E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of Patients with Epithelial Ovarian Cancer
Abstract
:1. Introduction
E-Cadherin in Cancers
2. E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of EOC Patients
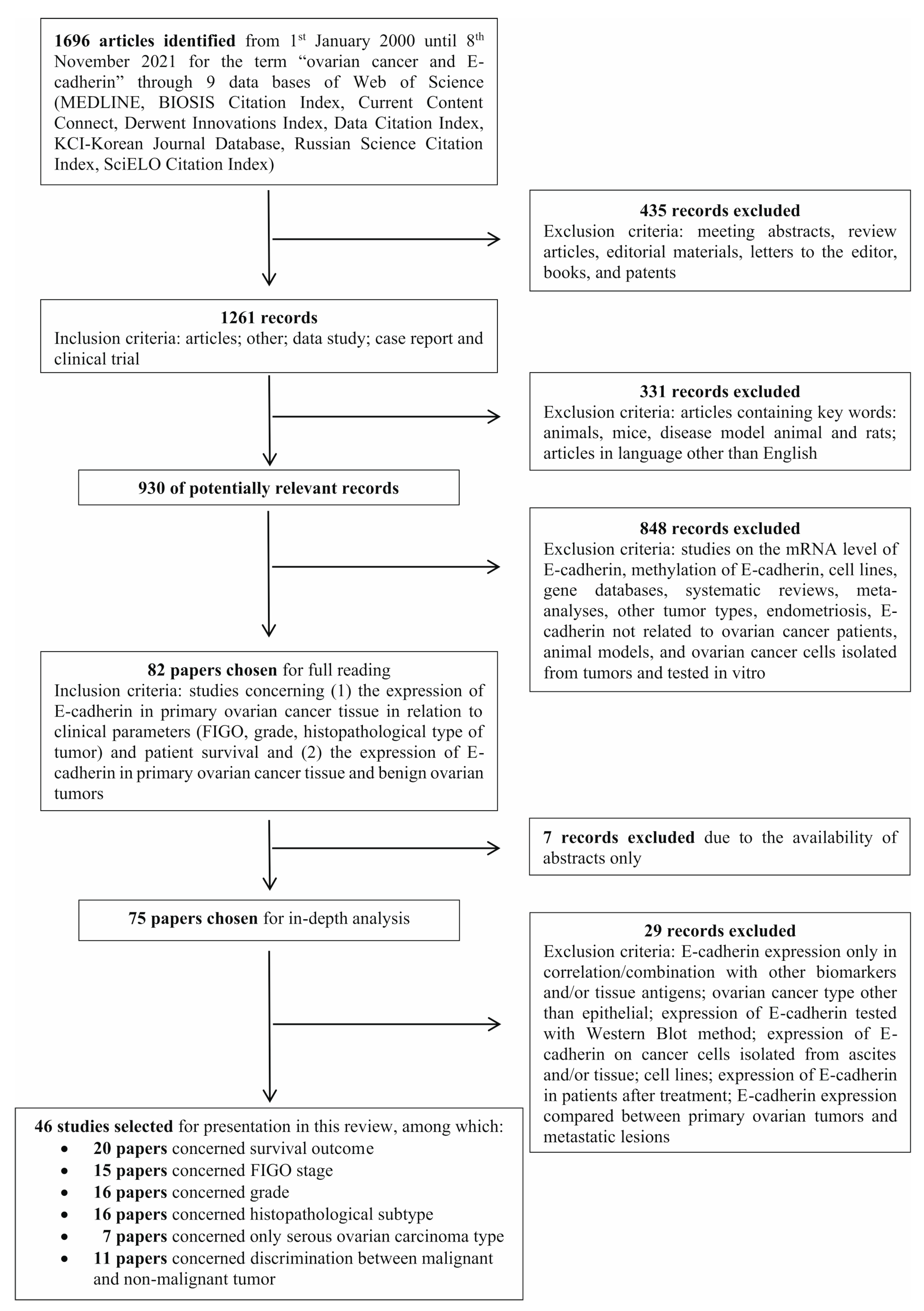
2.1. Literature Search and Selection Criteria
2.2. Results
2.2.1. Association of E-Cadherin Expression with Patient Survival
2.2.2. Association of E-Cadherin Expression with Clinicopathological Parameters
FIGO Stage
Grading
Histopathological Type (HP)
