MR1- and HLA-E-Dependent Antigen Presentation of Mycobacterium tuberculosis
Abstract
1. Introduction
2. MR1 and MR1-Restricted T Cells
3. MR1 Ligands and Antigens
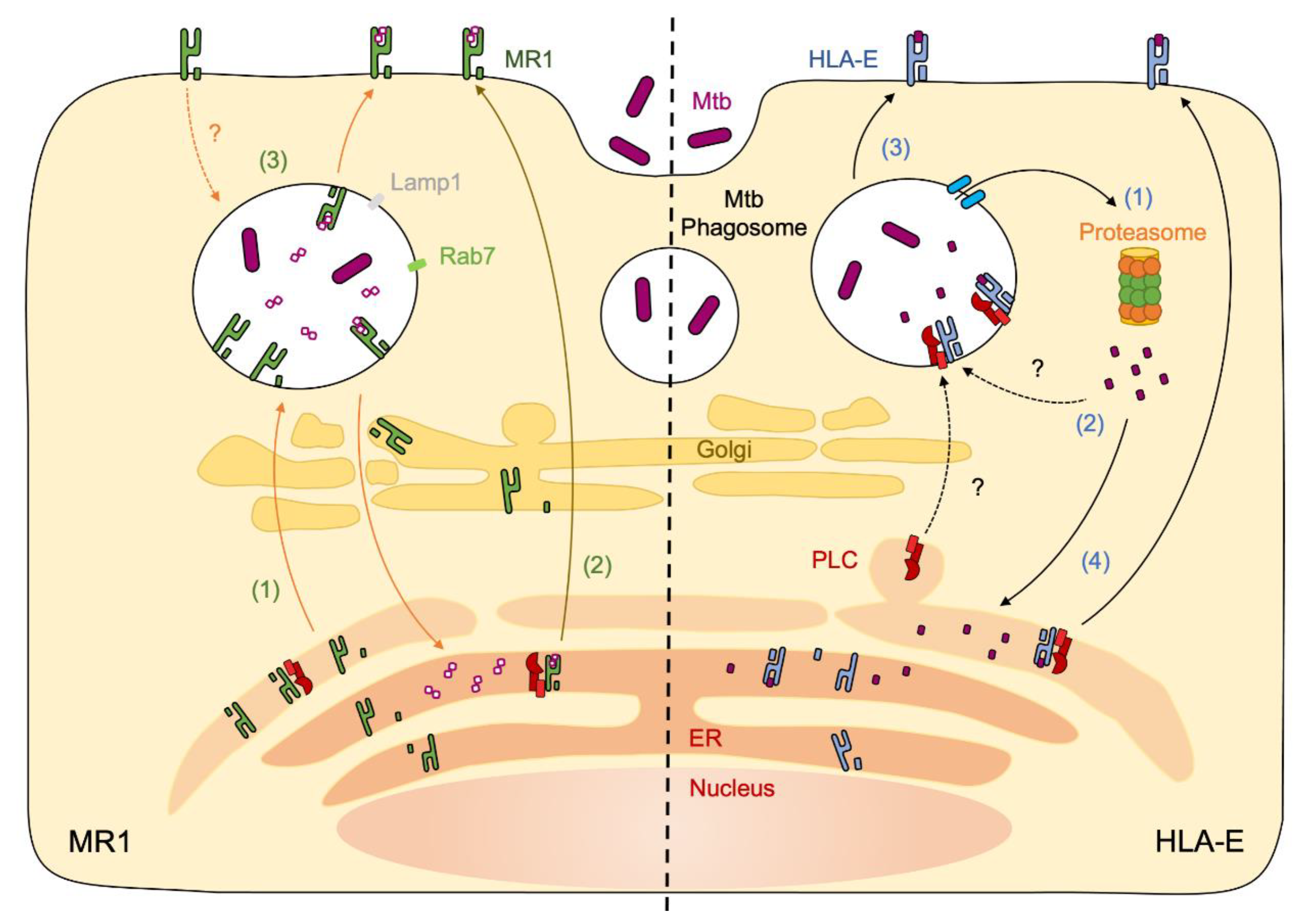
4. MR1 Antigen Presentation of Mycobacteria
5. Clinical Implications of MR1 and Mycobacterial Infections
6. HLA-E and HLA-E-Restricted T Cells
7. HLA-E Antigen Presentation of Mycobacteria
8. Clinical Implications of HLA-E and Mycobacterial Infections
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019, 393, 1331–1384. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Goldstein, M.M.; Triebold, K.J.; Koller, B.; Bloom, B.R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 1992, 89, 12013–12017. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Dascher, C.C.; Grusby, M.J.; Wang, C.R.; Brenner, M.B. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 1999, 189, 1973–1980. [Google Scholar] [CrossRef]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.; Kumararatne, D.; Casanova, J.L. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 1998, 19, 491–494. [Google Scholar] [CrossRef]
- Joosten, S.A.; Ottenhoff, T.H.M.; Lewinsohn, D.M.; Hoft, D.F.; Moody, D.B.; Seshadri, C.; Collaboration for Tuberculosis Vaccine Discovery - Donor-Unrestricted T-cells Working Group, B.; Melinda Gates, F. Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine 2019, 37, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.O.; Mazzaccaro, R.J.; Russell, R.G.; Lee, F.K.; Turner, O.C.; Hong, S.; Van Kaer, L.; Bloom, B.R. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 4204–4208. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, D.M.; Briden, A.L.; Reed, S.G.; Grabstein, K.H.; Alderson, M.R. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: The relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 2000, 165, 925–930. [Google Scholar] [CrossRef]
- Van Rhijn, I.; Moody, D.B. CD1 and mycobacterial lipids activate human T cells. Immunol. Rev. 2015, 264, 138–153. [Google Scholar] [CrossRef]
- Gay, L.; Mezouar, S.; Cano, C.; Frohna, P.; Madakamutil, L.; Mege, J.L.; Olive, D. Role of Vgamma9vdelta2 T lymphocytes in infectious diseases. Front. Immunol. 2022, 13, 928441. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hirai, M.; Kurosawa, Y. A gene outside the human MHC related to classical HLA class I genes. Science 1995, 269, 693–695. [Google Scholar] [CrossRef]
- Morandi, F.; Pistoia, V. Interactions between HLA-G and HLA-E in Physiological and Pathological Conditions. Front. Immunol. 2014, 5, 394. [Google Scholar] [CrossRef]
- Riegert, P.; Wanner, V.; Bahram, S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J. Immunol. 1998, 161, 4066–4077. [Google Scholar]
