The Hidden Secrets of the Dental Calculus: Calibration of a Mass Spectrometry Protocol for Dental Calculus Protein Analysis
Abstract
1. Introduction
1.1. Mass Spectrometry (MS) for Quantitative Proteomics Analyses
1.2. Limitations
2. Results
2.1. Decalcification Optimization
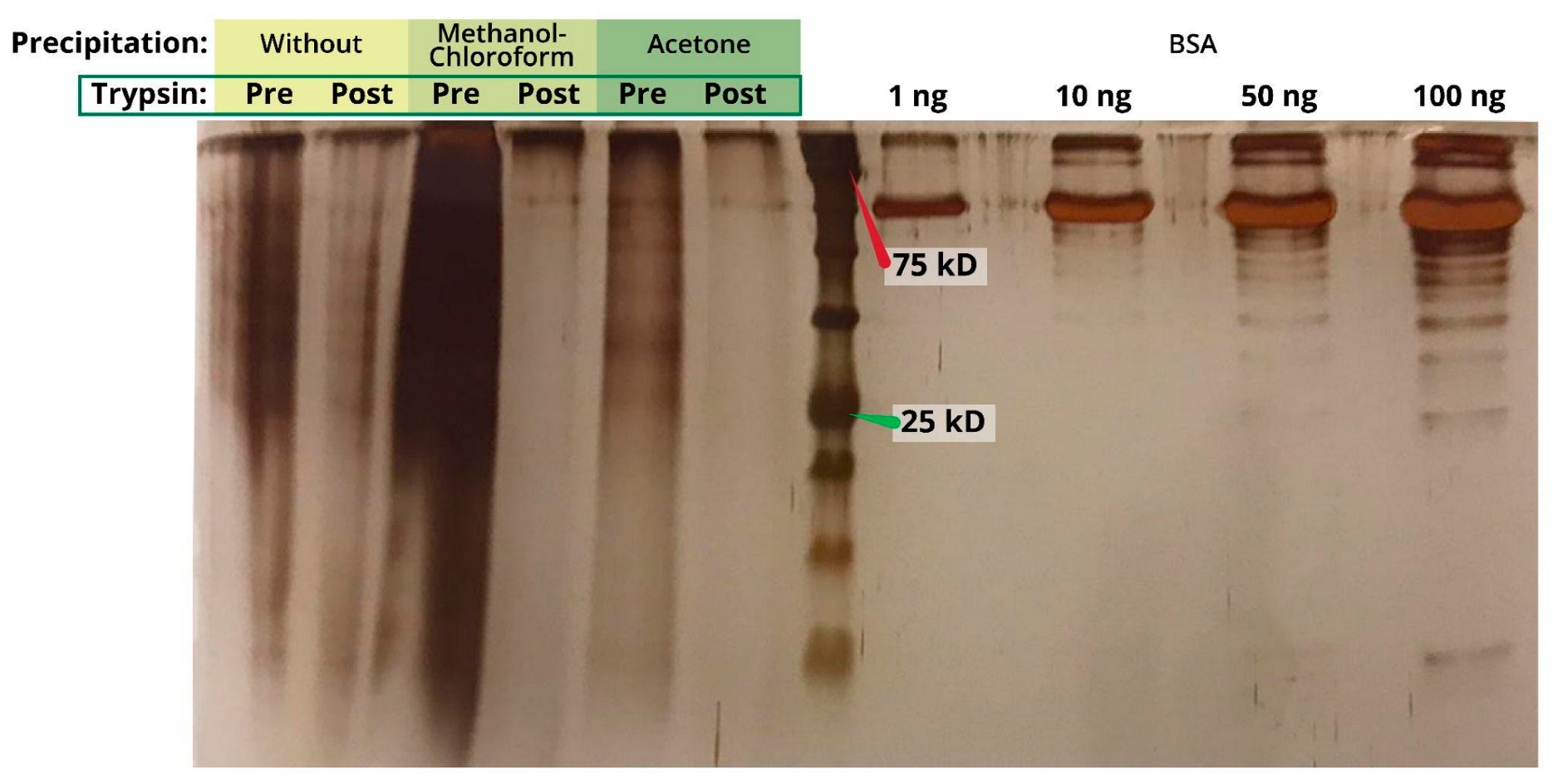
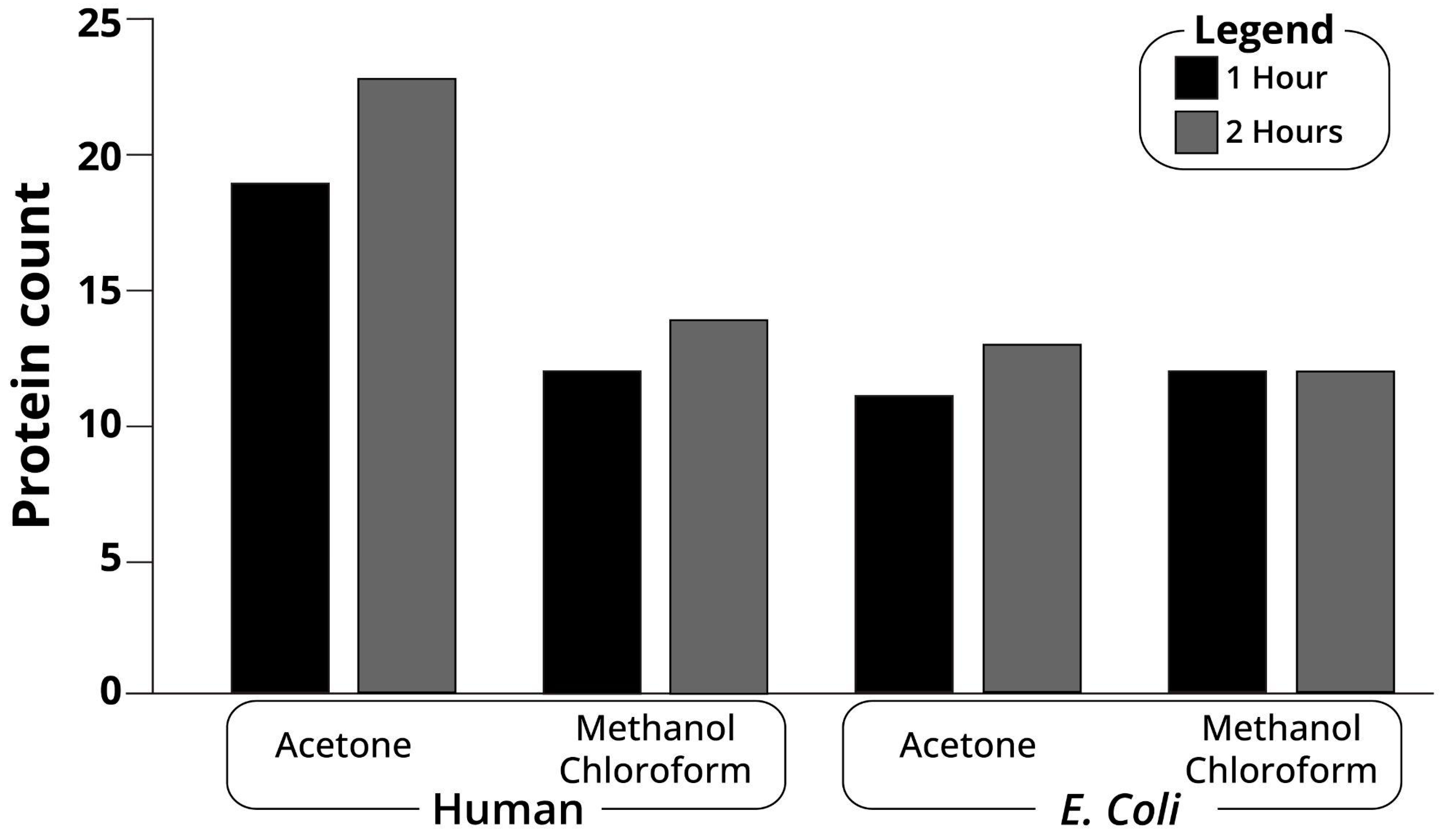