2.2.3. Can E-Cadherin Expression Be a Helpful for Discriminating Malignant and Nonmalignant Ovarian Tumors?
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeichi, M. Functional Correlation between Cell Adhesive Properties and Some Cell Surface Proteins. J. Cell Biol. 1977, 75, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorian, I.Y.; Linkova, N.S.; Polyakova, V.O.; Paltseva, E.M.; Kozlov, K.L. Signaling Molecules of the Endometrium: Gerontological and General Pathological Aspects. Adv. Gerontol. 2016, 6, 36–43. [Google Scholar] [CrossRef]
- Van Roy, F.; Berx, G. The Cell-Cell Adhesion Molecule E-Cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Cadwell, C.M.; Su, W.; Kowalczyk, A.P. Cadherin Tales: Regulation of Cadherin Function by Endocytic Membrane Trafficking. Traffic 2016, 17, 1262–1271. [Google Scholar] [CrossRef]
- Yap, A.S. The Morphogenetic Role of Cadherin Cell Adhesion Molecules in Human Cancer: A Thematic Review. Cancer Investig. 1998, 16, 252–261. [Google Scholar] [CrossRef]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef]
- Berx, G.; Becker, K.F.; Höfler, H.; van Roy, F. Mutations of the Human E-Cadherin (CDH1) Gene. Hum. Mutat. 1998, 12, 226–237. [Google Scholar] [CrossRef]
- Bruner, H.C.; Derksen, P.W.B. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb. Perspect. Med. 2018, 10, a029330. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, T.; Rizvi, A.; Batta, S.P.R.; Kataria, S.; Jamora, C. Signaling and Mechanical Roles of E-Cadherin. Cell Commun. Adhes. 2013, 20, 189–199. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and BHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sun, L.; Li, Q.; Han, X.; Lei, L.; Zhang, H.; Shang, Y. SET8 Promotes Epithelial-Mesenchymal Transition and Confers TWIST Dual Transcriptional Activities. EMBO J. 2012, 31, 110–123. [Google Scholar] [CrossRef] [Green Version]
- Hazan, R.B.; Qiao, R.; Keren, R.; Badano, I.; Suyama, K. Cadherin Switch in Tumor Progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rosivatz, E.; Becker, I.; Specht, K.; Fricke, E.; Luber, B.; Busch, R.; Höfler, H.; Becker, K.-F. Differential Expression of the Epithelial-Mesenchymal Transition Regulators Snail, SIP1, and Twist in Gastric Cancer. Am. J. Pathol. 2002, 161, 1881–1891. [Google Scholar] [CrossRef]
- Elloul, S.; Elstrand, M.B.; Nesland, J.M.; Tropé, C.G.; Kvalheim, G.; Goldberg, I.; Reich, R.; Davidson, B. Snail, Slug, and Smad-Interacting Protein 1 as Novel Parameters of Disease Aggressiveness in Metastatic Ovarian and Breast Carcinoma. Cancer 2005, 103, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, S.; Schmalhofer, O.; Wahlbuhl, M.; Dimmler, A.; Bauer, K.; Sultan, A.; Hlubek, F.; Jung, A.; Strand, D.; Eger, A.; et al. The Transcriptional Repressor ZEB1 Promotes Metastasis and Loss of Cell Polarity in Cancer. Cancer Res. 2008, 68, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-J.; Liu, H.; Zhang, P.; Zhang, X.-D.; Jiang, Z.-W.; Jiang, C.-C. MiR-10b Promotes Migration and Invasion in Nasopharyngeal Carcinoma Cells. Asian Pac. J. Cancer Prev. 2013, 14, 5533–5537. [Google Scholar] [CrossRef] [Green Version]
- Le, T.L.; Yap, A.S.; Stow, J.L. Recycling of E-Cadherin: A Potential Mechanism for Regulating Cadherin Dynamics. J. Cell Biol. 1999, 146, 219–232. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef] [Green Version]
- Na, T.-Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The Functional Activity of E-Cadherin Controls Tumor Cell Metastasis at Multiple Steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Hollestelle, A.; Peeters, J.K.; Smid, M.; Timmermans, M.; Verhoog, L.C.; Westenend, P.J.; Heine, A.A.J.; Chan, A.; Sieuwerts, A.M.; Wiemer, E.A.C.; et al. Loss of E-Cadherin Is Not a Necessity for Epithelial to Mesenchymal Transition in Human Breast Cancer. Breast Cancer Res. Treat. 2013, 138, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Han, S.-M.; Tang, X.-Y.; Han, L.; Li, C.-Z. Overexpressed FOXC2 in Ovarian Cancer Enhances the Epithelial-to-Mesenchymal Transition and Invasion of Ovarian Cancer Cells. Oncol. Rep. 2014, 31, 2545–2554. [Google Scholar] [CrossRef] [Green Version]