- Yamaguchi, H.; Hashimoto, K. Association of MR1 protein, an MHC class I-related molecule, with beta(2)-microglobulin. Biochem. Biophys. Res. Commun. 2002, 290, 722–729. [Google Scholar] [CrossRef]
- Miley, M.J.; Truscott, S.M.; Yu, Y.Y.; Gilfillan, S.; Fremont, D.H.; Hansen, T.H.; Lybarger, L. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J. Immunol. 2003, 170, 6090–6098. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.J.; Yu, Y.Y.; et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Martin, E.; Peguillet, I.; Guihot, A.; Froux, N.; Core, M.; Levy, E.; Dusseaux, M.; Meyssonnier, V.; Premel, V.; et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gilfillan, S.; Kim, S.; Thompson, B.; Wang, X.; Sant, A.J.; Fremont, D.H.; Lantz, O.; Hansen, T.H. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J. Exp. Med. 2008, 205, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.J.; Eckle, S.B.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef]
- Legoux, F.; Bellet, D.; Daviaud, C.; El Morr, Y.; Darbois, A.; Niort, K.; Procopio, E.; Salou, M.; Gilet, J.; Ryffel, B.; et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 2019, 366, 494–499. [Google Scholar] [CrossRef]
- Eckle, S.B.; Birkinshaw, R.W.; Kostenko, L.; Corbett, A.J.; McWilliam, H.E.; Reantragoon, R.; Chen, Z.; Gherardin, N.A.; Beddoe, T.; Liu, L.; et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 2014, 211, 1585–1600. [Google Scholar] [CrossRef]
- Gold, M.C.; McLaren, J.E.; Reistetter, J.A.; Smyk-Pearson, S.; Ladell, K.; Swarbrick, G.M.; Yu, Y.Y.; Hansen, T.H.; Lund, O.; Nielsen, M.; et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J. Exp. Med. 2014, 211, 1601–1610. [Google Scholar] [CrossRef]
- Gherardin, N.A.; Keller, A.N.; Woolley, R.E.; Le Nours, J.; Ritchie, D.S.; Neeson, P.J.; Birkinshaw, R.W.; Eckle, S.B.G.; Waddington, J.N.; Liu, L.; et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity 2016, 44, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Meermeier, E.W.; Laugel, B.F.; Sewell, A.K.; Corbett, A.J.; Rossjohn, J.; McCluskey, J.; Harriff, M.J.; Franks, T.; Gold, M.C.; Lewinsohn, D.M. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat. Commun. 2016, 7, 12506. [Google Scholar] [CrossRef] [PubMed]
- Harriff, M.J.; McMurtrey, C.; Froyd, C.A.; Jin, H.; Cansler, M.; Null, M.; Worley, A.; Meermeier, E.W.; Swarbrick, G.; Nilsen, A.; et al. MR1 displays the microbial metabolome driving selective MR1-restricted T cell receptor usage. Sci. Immunol. 2018, 3, eaao2556. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.N.; Eckle, S.B.; Xu, W.; Liu, L.; Hughes, V.A.; Mak, J.Y.; Meehan, B.S.; Pediongco, T.; Birkinshaw, R.W.; Chen, Z.; et al. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat. Immunol. 2017, 18, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M. Regulation of the Cell Biology of Antigen Cross-Presentation. Annu. Rev. Immunol. 2018, 36, 717–753. [Google Scholar] [CrossRef]