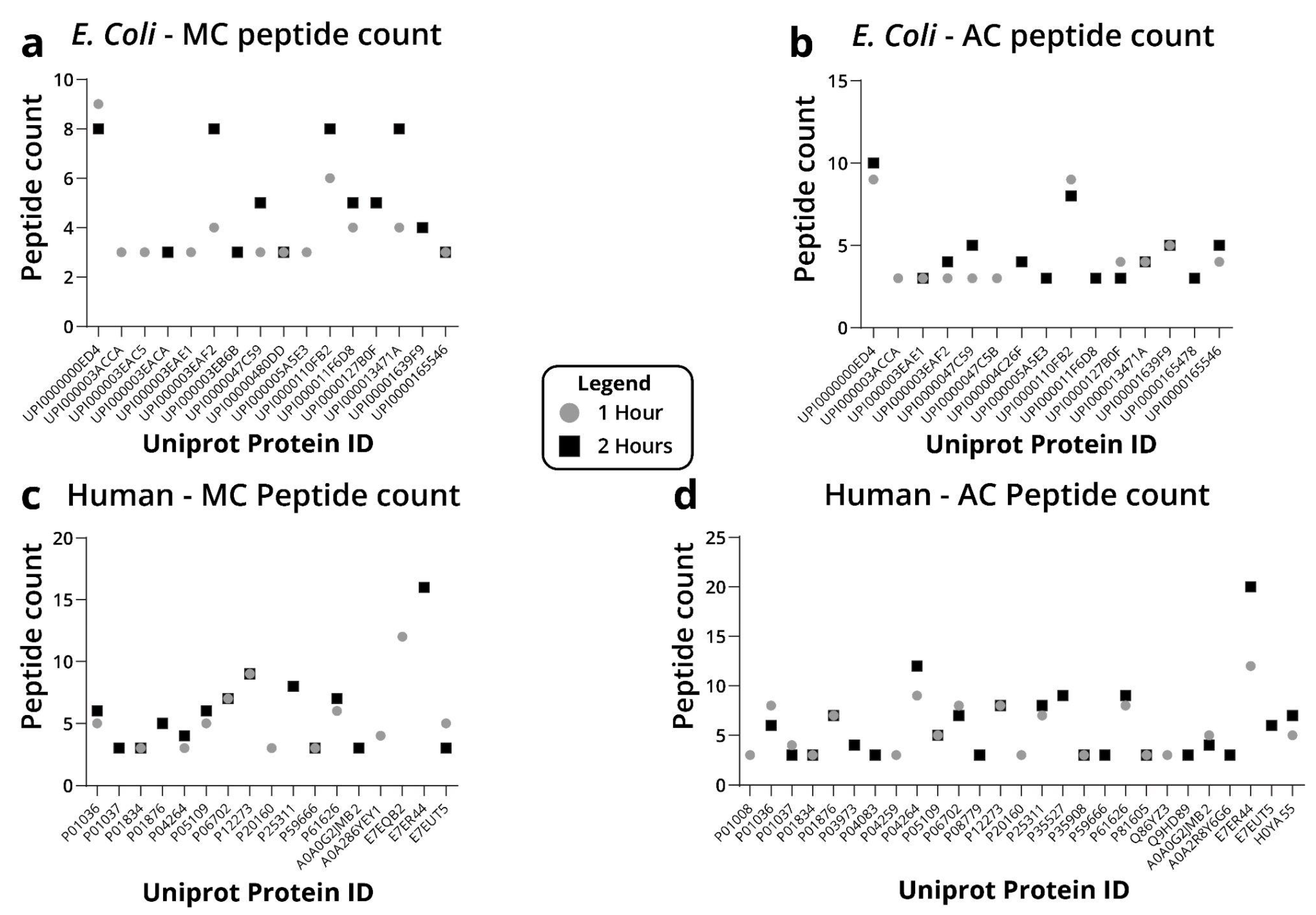
2.2. Protein Precipitation
2.3. Gradient Time Optimization
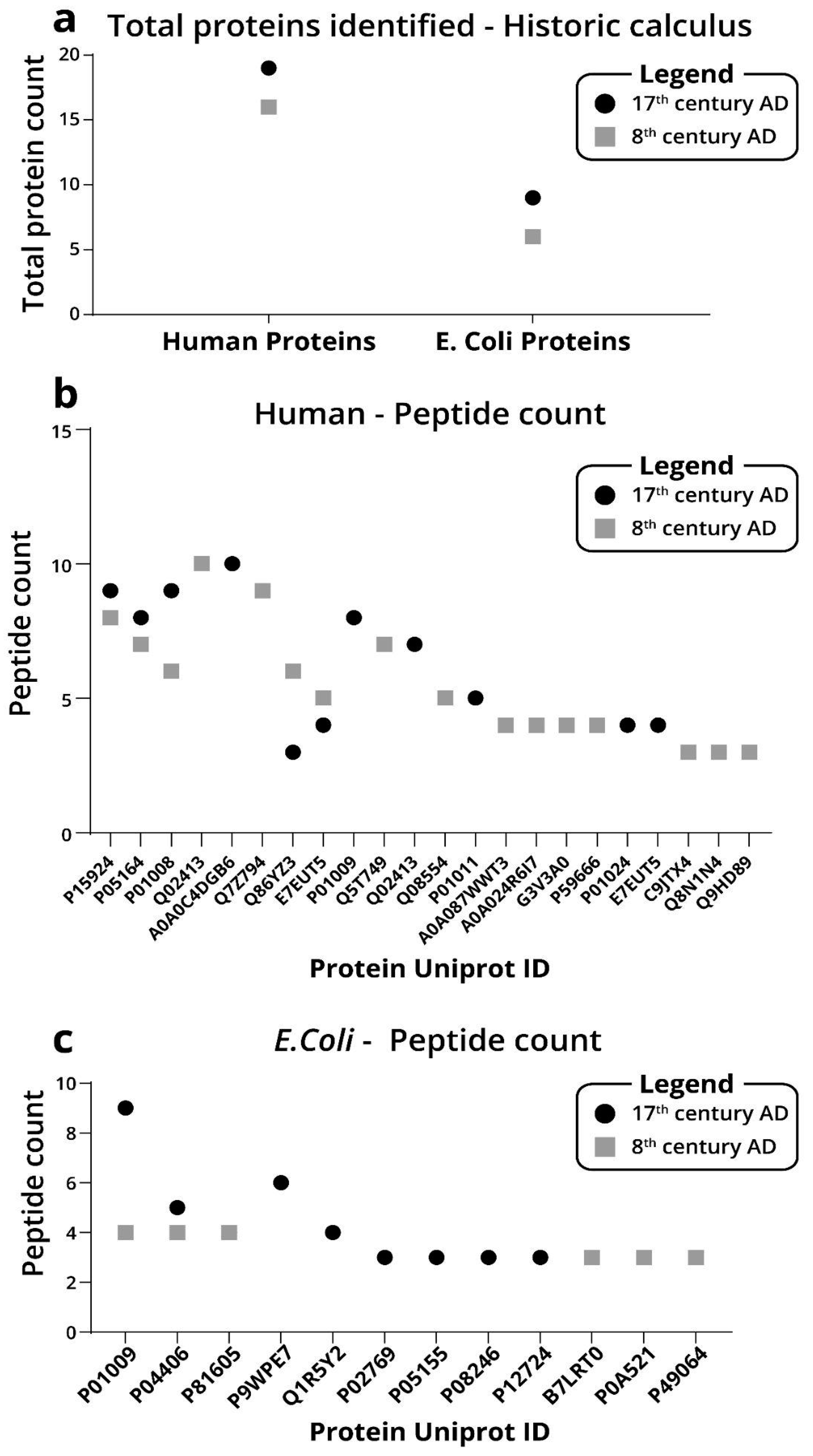
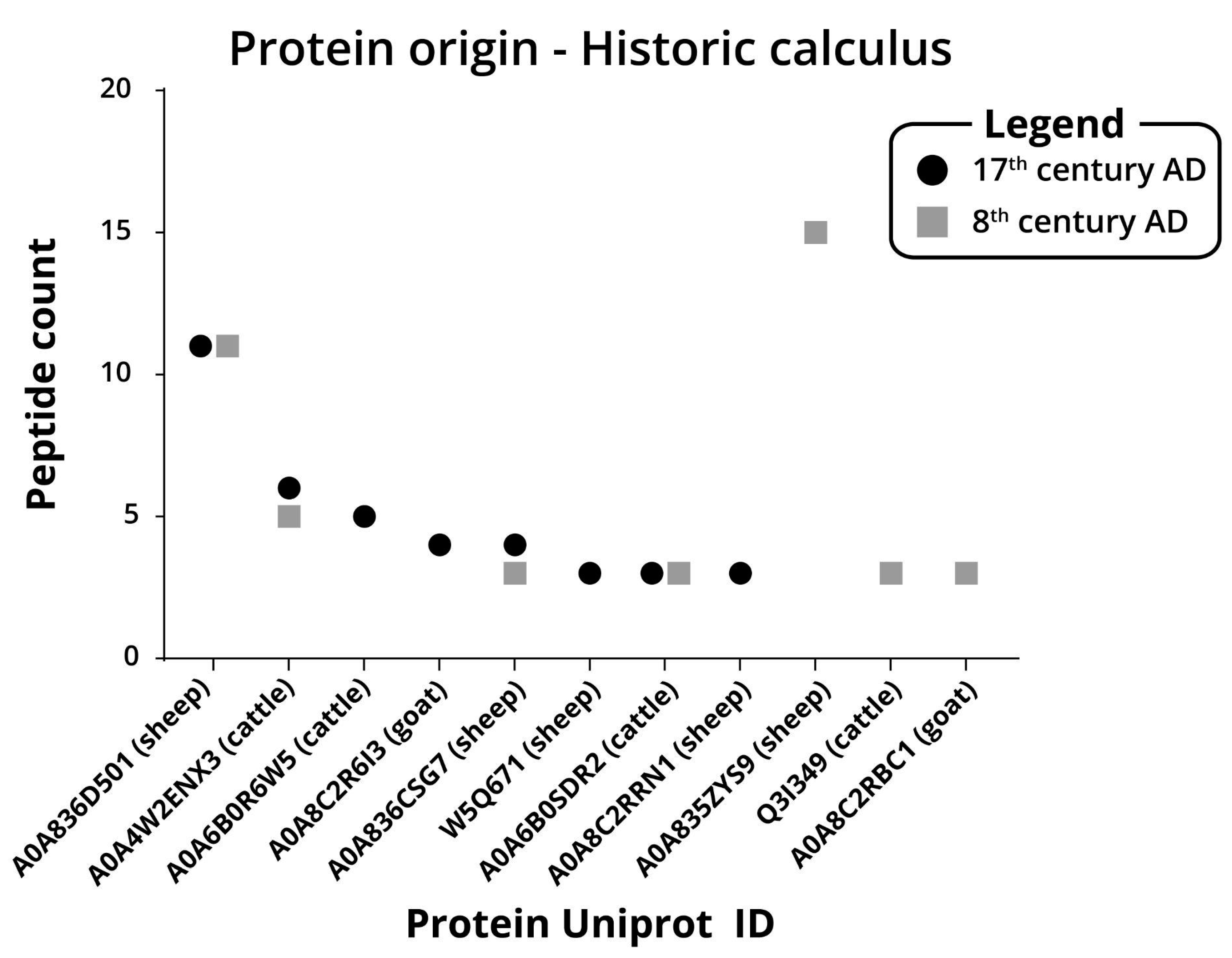