- Choi, P.-W.; Yang, J.; Ng, S.-K.; Feltmate, C.; Muto, M.G.; Hasselblatt, K.; Lafferty-Whyte, K.; JeBailey, L.; MacConaill, L.; Welch, W.R.; et al. Loss of E-Cadherin Disrupts Ovarian Epithelial Inclusion Cyst Formation and Collective Cell Movement in Ovarian Cancer Cells. Oncotarget 2015, 7, 4110–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuecheng, Y.; Hongmei, L.; Xiaoyan, X. Clinical Evaluation of E-Cadherin Expression and Its Regulation Mechanism in Epithelial Ovarian Cancer. Clin. Exp. Metastasis 2006, 23, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Majem, B.; Devis, L.; Lapyckyj, L.; Besso, M.J.; Llauradó, M.; Abascal, M.F.; Matos, M.L.; Lanau, L.; Castellví, J.; et al. E-Cadherin: A Determinant Molecule Associated with Ovarian Cancer Progression, Dissemination and Aggressiveness. PLoS ONE 2017, 12, e0184439. [Google Scholar] [CrossRef] [Green Version]
- Jäger, T.; Becker, M.; Eisenhardt, A.; Tilki, D.; Tötsch, M.; Schmid, K.W.; Romics, I.; Rübben, H.; Ergün, S.; Szarvas, T. The Prognostic Value of Cadherin Switch in Bladder Cancer. Oncol. Rep. 2010, 23, 1125–1132. [Google Scholar] [CrossRef]
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B. Prognostic Value of Reduced E-Cadherin Expression in Breast Cancer: A Meta-Analysis. Oncotarget 2017, 8, 16445–16455. [Google Scholar] [CrossRef] [Green Version]
- Zhai, B.; Yan, H.-X.; Liu, S.-Q.; Chen, L.; Wu, M.-C.; Wang, H.-Y. Reduced Expression of E-Cadherin/Catenin Complex in Hepatocellular Carcinomas. World J. Gastroenterol. 2008, 14, 5665–5673. [Google Scholar] [CrossRef]
- Koensgen, D.; Freitag, C.; Klaman, I.; Dahl, E.; Mustea, A.; Chekerov, R.; Braicu, I.; Lichtenegger, W.; Sehouli, J. Expression and Localization of E-Cadherin in Epithelial Ovarian Cancer. Anticancer Res. 2010, 30, 2525–2530. [Google Scholar]
- Alshenawy, H.A. Immunohistochemical Expression of Epidermal Growth Factor Receptor, E-Cadherin, and Matrix Metalloproteinase-9 in Ovarian Epithelial Cancer and Relation to Patient Deaths. Ann. Diagn. Pathol. 2010, 14, 387–395. [Google Scholar] [CrossRef]
- Cho, E.Y.; Choi, Y.; Chae, S.W.; Sohn, J.H.; Ahn, G.H. Immunohistochemical Study of the Expression of Adhesion Molecules in Ovarian Serous Neoplasms. Pathol. Int. 2006, 56, 62–70. [Google Scholar] [CrossRef]
- Miše, B.P.; Telesmanić, V.D.; Tomić, S.; Šundov, D.; Čapkun, V.; Vrdoljak, E. Correlation between E-Cadherin Immunoexpression and Efficacy of First Line Platinum-Based Chemotherapy in Advanced High Grade Serous Ovarian Cancer. Pathol. Oncol. Res 2015, 21, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Bačić, B.; Haller, H.; Mrklić, I.; Košta, V.; Čarić, A.; Tomić, S. Prognostic Role of E-Cadherin in Patients with Advanced Serous Ovarian Cancer. Arch. Gynecol. Obs. 2013, 287, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Communal, L.; Roy, N.; Cahuzac, M.; Rahimi, K.; Köbel, M.; Provencher, D.M.; Mes-Masson, A.-M. A Keratin 7 and E-Cadherin Signature Is Highly Predictive of Tubo-Ovarian High-Grade Serous Carcinoma Prognosis. Int. J. Mol. Sci. 2021, 22, 5325. [Google Scholar] [CrossRef]
- Song, I.H.; Kim, K.-R.; Lim, S.; Kim, S.-H.; Sung, C.O. Expression and Prognostic Significance of Epithelial-Mesenchymal Transition-Related Markers and Phenotype in Serous Ovarian Cancer. Pathol. Res. Pract. 2018, 214, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, L.; Green, A.R.; Martin, S.; Durrant, L.; Deen, S. The Cadherin Switch in Ovarian High-Grade Serous Carcinoma Is Associated with Disease Progression. Virchows Arch. 2011, 459, 21–29. [Google Scholar] [CrossRef]
- Taşkin, S.; Dünder, I.; Erol, E.; Taşkin, E.A.; Kiremitçi, S.; Öztuna, D.; Sertçelik, A. Roles of E-Cadherin and Cyclooxygenase Enzymes in Predicting Different Survival Patterns of Optimally Cytoreduced Serous Ovarian Cancer Patients. Asian Pac. J. Cancer Prev. 2012, 13, 5715–5719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dian, D.; Brüning, A.; Mylonas, I. E-Cadherin as a Prognostic Marker in Human Serous Carcinomas of the Ovary: An Immunohistochemical Analysis. Arch. Gynecol. Obs. 2011, 284, 437–443. [Google Scholar] [CrossRef]
- Blechschmidt, K.; Sassen, S.; Schmalfeldt, B.; Schuster, T.; Höfler, H.; Becker, K.-F. The E-Cadherin Repressor Snail Is Associated with Lower Overall Survival of Ovarian Cancer Patients. Br. J. Cancer 2008, 98, 489–495. [Google Scholar] [CrossRef]
