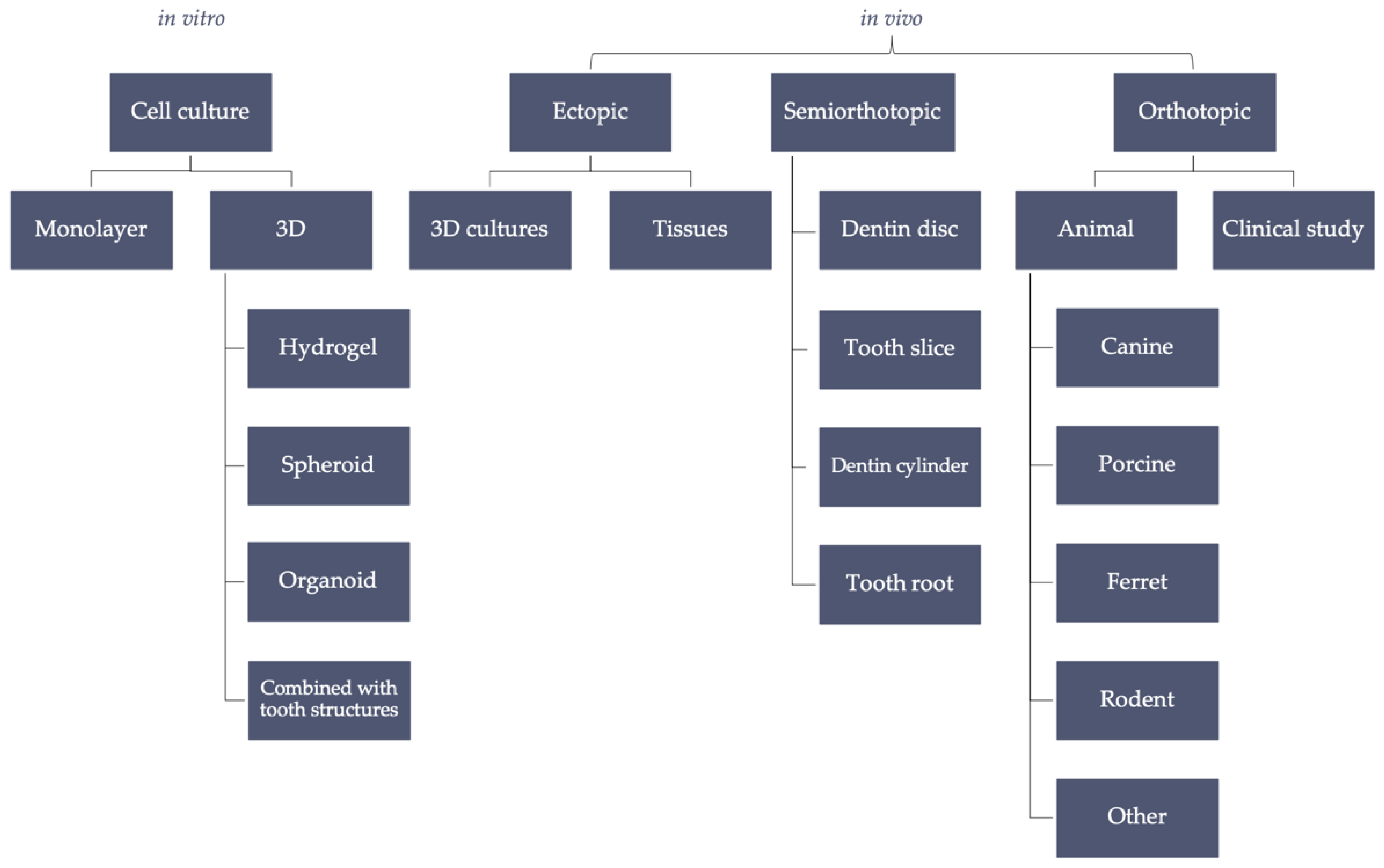
:1. Introduction
2. In Vitro
2.1. Monolayer Cell Culture
2.2. Three-Dimensional (3D) Cell Culture
2.2.1. Hydrogels
2.2.2. Spheroids
2.2.3. Organoids
2.2.4. Bioreactors
2.2.5. Tooth-on-a-Chip Model
3. In Vivo Ectopic and Semiorthotopic Models
3.1. Dentin Disk and Tooth Slice
3.2. Dentin Cylinder and Tooth Root
4. In Vivo Orthotopic Model
4.1. Large Animal Models
4.1.1. Dogs
4.1.2. Pigs
4.2. Small Animal Models
4.2.1. Rodents
4.2.2. Ferrets
4.3. Untypical or Inappropriate Models
4.3.1. Feline Model
4.3.2. Ovine Model
4.3.3. Primate Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caplan, D.J.; Cai, J.; Yin, G.; White, B.A. Root canal filled versus non-root canal filled teeth: A retrospective comparison of survival times. J. Public Health Dent. 2005, 65, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Montoya, C.; Øilo, M.; Ossa, A.; Paranjpe, A.; Zhang, H.; Arola, D.D. Contribution of root canal treatment to the fracture resistance of dentin. J. Endod. 2019, 45, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, C.; Marcé-Nogué, J.; Galler, K.M.; Widbiller, M. Biomechanical performance of an immature maxillary central incisor after revitalization: A finite element analysis. Int. Endod. J. 2019, 52, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with guttapercha: A retrospective clinical study. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef]
- Widbiller, M.; Schmalz, G. Endodontic regeneration: Hard shell, soft core. Odontology 2021, 109, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [Green Version]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-based regenerative endodontics for treatment of periapical lesions: A randomized, controlled phase I/II clinical trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef]
- Widbiller, M.; Knüttel, H.; Meschi, N.; Durán-Sindreu Terol, F. Effectiveness of endodontic tissue engineering in treatment of apical periodontitis: A systematic review. Int. Endod. J. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem. Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Gay, I.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofac. Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef]
- Nakashima, M. Establishment of primary cultures of pulp cells from bovine permanent incisors. Arch. Oral Biol. 1991, 36, 655–663. [Google Scholar] [CrossRef]
- Yang, X.; van den Dolder, J.; Walboomers, X.F.; Zhang, W.; Bian, Z.; Fan, M.; Jansen, J.A. The odontogenic potential of stro-1 sorted rat dental pulp stem cells in vitro. J. Tissue Eng. Regen. Med. 2007, 1, 66–73. [Google Scholar] [CrossRef]
- Bucchi, C.; Ohlsson, E.; de Anta, J.M.; Woelflick, M.; Galler, K.; Manzanares-Cespedes, M.C.; Widbiller, M. Human amnion epithelial cells: A potential cell source for pulp regeneration? Int. J. Mol. Sci. 2022, 23, 2830. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Sonoyama, W.; Chen, J.; Park, S.H. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006, 324, 225–236. [Google Scholar] [CrossRef]
- Kang, S.; Kim, D.; Lee, J.; Takayama, S.; Park, J.Y. Engineered microsystems for spheroid and organoid studies. Adv. Healthc. Mater. 2021, 10, 2001284. [Google Scholar] [CrossRef]