- Kulicke, C.; Karamooz, E.; Lewinsohn, D.; Harriff, M. Covering All the Bases: Complementary MR1 Antigen Presentation Pathways Sample Diverse Antigens and Intracellular Compartments. Front. Immunol. 2020, 11, 2034. [Google Scholar] [CrossRef]
- McWilliam, H.E.; Eckle, S.B.; Theodossis, A.; Liu, L.; Chen, Z.; Wubben, J.M.; Fairlie, D.P.; Strugnell, R.A.; Mintern, J.D.; McCluskey, J.; et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat. Immunol. 2016, 17, 531–537. [Google Scholar] [CrossRef]
- McWilliam, H.E.G.; Mak, J.Y.W.; Awad, W.; Zorkau, M.; Cruz-Gomez, S.; Lim, H.J.; Yan, Y.; Wormald, S.; Dagley, L.F.; Eckle, S.B.G.; et al. Endoplasmic reticulum chaperones stabilize ligand-receptive MR1 molecules for efficient presentation of metabolite antigens. Proc. Natl. Acad. Sci. USA 2020, 117, 24974–24985. [Google Scholar] [CrossRef]
- Harriff, M.J.; Cansler, M.E.; Toren, K.G.; Canfield, E.T.; Kwak, S.; Gold, M.C.; Lewinsohn, D.M. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS ONE 2014, 9, e97515. [Google Scholar] [CrossRef]
- Harriff, M.J.; Karamooz, E.; Burr, A.; Grant, W.F.; Canfield, E.T.; Sorensen, M.L.; Moita, L.F.; Lewinsohn, D.M. Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells. PLoS Pathog. 2016, 12, e1005524. [Google Scholar] [CrossRef]
- Huber, M.E.; Kurapova, R.; Heisler, C.M.; Karamooz, E.; Tafesse, F.G.; Harriff, M.J. Rab6 regulates recycling and retrograde trafficking of MR1 molecules. Sci. Rep. 2020, 10, 20778. [Google Scholar] [CrossRef] [PubMed]
- Karamooz, E.; Harriff, M.J.; Narayanan, G.A.; Worley, A.; Lewinsohn, D.M. MR1 recycling and blockade of endosomal trafficking reveal distinguishable antigen presentation pathways between Mycobacterium tuberculosis infection and exogenously delivered antigens. Sci. Rep. 2019, 9, 4797. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.B.; Gold, M.C.; Meermeier, E.W.; Xulu, B.Z.; Khuzwayo, S.; Sullivan, Z.A.; Mahyari, E.; Rogers, Z.; Kloverpris, H.; Sharma, P.K.; et al. TRAV1-2+ CD8+ T-cells including oligoconal expansions of MAIT cells are enriched in the airways in human tuberculosis. Commun. Biol. 2019, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.D.; Sallin, M.A.; Hoft, S.G.; Sakai, S.; Moore, R.; Wilder-Kofie, T.; Moore, I.N.; Sette, A.; Arlehamn, C.S.L.; Barber, D.L. Limited Pulmonary Mucosal-Associated Invariant T Cell Accumulation and Activation during Mycobacterium tuberculosis Infection in Rhesus Macaques. Infect. Immun. 2018, 86, e00431-18. [Google Scholar] [CrossRef]
- Seshadri, C.; Thuong, N.T.; Mai, N.T.; Bang, N.D.; Chau, T.T.; Lewinsohn, D.M.; Thwaites, G.E.; Dunstan, S.J.; Hawn, T.R. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 2017, 18, 8–14. [Google Scholar] [CrossRef]