- Faleiro-Rodrigues, C.; Macedo-Pinto, I.; Pereira, D.; Lopes, C.S. Prognostic Value of E-Cadherin Immunoexpression in Patients with Primary Ovarian Carcinomas. Ann. Oncol. 2004, 15, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Dionísio de Sousa, I.J.; Marques, D.S.; Príncipe, C.; Portugal, R.V.; Canberk, S.; Prazeres, H.; Lopes, J.M.; Gimba, E.R.P.; Lima, R.T.; Soares, P. Predictive Biomarkers and Patient Outcome in Platinum-Resistant (PLD-Treated) Ovarian Cancer. Diagnostics 2020, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, E.Y.; Yoon, M.S.; Suh, D.S.; Kim, K.H.; Lee, J.H.; Shin, D.H.; Kim, J.Y.; Sol, M.Y.; Choi, K.U. The Role of TWIST in Ovarian Epithelial Cancers. Korean J. Pathol. 2014, 48, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sundov, D.; Petric Mise, B.; Mrklic, I.; Bacic, B.; Vrdoljak, E.; Tomic, S. Prognostic Significance of MAPK, Topo IIα and E-Cadherin Immunoexpression in Ovarian Serous Carcinomas. Neoplasma 2017, 64, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Sui, L.-H. The Relevance and Role of Vascular Endothelial Growth Factor C, Matrix Metalloproteinase-2 and E-Cadherin in Epithelial Ovarian Cancer. Med. Oncol. 2012, 29, 318–323. [Google Scholar] [CrossRef]
- Liew, P.-L.; Hsu, C.-S.; Liu, W.-M.; Lee, Y.-C.; Lee, Y.-C.; Chen, C.-L. Prognostic and Predictive Values of Nrf2, Keap1, P16 and E-Cadherin Expression in Ovarian Epithelial Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 5642–5649. [Google Scholar]
- Nagy, B.; Tóth, L.; Molnár, P.; Méhes, G.; Thurzó, L.; Póka, R.; Hernádi, Z. Nuclear β-Catenin Positivity as a Predictive Marker of Long-Term Survival in Advanced Epithelial Ovarian Cancer. Pathol. Res. Pract. 2017, 213, 915–921. [Google Scholar] [CrossRef]
- Bodnar, L.; Stanczak, A.; Cierniak, S.; Smoter, M.; Cichowicz, M.; Kozlowski, W.; Szczylik, C.; Wieczorek, M.; Lamparska-Przybysz, M. Wnt/β-Catenin Pathway as a Potential Prognostic and Predictive Marker in Patients with Advanced Ovarian Cancer. J. Ovarian Res. 2014, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Voutilainen, K.A.; Anttila, M.A.; Sillanpää, S.M.; Ropponen, K.M.; Saarikoski, S.V.; Juhola, M.T.; Kosma, V.-M. Prognostic Significance of E-Cadherin-Catenin Complex in Epithelial Ovarian Cancer. J. Clin. Pathol. 2006, 59, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Toth, L.; Nagy, B.; Mehes, G.; Laszlo, E.; Molnar, P.P.; Poka, R.; Hernadi, Z. Cell Adhesion Molecule Profiles, Proliferation Activity and P53 Expression in Advanced Epithelial Ovarian Cancer Induced Malignant Ascites-Correlation of Tissue Microarray and Cytology Microarray. Pathol. Res. Pract. 2018, 214, 978–985. [Google Scholar] [CrossRef]
- Wu, X.; Zhuang, Y.; Hong, C.; Chen, J.; You, Y.; Zhang, F.; Huang, P.; Wu, M. Clinical Importance and Therapeutic Implication of E-Cadherin Gene Methylation in Human Ovarian Cancer. Med. Oncol. 2014, 31, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Hou, Y.Y.; Hou, J.Q.; Chen, Y.H.; Jiang, H.Y.; Chang, S.D.; Wang, G.Q. The Expression and Significance of P-STAT3, Twist, and E-Cadherin in Ovarian Epithelial Carcinoma. Eur. J. Gynaecol. Oncol. 2019, 40, 1019–1023. [Google Scholar] [CrossRef]
- Hayashi, A.; Horiuchi, A.; Kikuchi, N.; Hayashi, T.; Fuseya, C.; Suzuki, A.; Konishi, I.; Shiozawa, T. Type-Specific Roles of Histone Deacetylase (HDAC) Overexpression in Ovarian Carcinoma: HDAC1 Enhances Cell Proliferation and HDAC3 Stimulates Cell Migration with Downregulation of E-Cadherin. Int. J. Cancer 2010, 127, 1332–1346. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, Y.; Xu, Y.; Yang, H.; Fang, L.; Liu, Y.; Li, X.; Li, Q.; Yan, H. Expression and Clinical Significance of WWOX, Elf5, Snail1 and EMT Related Factors in Epithelial Ovarian Cancer. Oncol. Lett. 2020, 19, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Horiuchi, A.; Shiozawa, T.; Osada, R.; Kikuchi, N.; Ohira, S.; Oka, K.; Konishi, I. Elevated Expression of E-Cadherin and Alpha-, Beta-, and Gamma-Catenins in Metastatic Lesions Compared with Primary Epithelial Ovarian Carcinomas. Hum. Pathol. 2004, 35, 1469–1476. [Google Scholar] [CrossRef]