- Schmalz, G. Use of cell cultures for toxicity testing of dental materials—Advantages and limitations. J. Dent. 1994, 22, S6–S11. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Couble, M.-L.; Farges, J.-C.; Bleicher, F.; Perrat-Mabillon, B.; Boudeulle, M.; Magloire, H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif. Tissue Int. 2000, 66, 129–138. [Google Scholar] [CrossRef]
- Narayanan, K.; Srinivas, R.; Ramachandran, A.; Hao, J.; Quinn, B.; George, A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 2001, 98, 4516–4521. [Google Scholar] [CrossRef] [Green Version]
- Seki, D.; Takeshita, N.; Oyanagi, T.; Sasaki, S.; Takano, I.; Hasegawa, M.; Takano-Yamamoto, T. Differentiation of odontoblast-like cells from mouse induced pluripotent stem cells by Pax9 and Bmp4 transfection. Stem Cells Transl. Med. 2015, 4, 993–997. [Google Scholar] [CrossRef]
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine matrix proteins: Isolation and effects on human pulp cells. Int. Endod. J. 2018, 51, e278–e290. [Google Scholar] [CrossRef] [Green Version]
- Bento, L.W.; Zhang, Z.; Imai, A.; Nör, F.; Dong, Z.; Shi, S.; Araujo, F.B.; Nör, J.E. Endothelial differentiation of SHED requires MEK1/ERK signaling. J. Dent. Res. 2013, 92, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Nör, F.; Oh, M.; Cucco, C.; Shi, S.; Nör, J.E. Wnt/β-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells 2016, 34, 1576–1587. [Google Scholar] [CrossRef]
- Liu, L.; Wei, X.; Ling, J.; Wu, L.; Xiao, Y. Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J. Endod. 2011, 37, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Smeda, M.; Galler, K.M.; Woelflick, M.; Rosendahl, A.; Moehle, C.; Lenhardt, B.; Buchalla, W.; Widbiller, M. Molecular biological comparison of dental pulp- and apical papilla-derived stem cells. Int. J. Mol. Sci. 2022, 23, 2615. [Google Scholar] [CrossRef]
- Wei, X.; Ling, J.; Wu, L.; Liu, L.; Xiao, Y. Expression of Mineralization Markers in Dental Pulp Cells. J. Endod. 2007, 33, 703–708. [Google Scholar] [CrossRef]
- Magne, D.; Bluteau, G.; Lopez-Cazaux, S.; Weiss, P.; Pilet, P.; Ritchie, H.H.; Daculsi, G.; Guicheux, J. Development of an odontoblast in vitro model to study dentin mineralization. Connect. Tissue Res. 2004, 45, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Riccio, M.; Resca, E.; Maraldi, T.; Pisciotta, A.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures. Eur. J. Histochem. 2010, 54, 46. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef]
- Widbiller, M.; Schweikl, H.; Bruckmann, A.; Rosendahl, A.; Hochmuth, E.; Lindner, S.R.; Buchalla, W.; Galler, K.M. Shotgun proteomics of human dentin with different prefractionation methods. Sci. Rep. 2019, 9, 4457. [Google Scholar] [CrossRef] [Green Version]
- Knowlton, S.; Cho, Y.; Li, X.-J.; Khademhosseini, A.; Tasoglu, S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomater. Sci. 2016, 4, 768–784. [Google Scholar] [CrossRef]