2.4. Archaeological Dental Calculus Analysis
3. Discussion
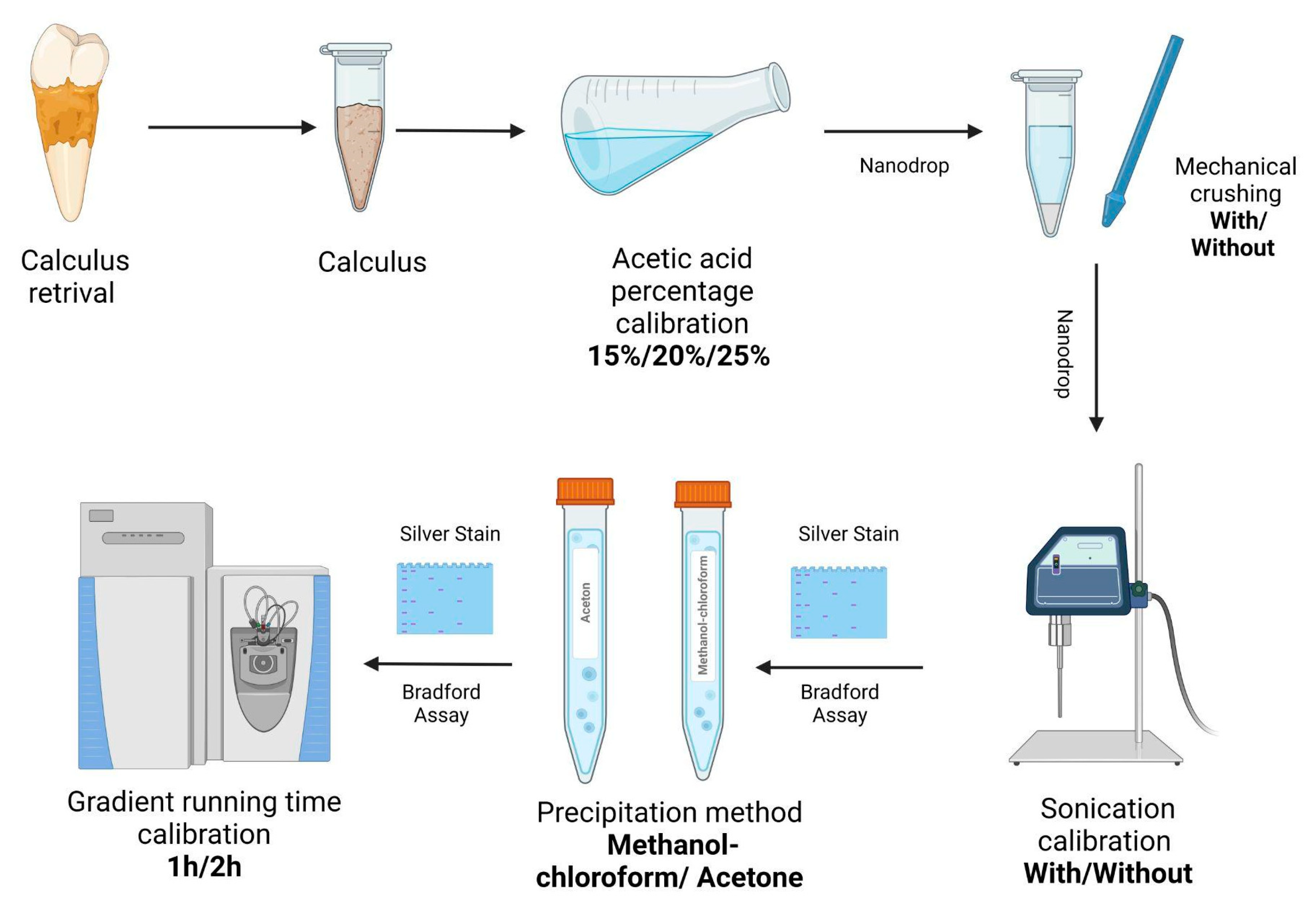
4. Material and Methods
4.1. Sample Preparation
4.2. Decalcification
4.3. Residual Protein Loss during Decalcification
4.4. Protein Precipitation
4.4.1. Methanol-Chloroform
4.4.2. Acetone
4.5. Protein Processing
4.6. Solid Phase Extraction Stage Tips for Detergent Removal
4.7. MS Analysis
4.8. Silver Stain
4.9. Archeological Dental Calculus Samples
4.10. Bioinformatic Analysis
- Homo Sapiens (Human)—proteome ID UP000005640.
- Escherichia Coli—proteome ID UP000036496.