- Yoshida, J.; Horiuchi, A.; Kikuchi, N.; Hayashi, A.; Osada, R.; Ohira, S.; Shiozawa, T.; Konishi, I. Changes in the Expression of E-Cadherin Repressors, Snail, Slug, SIP1, and Twist, in the Development and Progression of Ovarian Carcinoma: The Important Role of Snail in Ovarian Tumorigenesis and Progression. Med. Mol. Morphol. 2009, 42, 82–91. [Google Scholar] [CrossRef]
- Bhagat, R.; Premalata, C.S.; Shilpa, V.; Pallavi, V.R.; Ramesh, G.; Vijay, C.R.; Krishnamoorthy, L. Altered Expression of β-Catenin, E-Cadherin, and E-Cadherin Promoter Methylation in Epithelial Ovarian Carcinoma. Tumour Biol. 2013, 34, 2459–2468. [Google Scholar] [CrossRef]
- Marques, F.R.; Fonsechi-Carvasan, G.A.; De Angelo Andrade, L.A.L.; Böttcher-Luiz, F. Immunohistochemical Patterns for Alpha- and Beta-Catenin, E- and N-Cadherin Expression in Ovarian Epithelial Tumors. Gynecol. Oncol. 2004, 94, 16–24. [Google Scholar] [CrossRef]
- Elbasateeny, S.S.; Elnagar, W.M.; Yassin, M.A.; Rashad, H.M. IMP3 Expression in Ovarian Serous Tumours: Correlation with Epithelial-Mesenchymal Transition-Related Markers (E-Cadherin and Vimentin) and Clinicopathological Factors. J. Clin. Diagn. Res. 2020, 14, 16–20. [Google Scholar] [CrossRef]
- Dochiţ, C.M.; Stepan, A.E.; Mărgăritescu, C.; Florescu, M.M.; Simionescu, C.E. Immunoexpression of E-, P- and N-Cadherins in Ovarian Serous Malignant Tumors. Rom. J. Morphol. Embryol. 2019, 60, 1215–1219. [Google Scholar]
- Ryabtseva, O.D.; Lukianova, N.Y.; Shmurakov, Y.A.; Polishchuk, L.Z.; Antipova, S.V. Significance of Adhesion Molecules Expression for Estimation of Serous Ovarian Cancer Prognosis. Exp. Oncol. 2013, 35, 211–218. [Google Scholar] [PubMed]
- Xuyin, Z.; Jingxin, D.; Xiang, T.; Luoqi, J.; Keqin, H. Biomarker Expression in Normal Fimbriae: Comparison of High- and Low-Grade Serous Ovarian Carcinoma. Oncol. Lett. 2012, 4, 1008–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.-K.; Lu, K.H.; Malpica, A.; Bodurka, D.C.; Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly Greater Expression of ER, PR, and ECAD in Advanced-Stage Low-Grade Ovarian Serous Carcinoma as Revealed by Immunohistochemical Analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.H.; Kalloger, S.E.; Santos, J.L.; Swenerton, K.D.; Gilks, C.B. Stage II to IV Low-Grade Serous Carcinoma of the Ovary Is Associated with a Poor Prognosis: A Clinicopathologic Study of 32 Patients from a Population-Based Tumor Registry. Int. J. Gynecol. Pathol. 2013, 32, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, Y.; Mi, C. Expressions of Beta-Catenin, E-Cadherin and MMP-7 in Ovarian Epithelial Tumors. Chin. J. Cancer Res. 2008, 20, 190–193. [Google Scholar] [CrossRef]
- Houle, C.D.; Ding, X.-Y.; Foley, J.F.; Afshari, C.A.; Barrett, J.C.; Davis, B.J. Loss of Expression and Altered Localization of KAI1 and CD9 Protein Are Associated with Epithelial Ovarian Cancer Progression. Gynecol. Oncol. 2002, 86, 69–78. [Google Scholar] [CrossRef]
- Schlosshauer, P.W.; Ellenson, L.H.; Soslow, R.A. Beta-Catenin and E-Cadherin Expression Patterns in High-Grade Endometrial Carcinoma Are Associated with Histological Subtype. Mod. Pathol. 2002, 15, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Adham, S.A.I.; Al Harrasi, I.; Al Haddabi, I.; Al Rashdi, A.; Al Sinawi, S.; Al Maniri, A.; Ba-Omar, T.; Coomber, B.L. Immunohistological Insight into the Correlation between Neuropilin-1 and Epithelial-Mesenchymal Transition Markers in Epithelial Ovarian Cancer. J. Histochem. Cytochem. 2014, 62, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Sarrió, D.; Moreno-Bueno, G.; Sánchez-Estévez, C.; Bañón-Rodríguez, I.; Hernández-Cortés, G.; Hardisson, D.; Palacios, J. Expression of Cadherins and Catenins Correlates with Distinct Histologic Types of Ovarian Carcinomas. Hum. Pathol. 2006, 37, 1042–1049. [Google Scholar] [CrossRef]
- Faleiro-Rodrigues, C.; Macedo-Pinto, I.; Pereira, D.; Ferreira, V.M.; Lopes, C.S. Association of E-Cadherin and Beta-Catenin Immunoexpression with Clinicopathologic Features in Primary Ovarian Carcinomas. Hum. Pathol. 2004, 35, 663–669. [Google Scholar] [CrossRef]
- Bhuyan, G.; Arora, R.; Ahluwalia, C.; Sharma, P. Epithelial-Mesenchymal Transition in Serous and Mucinous Epithelial Tumors of the Ovary. J. Cancer Res. 2019, 15, 1309–1315. [Google Scholar] [CrossRef]