- Qu, T.; Liu, X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J. Mater. Chem. B 2013, 1, 4764. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery (review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-T.; Hsu, T.-T.; Liu, Y.-W.; Kao, C.-T.; Huang, T.-H. Bidirectional differentiation of human-derived stem cells induced by biomimetic calcium silicate-reinforced gelatin methacrylate bioink for odontogenic regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, H.; Zhang, Z.; Yang, W.; Liu, W.; Li, Y.; Li, L. Biomaterial Stiffness Determines Stem Cell Fate. Life Sci. 2017, 178, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nör, J.E. Dentin-derived BMP-2 and odontoblast differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.M.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–997. [Google Scholar] [CrossRef] [Green Version]
- Chiu, L.L.Y.; Chu, Z.; Radisic, M. Tissue Engineering. In Comprehensive Nanoscience and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 175–211. ISBN 978-0-12-374396-1. [Google Scholar]
- Galler, K.M.; Widbiller, M. Perspectives for cell-homing approaches to engineer dental pulp. J. Endod. 2017, 43, S40–S45. [Google Scholar] [CrossRef]
- Yildirimer, L.; Seifalian, A.M. Three-dimensional biomaterial degradation—Material choice, design and extrinsic factor considerations. Biotechnol. Adv. 2014, 32, 984–999. [Google Scholar] [CrossRef]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.G.; Anovazzi, G.; Bordini, E.A.F.; Zuta, U.O.; Silva Leite, M.L.A.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Biological analysis of simvastatin-releasing chitosan scaffold as a cell-free system for pulp-dentin regeneration. J. Endod. 2018, 44, 971–976.e1. [Google Scholar] [CrossRef]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2016, 20, 237–246. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ye, D.; Zhang, J.; Guo, Y.; You, R.; Yan, S.; Li, M.; Qu, J. Fabrication and characterization of electrospun PCL/ antheraea pernyi silk fibroin nanofibrous scaffolds. Polym. Eng. Sci. 2017, 57, 206–213. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of Three-Dimensional Dentin–Pulp Complex with Local Differentiation of Human Dental Pulp Stem Cells. J. Tissue Eng. 2019, 10, 204173141984584. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A Dentin-Derived Hydrogel Bioink for 3D Bioprinting of Cell Laden Scaffolds for Regenerative Dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, C.-H. 3-Dimensional Bioprinting for Tissue Engineering Applications. Biomater. Res. 2016, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kawashima, N.; Takashino, N.; Koizumi, Y.; Takimoto, K.; Suzuki, N.; Saito, M.; Suda, H. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch. Oral Biol. 2014, 59, 310–331. [Google Scholar] [CrossRef]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.Y.; Lee, S.; Choi, W.H.; Jee, J.H.; Kim, H.-R.; Yoo, J. Fabrication of dentin-pulp-like organoids using dental-pulp stem cells. Cells 2020, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Abbott, A. Biology’s New Dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Izpisua Belmonte, J.C. Organoids—Preclinical models of human disease. N. Engl. J. Med. 2019, 380, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, Y.; Liao, L.; Tian, W. Oral organoids: Progress and challenges. J. Dent. Res. 2021, 100, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Kumar, R.; Buragohain, L.; Kumari, A.; Ghosh, M. Organoid technology: A reliable developmental biology tool for organ-specific nanotoxicity evaluation. Front. Cell Dev. Biol. 2021, 9, 696668. [Google Scholar] [CrossRef] [PubMed]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Ai, X.; Tang, Y.; Yang, D.; Dou, L. Human three-dimensional dental pulp organoid model for toxicity screening of dental materials on dental pulp cells and tissue. Int. Endod. J. 2022, 55, 79–88. [Google Scholar] [CrossRef]