- Bovidae—proteome ID UP000009895.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, D.J. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of su-pragingival and subgingival deposits. Eur. J. Oral Sci. 1997, 105, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G. A survey of the oral health of Navajo Indian children. Arch. Oral Biol. 1960, 1, 193–205. [Google Scholar] [CrossRef]
- Jin, Y.; Yip, H.-K. Supragingival Calculus: Formation and Control. Crit. Rev. Oral Biol. Med. 2002, 13, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Friskopp, J.; Isacsson, G. A quantitative microradiographic study of mineral content of supragingival and subgingival dental calculus. Scand. J. Dent. Res. 1984, 92, 25–32. [Google Scholar] [CrossRef]
- Fagernäs, Z.; García-Collado, M.I.; Hendy, J.; Hofman, C.A.; Speller, C.; Velsko, I.; Warinner, C. A unified protocol for simultaneous extraction of DNA and proteins from archaeological dental calculus. J. Archaeol. Sci. 2020, 118, 105135. [Google Scholar] [CrossRef]
- Ziesemer, K.A.; Ramos-Madrigal, J.; Mann, A.E.; Brandt, B.W.; Sankaranarayanan, K.; Ozga, A.T.; Hoogland, M.; Hofman, C.A.; Salazar-García, D.C.; Frohlich, B.; et al. The efficacy of whole human genome capture on ancient dental calculus and dentin. Am. J. Phys. Anthr. 2018, 168, 496–509. [Google Scholar] [CrossRef]
- Velsko, I.M.; Yates, J.A.F.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef]
- Hendy, J.; Warinner, C.; Bouwman, A.; Collins, M.J.; Fiddyment, S.; Fischer, R.; Hagan, R.; Hofman, C.A.; Holst, M.; Chaves, E.; et al. Proteomic evidence of dietary sources in ancient dental calculus. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180977. [Google Scholar] [CrossRef]
- Gismondi, A.; D’Agostino, A.; Canuti, L.; Di Marco, G.; Martínez-Labarga, C.; Angle, M.; Rickards, O.; Canini, A. Dental calculus reveals diet habits and medicinal plant use in the Early Medieval Italian population of Colonna. J. Archaeol. Sci. Rep. 2018, 20, 556–564. [Google Scholar] [CrossRef]
- Radini, A.; Tromp, M.; Beach, A.; Tong, E.; Speller, C.; McCormick, M.; Dudgeon, J.V.; Collins, M.J.; Rühli, F.; Kröger, R.; et al. Medieval women’s early involvement in manuscript production suggested by lapis lazuli identification in dental calculus. Sci. Adv. 2019, 5, eaau7126. [Google Scholar] [CrossRef]
- Molnar, S. Human tooth wear, tooth function and cultural variability. Am. J. Phys. Anthr. 1971, 34, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.G.; Brooks, A.S.; Piperno, D.R. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium). Proc. Natl. Acad. Sci. USA 2011, 108, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Geber, J.; Tromp, M.; Scott, A.; Bouwman, A.; Nanni, P.; Grossmann, J.; Hendy, J.; Warinner, C. Relief food subsistence revealed by microparticle and proteomic analyses of dental calculus from victims of the Great Irish Famine. Proc. Natl. Acad. Sci. USA 2019, 116, 19380–19385. [Google Scholar] [CrossRef] [PubMed]
- Warinner, C.; Rodrigues, J.F.M.; Vyas, R.; Trachsel, C.; Shved, N.; Grossmann, J.; Radini, A.; Hancock, Y.; Tito, R.Y.; Fiddyment, S.; et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 2014, 46, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Akcalı, A.; Lang, N.P. Dental calculus: The calcified biofilm and its role in disease development. Periodontology 2000 2018, 76, 109–115. [Google Scholar] [CrossRef]
- Kenward, H.; Hall, A. Environmental Archaeology Unit Reports. Available online: https://www.york.ac.uk/inst/chumpal/EAU-reps/eaureps-web.htm (accessed on 16 November 2022).