- Wu, D.I.; Liu, L.; Ren, C.; Kong, D.; Zhang, P.; Jin, X.; Wang, T.; Zhang, G. Epithelial-Mesenchymal Interconversions and the Regulatory Function of the ZEB Family during the Development and Progression of Ovarian Cancer. Oncol. Lett. 2016, 11, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Faleiro-Rodrigues, C.; Macedo-Pinto, I.M.; Maia, S.S.; Vieira, R.H.; Lopes, C.S. Biological Relevance of E-Cadherin-Catenin Complex Proteins in Primary Epithelial Ovarian Tumours. Gynecol. Obstet. Investig. 2005, 60, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Burandt, E.; Lübbersmeyer, F.; Gorbokon, N.; Büscheck, F.; Luebke, A.M.; Menz, A.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; et al. E-Cadherin Expression in Human Tumors: A Tissue Microarray Study on 10,851 Tumors. Biomark. Res. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef] [Green Version]
- Strauss, R.; Li, Z.-Y.; Liu, Y.; Beyer, I.; Persson, J.; Sova, P.; Möller, T.; Pesonen, S.; Hemminki, A.; Hamerlik, P.; et al. Analysis of Epithelial and Mesenchymal Markers in Ovarian Cancer Reveals Phenotypic Heterogeneity and Plasticity. PLoS ONE 2011, 6, e16186. [Google Scholar] [CrossRef]
- Chirshev, E.; Hojo, N.; Bertucci, A.; Sanderman, L.; Nguyen, A.; Wang, H.; Suzuki, T.; Brito, E.; Martinez, S.R.; Castañón, C.; et al. Epithelial/Mesenchymal Heterogeneity of High-Grade Serous Ovarian Carcinoma Samples Correlates with MiRNA Let-7 Levels and Predicts Tumor Growth and Metastasis. Mol. Oncol. 2020, 14, 2796–2813. [Google Scholar] [CrossRef]
- Imai, T.; Horiuchi, A.; Wang, C.; Oka, K.; Ohira, S.; Nikaido, T.; Konishi, I. Hypoxia Attenuates the Expression of E-Cadherin via up-Regulation of SNAIL in Ovarian Carcinoma Cells. Am. J. Pathol. 2003, 163, 1437–1447. [Google Scholar] [CrossRef]
| E-Cadherin Expression | Number of Patients | Follow-Up (Months) | Median OS (Months) | Number of Deaths | p Value | HR | 95% CI | Localiza-tion | Cutoff [%] | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Preserved Reduced | 74 24 | 140 | 61 22 | <0.001 | 2.7 | 1.3–5.9 | M | 10 | [33] | |
| Preserved Reduced | 35 19 | 168 | 55 23 | 0.001 | 3.084 | 1.541–6.175 | M | 10 | [34] | |
| Preserved Reduced | 24 72 | 175 | 99 41 | 0.043 | 3.084 | 1.541–6.175 | NL | Nd | [35] | |
| Preserved Reduced | 72 126 | 180 | 0.213 | 1.245 | 0.882–1.756 | NL | Nd | [36] | ||
Preserved Reduced | 177 | 36 | Mean months 27 19 | 0.008 | M | Nd | [37] | |||
| Preserved Reduced | 14 16 | 124 | <0.001 | 9.6 | 2.1–43.6 | M + C | 1 | [38] | ||
| Weak * Moderate ** Strong *** | 53 41 6 | 160 | 0.635 (* vs. **) 0.103 (* vs. ***) 0.070 (** vs. ***) | NL | Nd | [39] | ||||
| Preserved Reduced | 36 12 | 60 | 48.8 17.9 | 0.008 | 2.82 | 1.3–6.3 | NL | <10 | [40] | |
| Preserved Reduced | 97 7 | 60 | (29%) (66%) | 0.006 | 4.83 (p = 0.014) | 1.3795–16.9259 | M | 10 | [41] | |
| Preserved Reduced | 37 12 | 200 | 0.02 | M + C | 5 | [42] | ||||
| Preserved Reduced | 60 63 | 175 | 12 (19%) 38 (63%) | 0.000 | M | 10 | [43] | |||
| Preserved Reduced | 46 35 | 180 | 52 21 | <0.001 | 1.9–5.8 | M | 10 | [44] | ||
| Preserved Reduced | 120 16 | 67 | 0.6547 | 1.147 | 0.629–2.091 | NL | 6 | [45] | ||
| Preserved Reduced | 148 | 0.691 | 1.151 1.00 | 0.575–2.307 | M | 10 | [46] | |||
Preserved Reduced | 34 10 | Mean months 79 98 | 0.491 | M | 10 | [47] | ||||
| Preserved Reduced | 39 7 | 50 36 | 0.472 | M | 10 | [48] | ||||
| Mean RFS (patients) | ||||||||||
| Preserved Reduced | 78 204 | 120 | 43 106 | 0.038 | M | 5 | [49] | |||
| Median PFS (Months) | ||||||||||
| Preserved Reduced | 74 24 | 160 | 24 9 | <0.001 | 4.3 | 2.5–7 | M | 10 | [33] | |
| Preserved Reduced | 24 72 | 175 | 38 17 | 0.007 | NL | Nd | [35] | |||
| Preserved Reduced | 177 | 36 | 23 9 | 0.000 | M | Nd | [37] | |||
| Preserved Reduced | 14 16 | 0.064 | M + C | 1 | [38] | |||||
| Weak * Moderate ** Strong *** | 53 41 6 | 160 | 0.337 (* vs. **) 0.679 (* vs. ***) 0.532 (** vs. ***) | NL | Nd | [39] | ||||
| Preserved Reduced | 42 21 | 175 | 0.001 | M | 10 | [43] | ||||
Preserved Reduced | 34 10 | Mean months 42 46 | 0.967 | M | 10 | [47] | ||||
| Preserved Reduced | 39 7 | 17 18 | 0.775 | M | 10 | [48] | ||||