- Modino, S.A.C.; Sharpe, P.T. Tissue engineering of teeth using adult stem cells. Arch. Oral Biol. 2005, 50, 255–258. [Google Scholar] [CrossRef]
- Smith, E.E.; Zhang, W.; Schiele, N.R.; Khademhosseini, A.; Kuo, C.K.; Yelick, P.C. Developing a biomimetic tooth bud model: Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017, 11, 3326–3336. [Google Scholar] [CrossRef]
- Rosowski, J.; Bräunig, J.; Amler, A.-K.; Strietzel, F.P.; Lauster, R.; Rosowski, M. Emulating the early phases of human tooth development in vitro. Sci. Rep. 2019, 9, 7057. [Google Scholar] [CrossRef] [Green Version]
- Tayebi, L. Applications of Biomedical Engineering in Dentistry; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-21582-8. [Google Scholar]
- Botchwey, E.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J. Biomed. Mater. Res. 2001, 55, 242–253. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Barrias, C.C.; Lourenço, A.H.; Gonçalves, R.M. A multicompartment holder for spinner flasks improves expansion and osteogenic differentiation of mesenchymal stem cells in three-dimensional scaffolds. Tissue Eng. Part C Methods 2014, 20, 984–993. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.; Konrad, L.; Gotzen, L.; Printz, H.; Ramaswamy, A.; Hofmann, C. Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J. Biomed. Mater. Res. 2003, 67, 191–199. [Google Scholar] [CrossRef]
- Reinwald, Y.; El Haj, A.J. Hydrostatic pressure in combination with topographical cues affects the fate of bone marrow-derived human mesenchymal stem cells for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Song, K.-D.; Liu, T.-Q.; Li, X.-Q.; Cui, Z.-F.; Sun, X.-Y.; Ma, X.-H. Three-dimensional expansion: In suspension culture of SD rat’s osteoblasts in a rotating wall vessel bioreactor. Biomed. Environ. Sci. BES 2007, 20, 91–98. [Google Scholar]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- França, C.M.; Tahayeri, A.; Rodrigues, N.S.; Ferdosian, S.; Puppin Rontani, R.M.; Sereda, G.; Ferracane, J.L.; Bertassoni, L.E. The tooth on-a-chip: A microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip 2020, 20, 405–413. [Google Scholar] [CrossRef]
- Buurma, B.; Gu, K.; Rutherford, R.B. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds: Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur. J. Oral Sci. 1999, 107, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Lee, D.-S.; Choung, H.-W.; Shon, W.-J.; Seo, B.-M.; Lee, E.-H.; Cho, J.-Y.; Park, J.-C. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 2011, 32, 9696–9706. [Google Scholar] [CrossRef]