- Jersie-Christensen, R.R.; Lanigan, L.T.; Lyon, D.; Mackie, M.; Belstrøm, D.; Kelstrup, C.D.; Fotakis, A.K.; Willerslev, E.; Lynnerup, N.; Jensen, L.J.; et al. Quantitative metaproteomics of medieval dental calculus reveals individual oral health status. Nat. Commun. 2018, 9, 4744. [Google Scholar] [CrossRef]
- Hardy, K.; Blakeney, T.; Copeland, L.; Kirkham, J.; Wrangham, R.; Collins, M. Starch granules, dental calculus and new perspectives on ancient diet. J. Archaeol. Sci. 2009, 36, 248–255. [Google Scholar] [CrossRef]
- Bleasdale, M.; Richter, K.K.; Janzen, A.; Brown, S. Ancient proteins provide evidence of dairy consumption in eastern Africa. Nature 2021, 12, 632. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, X.; Zhang, K. Cadmium Profiles in Dental Calculus: A Cross-Sectional Population-Based Study in Hunan Province of China. Biol. Trace Elem. Res. 2018, 185, 63–70. [Google Scholar] [CrossRef]
- Yaprak, E.; Yolcubal, I.; Sinanoğlu, A.; Doğrul-Demiray, A.; Guzeldemir-Akcakanat, E.; Marakoğlu, I. High levels of heavy metal accumulation in dental calculus of smokers: A pilot inductively coupled plasma mass spectrometry study. J. Periodontal Res. 2017, 52, 83–88. [Google Scholar] [CrossRef]
- Gismondi, A.; Baldoni, M.; Gnes, M.; Scorrano, G.; D’Agostino, A.; Di Marco, G.; Calabria, G.; Petrucci, M.; Müldner, G.; Von Tersch, M.; et al. A multidisciplinary approach for investigating dietary and medicinal habits of the Medieval population of Santa Severa (7th–15th centuries, Rome, Italy). PLoS ONE 2020, 15, e0227433. [Google Scholar] [CrossRef] [PubMed]

| Acetic Acid Concentration | Protein Concentration mg/mL before Mechanical Crushing | Protein Concentration mg/mL after Mechanical Crushing |
|---|---|---|
| 20% | 0.13 | 0.23 |
| 25% | 0.23 | 0.27 |
| Acetic Acid Concentration | Protein Concentration μg/mL before Precipitation | Protein Concentration μg/mL after Acetone Precipitation | Protein Concentration μg/mL after Methanol—Chloroform Precipitation |
|---|---|---|---|
| 20% | 134 | 130 | 126 |
| 25% | 154 | 138 | 141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, O.; Megreli, D.; Gavish, T.; Meyrom, N.; Zamir, N.; May, H.; Sarig, R.; Bar, D.Z. The Hidden Secrets of the Dental Calculus: Calibration of a Mass Spectrometry Protocol for Dental Calculus Protein Analysis. Int. J. Mol. Sci. 2022, 23, 14387. https://doi.org/10.3390/ijms232214387
Bender O, Megreli D, Gavish T, Meyrom N, Zamir N, May H, Sarig R, Bar DZ. The Hidden Secrets of the Dental Calculus: Calibration of a Mass Spectrometry Protocol for Dental Calculus Protein Analysis. International Journal of Molecular Sciences. 2022; 23(22):14387. https://doi.org/10.3390/ijms232214387
Chicago/Turabian StyleBender, Omer, Dana Megreli, Talia Gavish, Noa Meyrom, Neta Zamir, Hila May, Rachel Sarig, and Daniel Z. Bar. 2022. "The Hidden Secrets of the Dental Calculus: Calibration of a Mass Spectrometry Protocol for Dental Calculus Protein Analysis" International Journal of Molecular Sciences 23, no. 22: 14387. https://doi.org/10.3390/ijms232214387
APA StyleBender, O., Megreli, D., Gavish, T., Meyrom, N., Zamir, N., May, H., Sarig, R., & Bar, D. Z. (2022). The Hidden Secrets of the Dental Calculus: Calibration of a Mass Spectrometry Protocol for Dental Calculus Protein Analysis. International Journal of Molecular Sciences, 23(22), 14387. https://doi.org/10.3390/ijms232214387