- Howson, L.J.; Awad, W.; von Borstel, A.; Lim, H.J.; McWilliam, H.E.G.; Sandoval-Romero, M.L.; Majumdar, S.; Hamzeh, A.R.; Andrews, T.D.; McDermott, D.H.; et al. Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci. Immunol. 2020, 5, eabc9492. [Google Scholar] [CrossRef]
- Yu, H.; Yang, A.; Derrick, S.; Mak, J.Y.W.; Liu, L.; Fairlie, D.P.; Cowley, S. Artificially induced MAIT cells inhibit M. bovis BCG but not M. tuberculosis during in vivo pulmonary infection. Sci. Rep. 2020, 10, 13579. [Google Scholar]
- Vorkas, C.K.; Levy, O.; Skular, M.; Li, K.; Aube, J.; Glickman, M.S. Efficient 5-OP-RU-Induced Enrichment of Mucosa-Associated Invariant T Cells in the Murine Lung Does Not Enhance Control of Aerosol Mycobacterium tuberculosis Infection. Infect. Immun. 2020, 89, e00524-20. [Google Scholar] [CrossRef]
- Sakai, S.; Kauffman, K.D.; Oh, S.; Nelson, C.E.; Barry, C.E.; Barber, D.L. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021, 14, 199–208. [Google Scholar] [CrossRef]
- Gela, A.; Murphy, M.; Rodo, M.; Hadley, K.; Hanekom, W.A.; Boom, W.H.; Johnson, J.L.; Hoft, D.F.; Joosten, S.A.; Ottenhoff, T.H.M.; et al. Effects of BCG vaccination on donor unrestricted T cells in two prospective cohort studies. EBioMedicine 2022, 76, 103839. [Google Scholar] [CrossRef]
- Grimsley, C.; Ober, C. Population genetic studies of HLA-E: Evidence for selection. Hum. Immunol. 1997, 52, 33–40. [Google Scholar] [CrossRef]
- Strong, R.K.; Holmes, M.A.; Li, P.; Braun, L.; Lee, N.; Geraghty, D.E. HLA-E allelic variants. Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J. Biol. Chem. 2003, 278, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; Lopez-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef]
- Voogd, L.; Ruibal, P.; Ottenhoff, T.H.M.; Joosten, S.A. Antigen presentation by MHC-E: A putative target for vaccination? Trends Immunol. 2022, 43, 355–365. [Google Scholar] [CrossRef]
- Heinzel, A.S.; Grotzke, J.E.; Lines, R.A.; Lewinsohn, D.A.; McNabb, A.L.; Streblow, D.N.; Braud, V.M.; Grieser, H.J.; Belisle, J.T.; Lewinsohn, D.M. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J. Exp. Med. 2002, 196, 1473–1481. [Google Scholar] [CrossRef]
- Joosten, S.A.; van Meijgaarden, K.E.; van Weeren, P.C.; Kazi, F.; Geluk, A.; Savage, N.D.; Drijfhout, J.W.; Flower, D.R.; Hanekom, W.A.; Klein, M.R.; et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog. 2010, 6, e1000782. [Google Scholar] [CrossRef]
- van Meijgaarden, K.E.; Haks, M.C.; Caccamo, N.; Dieli, F.; Ottenhoff, T.H.; Joosten, S.A. Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS Pathog. 2015, 11, e1004671. [Google Scholar] [CrossRef]