- de Almeida, J.F.A.; Chen, P.; Henry, M.A.; Diogenes, A. Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng. Part A 2014, 20, 3089–3100. [Google Scholar] [CrossRef]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.; Dong, Z.; Bramante, C.; Holland, G.; Smith, A.; Nor, J. Tooth slice–based models for the study of human dental pulp angiogenesis. J. Endod. 2007, 33, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.S.; Alsanea, R.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; John, A.S.; George, A. In-vivo generation of dental pulp-like tissue using human pulpal stem cells, a collagen scaffold and dentin matrix protein 1 following subcutaneous transplantation in mice. J. Endod. 2009, 34, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Sakai, V.T.; Cordeiro, M.M.; Dong, Z.; Zhang, Z.; Zeitlin, B.D.; Nör, J.E. Tooth slice/scaffold model of dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 325–332. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef]
- Takeuchi, N.; Hayashi, Y.; Murakami, M.; Alvarez, F.; Horibe, H.; Iohara, K.; Nakata, K.; Nakamura, H.; Nakashima, M. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015, 21, 113–122. [Google Scholar] [CrossRef]
- Widbiller, M.; Driesen, R.B.; Eidt, A.; Lambrichts, I.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. J. Endod. 2018, 44, 956–962.e2. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Yamaza, T.; She, A.L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, J.; Yu, Z.; Chen, C.-A.; Aksel, H.; Azim, A.A.; Huang, G.T.-J. A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Eng. Part C Methods 2018, 24, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Bottino, M.C.; Fouad, A.F.; Nör, J.E.; Huang, G.T.-J. Animal Models for Stem Cell-Based Pulp Regeneration: Foundation for Human Clinical Applications. Tissue Eng. Part B Rev. 2019, 25, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, S.-J.; Song, Y.; Kim, E. In vivo experiments with dental pulp stem cells for pulp-dentin complex regeneration. Mediat. Inflamm. 2015, 2015, 409347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Deng, Z.; Shi, J.; Zhai, H.; Nie, X.; Zhuang, H.; Li, Y.; Jin, Y. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006, 12, 3097–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Backly, R.M.; Massoud, A.G.; El-Badry, A.M.; Sherif, R.A.; Marei, M.K. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in new zealand white rabbits. Aust. Endod. J. 2008, 34, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Ruangsawasdi, N.; Zehnder, M.; Patcas, R.; Ghayor, C.; Weber, F.E. Regenerative Dentistry: Animal model for regenerative endodontology. Transfus. Med. Hemotherapy 2016, 43, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Tsutsui, T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: Stem cells in the core: Stem cell distribution in spheroid. J. Cell Biochem. 2013, 114, 2624–2636. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, L.I.; Yan, M.; Gosau, M.; Smeets, R.; Kluwe, L.; Friedrich, R.E. Optimizing conditions for spheroid formation of dental pulp cells in cell culture. In Vivo 2021, 35, 1965–1972. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Lee, Y.-C.; Hung, C.-Y.; Yang, P.-J.; Lai, P.-C.; Feng, S.-W. Three-dimensional spheroid culture enhances multipotent differentiation and stemness capacities of human dental pulpderived mesenchymal stem cells by modulating MAPK and NF-KB signaling pathways. Stem. Cell Rev. Rep. 2021, 17, 1810–1826. [Google Scholar] [CrossRef]
- Sloan, A.J.; Shelton, R.M.; Hann, A.C.; Moxham, B.J.; Smith, A.J. An in vitro approach for the study of dentinogenesis by organ culture of the dentine–pulp complex from rat incisor teeth. Arch. Oral Biol. 1998, 43, 421–430. [Google Scholar] [CrossRef]