| Number of All Patients | FIGO | Number of Preserved/Positive Patients (%) | Number of Reduced/Negative Patients (%) | p Value | Localization | Cutoff [%] | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 80 | I/II III/IV | ≥++ 21 (72) * 20 (39) | + 6 (21) 16 (31) | − 3 (10) 14 (28) | * <0.05 | M | 1 | [25] |
| 73 | I II III IV | 19 (70) 6 (60) 13 (39) 1 (33) | 8 (30) * 4 (40) 20 (61) 2 (67) | * 0.017 (vs. FIGO IV) | M+C | Nd | [26] | |
| 76 | I II III IV | 13 (48) 5 (50) 9 (25) 0 (0) | 14 (52) * 5 (50) 27(75) 3 (100) | * 0.027 (vs. FIGO IV) | M | Nd | [26] | |
| 74 | I II III IV | 2 (7) 1 (10) 3 (9) 0 (0) | 25 (93) 9 (90) 31 (91) 3(100) | 1.000 | C | Nd | [26] | |
| 95 | I II III IV I/II III/IV | 39 (91) 3 (100) 62 (87) 12 (71) 42 (89) 74 (84) | nd | ns | M | 10 | [32] | |
| 123 | I/II III/IV | 41 (65) 22 (37) | 0.002 | M | 10 | [43] | ||
| 282 | I II III IV | 27 (35) 10 (23) 36 (27) 5 (19) | 56 (65) 34 (77) 98 (73) 22 (81) | 0.12 | M | 5 | [49] | |
| 50 | I/II III/IV | 8 (67) 12 (32) * | 4 (33) 26 (68) * | 0.044 | NL | 10 | [51] | |
| 60 | I/II III/IV | 12 (67) 5 (12) | nd | <0.01 | NL | 25 | [52] | |
| 75 | I II III IV | 116.9 ± 86.6 * 106.1 ± 67.8 ** 101.0 ± 74.8 ** 52.7 ± 15.0 | nd | * ≤0.05 ** ≤0.01 (vs. FIGO IV) | C | Nd | [53] | |
| 300 | I/II III/IV | 80 (56) 11 (7) | 63 (44) 146 (93) | <0.001 | NL | 5 | [54] | |
| 77 | I/II III/IV | ++ 40 (83) * 17 (59) | + 8 (17) 10 (34) | − 0 2 (7) | * 0.05 | NL | 1 | [55] |
| 68 | I II III IV | ++ 10 (27) 3 (30) 9 (50) * 0 | + 19 (51) 5 (50) 6 (33) 1 (33) | − 8 (22) 2 (20) 3 (17) 2 (67) | * <0.01 | NL | Nd | [56] |
| 64 | I II III IV | nd | 5 (42) 2 (40) 12 (27) 0 | 0.63 | M | 25 | [57] | |
| 46 | I/II III/IV | 14 (70) 15 (53) | 6 (30) 13 (46) | 0.35 | NL | 10 | [58] | |
| Number of All Patients | Serous Type (FIGO) | Number of Preserved/Positive Patients (%) | Number of Reduced/Negative Patients (%) | p Value | Localization | Cutoff [%] | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| 100 | I/II III/IV | + 9 (60) 44 (52) | ++ 3 (20) 37 (45) | +++ 3 (20) 3 (3) | Nd | 0.02 | NL | Nd | [39] |
| 43 | I/II III/IV | 12 (75) 8 (30) | 4 (25) 19 (70) | 0.004 | M + C | 5 | [59] | ||
| 50 | I/II III/IV | IRS 9.1 2.3 | Nd | 0.001 | NL | 6 | [60] | ||
| 72 | I II III | 12 (70.5) 10 (43) 12 (37.5) | Nd | <0.05 | NL | 5 | [61] | ||
| Number of All Patients | Grade | Number of Preserved/Positive Patients (%) | Number of Reduced/Negative Patients (%) | p Value | Localization | Cutoff [%] | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 80 | G1/G2 G3 | ≥++ 37 (58) 5 (31) | + 15 (23) 6 (38) | − 12 (19) 5 (31) | <0.05 | M | 1 | [25] |
| 73 | low high | 14 (56) 25 (52) | 11 (44) 23 (48) | 0.808 | M + C | Nd | [26] | |
| 76 | low high | 8 (30) 19 (39) | 19 (70) 30 (61) | 0.464 | M | Nd | [26] | |
| 74 | low high | 2 (8) 4 (8) | 24 (92) 44 (92) | 1.000 | C | Nd | [26] | |
| 95 | G1 G2 G3 | 11 (100) 19 (91) 47 (78) * | nd | * 0.03 | M | 10 | [32] | |
| 54 | low high | 5 (62) 30 (65) | 3 (38) 16 (35) | 0.801 | M | 10 | [34] | |
| 123 | G1 G2 G3 | Nd | 20 (57) 35 (56.5) 8 (32) | 0.063 | M | 10 | [43] | |
| 282 | G1 G2 G3 | 14 (38) 35 (35) 29 (20) | 23 (62) * 65 (65) * 116 (80) | 0.005 (G1 vs. G3; G2 vs. G3) | M | 5 | [49] | |
| 27 | low high | 9 (33.75 ± 32.71) 18 (30.44 ± 27.42) | nd | 1.00 | M | Nd | [50] | |
| 50 | G1/G2 G3 | 15 (47) 5 (17) | 17 (53) 13 (83) | 0.186 | NL | 10 | [51] | |
| 60 | G1/G2 G3 | 13 (42) 4 (14) | nd | 0.016 | NL | 25 | [52] | |
| 75 | G1 G2 G3 | % of positive cells 120.8 ± 83.7 120.7 ± 106.5 144.2 ± 83.2 | nd | ns | C | Nd | [53] | |
| 300 | G1/G2 G3 | 74 (60) 17 (10) | 50 (40) 159 (90) | <0.001 | NL | 5 | [54] | |
| 77 | G1 G2 G3 | ++ 27 (77) 20 (77) 10 (63) | + 8 (23) 6 (23) 4 (25) | − 0 0 2 (12.5) | ns | NL | 1 | [55] |
| 64 | G1 G2 G3 | Nd | 1 (11) 2 (13) 16 (40) | 0.08 | M | 25 | [57] | |
| 46 | G1 G2 G3 | 6 (74) 13 (62) 9 (56) | 2 (26) 8 (38) 7 (44) | 0.47 | NL | 10 | [58] | |
| Number of All Patients | Serous Type (Grade) | Number of Preserved/Positive Patients (%) | Number of Reduced/Negative Patients (%) | p Value | Localization | Cutoff [%] | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| 100 | G1 (23) G2 (38) G3 (35) | + 10 (43.5) 19 (50) 23 (65) | ++ 10 (43.5) 19 (50) 11 (31) | +++ 3 (13) 0 (0) 1 (3) | Nd | 0.001 | NL | Nd | [39] |