- Caccamo, N.; Pietra, G.; Sullivan, L.C.; Brooks, A.G.; Prezzemolo, T.; La Manna, M.P.; Di Liberto, D.; Joosten, S.A.; van Meijgaarden, K.E.; Di Carlo, P.; et al. Human CD8 T lymphocytes recognize Mycobacterium tuberculosis antigens presented by HLA-E during active tuberculosis and express type 2 cytokines. Eur. J. Immunol. 2015, 45, 1069–1081. [Google Scholar] [CrossRef]
- Prezzemolo, T.; van Meijgaarden, K.E.; Franken, K.; Caccamo, N.; Dieli, F.; Ottenhoff, T.H.M.; Joosten, S.A. Detailed characterization of human Mycobacterium tuberculosis specific HLA-E restricted CD8+ T cells. Eur. J. Immunol. 2018, 48, 293–305. [Google Scholar] [CrossRef]
- Braud, V.; Jones, E.Y.; McMichael, A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 1997, 27, 1164–1169. [Google Scholar] [CrossRef]
- O’Callaghan, C.A.; Tormo, J.; Willcox, B.E.; Braud, V.M.; Jakobsen, B.K.; Stuart, D.I.; McMichael, A.J.; Bell, J.I.; Jones, E.Y. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol. Cell 1998, 1, 531–541. [Google Scholar] [CrossRef]
- Hoare, H.L.; Sullivan, L.C.; Clements, C.S.; Ely, L.K.; Beddoe, T.; Henderson, K.N.; Lin, J.; Reid, H.H.; Brooks, A.G.; Rossjohn, J. Subtle changes in peptide conformation profoundly affect recognition of the non-classical MHC class I molecule HLA-E by the CD94-NKG2 natural killer cell receptors. J. Mol. Biol. 2008, 377, 1297–1303. [Google Scholar] [CrossRef]
- McMurtrey, C.; Harriff, M.J.; Swarbrick, G.M.; Duncan, A.; Cansler, M.; Null, M.; Bardet, W.; Jackson, K.W.; Lewinsohn, D.A.; Hildebrand, W.; et al. T cell recognition of Mycobacterium tuberculosis peptides presented by HLA-E derived from infected human cells. PLoS ONE 2017, 12, e0188288. [Google Scholar] [CrossRef]
- Harriff, M.J.; Wolfe, L.M.; Swarbrick, G.; Null, M.; Cansler, M.E.; Canfield, E.T.; Vogt, T.; Toren, K.G.; Li, W.; Jackson, M.; et al. HLA-E Presents Glycopeptides from the Mycobacterium tuberculosis Protein MPT32 to Human CD8+ T cells. Sci. Rep. 2017, 7, 4622. [Google Scholar] [CrossRef]
- Ruibal, P.; Franken, K.; van Meijgaarden, K.E.; van Wolfswinkel, M.; Derksen, I.; Scheeren, F.A.; Janssen, G.M.C.; van Veelen, P.A.; Sarfas, C.; White, A.D.; et al. Identification of HLA-E Binding Mycobacterium tuberculosis-Derived Epitopes through Improved Prediction Models. J. Immunol. 2022, 209, 1555–1565. [Google Scholar] [CrossRef]
- Walters, L.C.; Harlos, K.; Brackenridge, S.; Rozbesky, D.; Barrett, J.R.; Jain, V.; Walter, T.S.; O’Callaghan, C.A.; Borrow, P.; Toebes, M.; et al. Pathogen-derived HLA-E bound epitopes reveal broad primary anchor pocket tolerability and conformationally malleable peptide binding. Nat. Commun. 2018, 9, 3137. [Google Scholar] [CrossRef]