- Widbiller, M.; Althumairy, R.I.; Diogenes, A. Direct and indirect effect of chlorhexidine on survival of stem cells from the apical papilla and its neutralization. J. Endod. 2019, 45, 156–160. [Google Scholar] [CrossRef]
- Atesci, A.A.; Avci, C.B.; Tuglu, M.I.; Ozates Ay, N.P.; Eronat, A.C. Effect of different dentin conditioning agents on growth factor release, mesenchymal stem cell attachment and morphology. J. Endod. 2020, 46, 200–208. [Google Scholar] [CrossRef]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Machado, M.A.A.M.; Shi, S.; Santos, C.F.; Nör, J.E. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef]
- Demarco, F.F.; Casagrande, L.; Zhang, Z.; Dong, Z.; Tarquinio, S.B.; Zeitlin, B.D.; Shi, S.; Smith, A.J.; Nör, J.E. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. J. Endod. 2010, 36, 1805–1811. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K. Regeneration of dental pulp by stem cells. Adv. Dent. Res. 2011, 23, 313–319. [Google Scholar] [CrossRef]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The angiogenic potential of dpscs and scaps in an in vivo model of dental pulp regeneration. Stem. Cells Int. 2017, 2017, 2582080. [Google Scholar] [CrossRef] [Green Version]
- Widbiller, M.; Rothmaier, C.; Saliter, D.; Wölflick, M.; Rosendahl, A.; Buchalla, W.; Schmalz, G.; Spruss, T.; Galler, K.M. Histology of human teeth: Standard and specific staining methods revisited. Arch. Oral Biol. 2021, 127, 105136. [Google Scholar] [CrossRef]
- Torabinejad, M.; Corr, R.; Buhrley, M.; Wright, K.; Shabahang, S. An animal model to study regenerative endodontics. J. Endod. 2011, 37, 197–202. [Google Scholar] [CrossRef]
- Muthanandam, S.; Muthu, J.; Mony, V.; Rl, P.; Lal K, P. Animal models in dental research—A Review. Int. Dent. J. Stud. Res. 2020, 8, 44–47. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: Potters Bar, Herts, 1992; ISBN 978-0-900767-78-4. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Wang, Y.; Zhao, Y.; Jia, W.; Yang, J.; Ge, L. Preliminary study on dental pulp stem cell-mediated pulp regeneration in canine immature permanent teeth. J. Endod. 2013, 39, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kikuchi, M.; Barroga, E.F.; Okumura, M.; Kadosawa, T.; Fujinaga, T. The formation of apical delta of the permanent teeth in dogs. J. Vet. Med. Sci. 2001, 63, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangione, F.; Salmon, B.; EzEldeen, M.; Jacobs, R.; Chaussain, C.; Vital, S. Characteristics of large animal models for current cell-based oral tissue regeneration. Tissue Eng. Part B Rev. 2021, 28, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of cd105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of cd31/cd146 side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. STEM CELLS Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef]
- Iohara, K.; Zayed, M.; Takei, Y.; Watanabe, H.; Nakashima, M. Treatment of pulpectomized teeth with trypsin prior to transplantation of mobilized dental pulp stem cells enhances pulp regeneration in aged dogs. Front. Bioeng. Biotechnol. 2020, 8, 983. [Google Scholar] [CrossRef]