| 43 | Low grade High grade | 13 (56.5) 7 (35) | 10 (43.5) 13 (65) | 0.158 | M + C | 5 | [59] | ||
| 50 | Low grade High grade | IRS 7.4 5.7 | Nd | 0.269 | NL | 6 | [60] | ||
| 72 | Low grade High grade | 23 (85) 11 (25) | Nd | <0.05 | NL | 5 | [61] | ||
| 52 | Low grade High grade | 20 (83) 6 (21) | Nd | 0.003 | NL | 25 | [62] | ||
| 93 | Low grade High grade | 21 (48) 12 (25) | Nd | 0.029 | NL | 25 | [63] | ||
| 452 | Low grade High grade | 17 (71) 256 (63) | Nd | 0.62 | M | 5 | [64] | ||
| Number of All Patients | Histopathological Type | Number of Preserved/Positive Patients (%) | Number of Reduced/Negative Patients (%) | p Value | Localization | Cutoff [%] | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 73 | Serous mucinous endometrioid clear cell | 9 (31) 9 (69) 11 (79) 7 (64) | 20 (69) * 4 (31) 3 (21) 4 (36) | * 0.023 | M + C | Nd | [26] | |
| 76 | Serous mucinous endometrioid clear cell | 5 (16) 6 (46) 7 (50) 6 (54.5) | 27 (84) * 7 (54) 7 (50) 5 (45.5) | * 0.028 | M | Nd | [26] | |
| 74 | Serous mucinous endometrioid clear cell | 1 (3) 2 (15) 1 (7) 2 (18) | 29 (97) 11 (85) 13 (93) 9 (82) | 0.311 | C | Nd | [26] | |
| 123 | Serous mucinous endometrioid clear cell | Nd | 28 (44) 16 (67) 6 (58) 13 (52) | 0.320 | M | 10 | [43] | |
| 282 | Serous mucinous endometrioid clear cell | 22 (22) 20 (67) 17 (23) 11 (37) | 80 (78) * 10 (33) 57 (77) ** 19 (63) | * <0.0005 ** <0.0005 | M | 5 | [49] | |
| 300 | Serous mucinous endometrioid clear cell | 52 (31) 19 (30) 16 (30) 4 (27) | 116 (69) 44 (70) 38 (70) 11 (73) | 0.982 | NL | 5 | [54] | |
| 77 | Serous mucinous endometrioid clear cell | Weak 8 0 5 5 | Strong 17 (63) 7 (100) 12 (71) 21 (81) | Nd | ns | NL | 1 | [55] |
| 68 | Serous mucinous endometrioid clear cell | Low 9 (39) 3 (43) 8 (47) 12 (57) | High 6 (26) 3 (43) $ 7 (41) 4 (19) | 8 (34) 1 (14) 2 (12) 5 (24) | $ <0.001 | NL | Nd | [56] |
| 64 | Serous mucinous endometrioid clear cell | Nd | 10 (36) 2 (20) 1 (17) 2 (50) | 0.71 | M | 25 | [57] | |
| 46 | Serous mucinous endometrioid | 7 (41) 5 (83) 6 (60) | 10 (59) 1 (17) 4 (40) | 0.36 | NL | 10 | [58] | |
| 73 | Serous mucinous endometrioid | IRS 5.29 ± 0.52 4.62 ± 1.00 5.03 ± 0.53 | Nd | ns | NL | 1 | [68] | |
| 84 | Serous mucinous endometrioid clear cell | 1 (4) 8 (61.5) # 1 (8) 5 (16) | 27 (96) * 5 (38.5) 11 (92) 26 (84) | * <0.001 # <0.001 | NL | 10 | [69] | |
| 104 | Serous mucinous endometrioid clear cell | 10 (27) 15 (40) 3 (8) 8 (22) | 46 (69) * 7 (10) 5 (8) 8 (12) | * 0.001 | M | 1 | [70] | |
| Number of Patients | E-Cad Positive Expression n(%) | p Value | IRS | p Value | Localization | Cutoff [%] | Ref |
|---|---|---|---|---|---|---|---|
| EOC = 39 BOT = 9 | 8 12 * | 0.024 | NL | 10 | [30] | ||
| EOC = 95 BOT = 23 | 79 (83) 23 (100) * | 0.05 | M | 10 | [32] | ||
| EOC = 136 BOT = 45 | 120 (88) # 7 (16) | <0.0001 | NL | 6 | [45] | ||
| EOC = 75 BOT = 23 | 124.1 ± 92.9 123.8 ± 106.8 | ns | C | Nd | [53] | ||
| EOC = 68 BOT = 14 | 52 (76) 10 (71) | ns | NL | Nd | [56] | ||
| EOC = 46 BOT = 13 | 28 (61) 6 (78) | =0.22 | NL | 10 | [58] | ||
| EOC = 63 BOT = 7 | 4.98 ± 0.68 # 2.71 ± 1.14 | ≤0.05 | NL | 1 | [68] | ||
| EOC = 30 BOT = 30 | 25 (83) 30 (100) * | <0.05 | M | 1 | [71] | ||
| EOC = 31 BOT = 12 | 1.61 ± 1.17 7.58 ± 2.97 * | <0.01 | M | 25 | [72] | ||
| EOC = 78 BOT = 17 | 25 (32) 5 (29) | =0.062 | M | 50 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielbik, M.; Szulc-Kielbik, I.; Klink, M. E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of Patients with Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 14383. https://doi.org/10.3390/ijms232214383
Kielbik M, Szulc-Kielbik I, Klink M. E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of Patients with Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2022; 23(22):14383. https://doi.org/10.3390/ijms232214383
Chicago/Turabian StyleKielbik, Michal, Izabela Szulc-Kielbik, and Magdalena Klink. 2022. "E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of Patients with Epithelial Ovarian Cancer" International Journal of Molecular Sciences 23, no. 22: 14383. https://doi.org/10.3390/ijms232214383