- Walters, L.C.; McMichael, A.J.; Gillespie, G.M. Detailed and atypical HLA-E peptide binding motifs revealed by a novel peptide exchange binding assay. Eur. J. Immunol. 2020, 50, 2075–2091. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; Wilson, D.; McMichael, A.J. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr. Biol. 1998, 8, 1–10. [Google Scholar] [CrossRef]
- Camilli, G.; Cassotta, A.; Battella, S.; Palmieri, G.; Santoni, A.; Paladini, F.; Fiorillo, M.T.; Sorrentino, R. Regulation and trafficking of the HLA-E molecules during monocyte-macrophage differentiation. J. Leukoc. Biol. 2016, 99, 121–130. [Google Scholar] [CrossRef]
- Grotzke, J.E.; Harriff, M.J.; Siler, A.C.; Nolt, D.; Delepine, J.; Lewinsohn, D.A.; Lewinsohn, D.M. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009, 5, e1000374. [Google Scholar] [CrossRef]
- Hansen, S.G.; Zak, D.E.; Xu, G.; Ford, J.C.; Marshall, E.E.; Malouli, D.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018, 24, 130–143. [Google Scholar] [CrossRef]
- Bian, Y.; Shang, S.; Siddiqui, S.; Zhao, J.; Joosten, S.A.; Ottenhoff, T.H.M.; Cantor, H.; Wang, C.R. MHC Ib molecule Qa-1 presents Mycobacterium tuberculosis peptide antigens to CD8+ T cells and contributes to protection against infection. PLoS Pathog. 2017, 13, e1006384. [Google Scholar] [CrossRef]
- La Manna, M.P.; Orlando, V.; Prezzemolo, T.; Di Carlo, P.; Cascio, A.; Delogu, G.; Poli, G.; Sullivan, L.C.; Brooks, A.G.; Dieli, F.; et al. HLA-E-restricted CD8+ T Lymphocytes Efficiently Control Mycobacterium tuberculosis and HIV-1 Coinfection. Am. J. Respir. Cell Mol. Biol. 2020, 62, 430–439. [Google Scholar] [CrossRef]
- Ussher, J.E.; van Wilgenburg, B.; Hannaway, R.F.; Ruustal, K.; Phalora, P.; Kurioka, A.; Hansen, T.H.; Willberg, C.B.; Phillips, R.E.; Klenerman, P. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur. J. Immunol. 2016, 46, 1600–1614. [Google Scholar] [CrossRef]
- Ernst, J.D. Mechanisms of M. tuberculosis Immune Evasion as Challenges to TB Vaccine Design. Cell Host Microbe 2018, 24, 34–42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Karamooz, E. MR1- and HLA-E-Dependent Antigen Presentation of Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 14412. https://doi.org/10.3390/ijms232214412
Kim S-J, Karamooz E. MR1- and HLA-E-Dependent Antigen Presentation of Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2022; 23(22):14412. https://doi.org/10.3390/ijms232214412
Chicago/Turabian StyleKim, Se-Jin, and Elham Karamooz. 2022. "MR1- and HLA-E-Dependent Antigen Presentation of Mycobacterium tuberculosis" International Journal of Molecular Sciences 23, no. 22: 14412. https://doi.org/10.3390/ijms232214412
APA StyleKim, S.-J., & Karamooz, E. (2022). MR1- and HLA-E-Dependent Antigen Presentation of Mycobacterium tuberculosis. International Journal of Molecular Sciences, 23(22), 14412. https://doi.org/10.3390/ijms232214412