- Ling, L.; Zhao, Y.M.; Wang, X.T.; Wen, Q.; Ge, L.H. Regeneration of dental pulp tissue by autologous grafting stem cells derived from inflammatory dental pulp tissue in immature premolars in a beagle dog. Chin. J. Dent. Res. J. Sci. Sect. Chin. Stomatol. Assoc. CSA 2020, 23, 143–150. [Google Scholar]
- Kodonas, K.; Gogos, C.; Papadimitriou, S.; Kouzi-Koliakou, K.; Tziafas, D. Experimental formation of dentin-like structure in the root canal implant model using cryopreserved swine dental pulp progenitor cells. J. Endod. 2012, 38, 913–919. [Google Scholar] [CrossRef]
- Mangione, F.; EzEldeen, M.; Bardet, C.; Lesieur, J.; Bonneau, M.; Decup, F.; Salmon, B.; Jacobs, R.; Chaussain, C.; Opsahl-Vital, S. Implanted dental pulp cells fail to induce regeneration in partial pulpotomies. J. Dent. Res. 2017, 96, 1406–1413. [Google Scholar] [CrossRef]
- Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab. Anim. 2010, 44, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, N.; Noiri, Y.; Matsui, S.; Kuremoto, K.; Maezono, H.; Ishimoto, T.; Nakano, T.; Ebisu, S.; Hayashi, M. Development of a root canal treatment model in the rat. Sci. Rep. 2017, 7, 3315. [Google Scholar] [CrossRef]
- Chicarelli, L.P.G.; Webber, M.B.F.; Amorim, J.P.A.; Rangel, A.L.C.A.; Camilotti, V.; Sinhoreti, M.A.C.; Mendonça, M.J. Effect of tricalcium silicate on direct pulp capping: Experimental study in rats. Eur. J. Dent. 2021, 15, 101–108. [Google Scholar] [CrossRef]
- Dammaschke, T.; Stratmann, U.; Fischer, R.-J.; Sagheri, D.; Schäfer, E. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. Berl. Ger. 2010, 41, e62–e71. [Google Scholar]
- Simon, S.; Cooper, P.; Smith, A.; Picard, B.; Ifi, C.N.; Berdal, A. Evaluation of a new laboratory model for pulp healing: Preliminary study. Int. Endod. J. 2008, 41, 781–790. [Google Scholar] [CrossRef]
- Almushayt, A.; Narayanan, K.; Zaki, A.E.; George, A. dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006, 13, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Fouad, A.F.; Walton, R.E.; Rittman, B.R. Healing of induced periapical lesions in ferret canines. J. Endod. 1993, 19, 123–129. [Google Scholar] [CrossRef]
- He, T.; Friede, H.; Kiliaridis, S. Dental eruption and exfoliation chronology in the ferret (mustela putorius furo). Arch. Oral Biol. 2002, 47, 619–623. [Google Scholar] [CrossRef]
- Alexander, A.; Torabinejad, M.; Vahdati, S.A.; Nosrat, A.; Verma, P.; Grandhi, A.; Shabahang, S. Regenerative endodontic treatment in immature noninfected ferret teeth using blood clot or synoss putty as scaffolds. J. Endod. 2020, 46, 209–215. [Google Scholar] [CrossRef]
- Bucchi, C.; Gimeno-Sandig, Á.; Valdivia-Gandur, I.; Manzanares-Céspedes, C.; De Anta, J.M. A Regenerative endodontic approach in mature ferret teeth using rodent preameloblast-conditioned medium. Vivo 2019, 33, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torabinejad, M.; Bakland, L.K. An animal model for the study of immunopathogenesis of periapical lesions. J. Endod. 1978, 4, 273–277. [Google Scholar] [CrossRef]
- Altaii, M.; Cathro, P.; Broberg, M.; Richards, L. Endodontic regeneration and tooth revitalization in immature infected sheep teeth. Int. Endod. J. 2017, 50, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Broberg, M.; Cathro, P.; Richards, L. Standardisation of sheep model for endodontic regeneration/revitalisation research. Arch. Oral Biol. 2016, 65, 87–94. [Google Scholar] [CrossRef]
- Danesh-Meyer, M.J.; Pack, A.R.C.; McMillan, M.D. A Comparison of 2 Polytetrafluoroethylene Membranes in Guided Tissue Regeneration in Sheep. J. Periodontal. Res. 1997, 32, 20–30. [Google Scholar] [CrossRef]
- Schliephake, H.; Knebel, J.W.; Aufderheide, M.; Tauscher, M. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: An experimental pilot study in sheep. Int. J. Oral. Maxillofac. Surg. 2001, 30, 531–537. [Google Scholar] [CrossRef]
- Carlsson, H.-E.; Schapiro, S.J.; Farah, I.; Hau, J. Use of primates in research: A global overview. Am. J. Primatol. 2004, 63, 225–237. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]





| In Vitro | In Vivo | |||
|---|---|---|---|---|
| Monolayer | 3D Culture | Ectopic | Semiorthotopic | |
| high cost | + | + | ++ | ++ |
| ethical concerns | + | + | +++ | +++ |
| literature experience | +++ | + | ++ | ++ |
| difficult implementation | + | ++ | ++ | ++ |
| reproducibility | +++ | ++ | + | + |
| mimicry of natural situation | + | ++ | ++ | +++ |
| Study Models | In Vitro | In Vivo | |
|---|---|---|---|
| Ectopic | Semiorthotopic | ||
| Scaffold culture | Wang et al., 2010 [81] Galler et al., 2012 [50] Qu and Liu, 2013 [40] Widbiller et al., 2016 [52] Lin et al., 2021 [42] | Buurma et al., 1999 [80] Gronthos et al., 2000 [12] Wang et al., 2010 [81] Lee et al., 2011 [82] De Almeidas et al., 2014 [83] | * |
| Spheroid and organoid | Xiao and Tsutsui, 2013 [99] Dissanayaka et al., 2014 [59] Jeong et al., 2020 [60] Zheng et al., 2021 [100] Chan et al., 2021 [101] | * | |
| Dentin disk | Sloan et al., 1998 [102] Huang et al., 2006 [19] Widbiller et al., 2019 [103] Atesci et al., 2020 [104] | * | Batouli et al., 2003 [84] Goncalves et al., 2007 [85] |
| Tooth slice | Casagrande et al., 2010 [45] | * | Cordeiro et al., 2008 [86] Prescott et al., 2009 [87] Sakai et al., 2010 [105] Casagrande et al., 2010 [45] Sakai et al., 2011 [88] Dissanayaka et al., 2014 [59] |
| Dentin cylinder and tooth root | Rosa et al., 2013 [46] | * | Galler et al., 2011 [89] Galler et al., 2012 [50] Rosa et al., 2013 [46] Takeuchi et al., 2015 [90] Widbiller et al., 2018 [91] With coronal plug: Huang et al., 2010 [92] Zhu et al., 2018 [93] |
| In Vivo Orthotopic | ||||
|---|---|---|---|---|
| Dog | Pig | Ferret | Rodent | |
| high cost | +++ | +++ | ++ | + |
| ethical concerns | +++ | ++ | ++ | ++ |
| literature experience | +++ | ++ | + | + |
| housing requirements | ++ | +++ | + | + |
| animal handling | +++ | + | ++ | +++ |
| similarity of tooth anatomy | ++ | ++ | + | + |
| similarity of tooth size | +++ | +++ | ++ | + |
| access to teeth | ++ | ++ | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohlsson, E.; Galler, K.M.; Widbiller, M. A Compilation of Study Models for Dental Pulp Regeneration. Int. J. Mol. Sci. 2022, 23, 14361. https://doi.org/10.3390/ijms232214361
Ohlsson E, Galler KM, Widbiller M. A Compilation of Study Models for Dental Pulp Regeneration. International Journal of Molecular Sciences. 2022; 23(22):14361. https://doi.org/10.3390/ijms232214361
Chicago/Turabian StyleOhlsson, Ella, Kerstin M. Galler, and Matthias Widbiller. 2022. "A Compilation of Study Models for Dental Pulp Regeneration" International Journal of Molecular Sciences 23, no. 22: 14361. https://doi.org/10.3390/ijms232214361






