Molecular Characterization of a B Cell Adaptor for Phosphoinositide 3-Kinase Homolog in Lamprey (Lampetra japonica) and Its Function in the Immune Response
Abstract
1. Introduction
2. Results
2.1. Identification of Lja-BCAP and Sequence Similarity Analysis
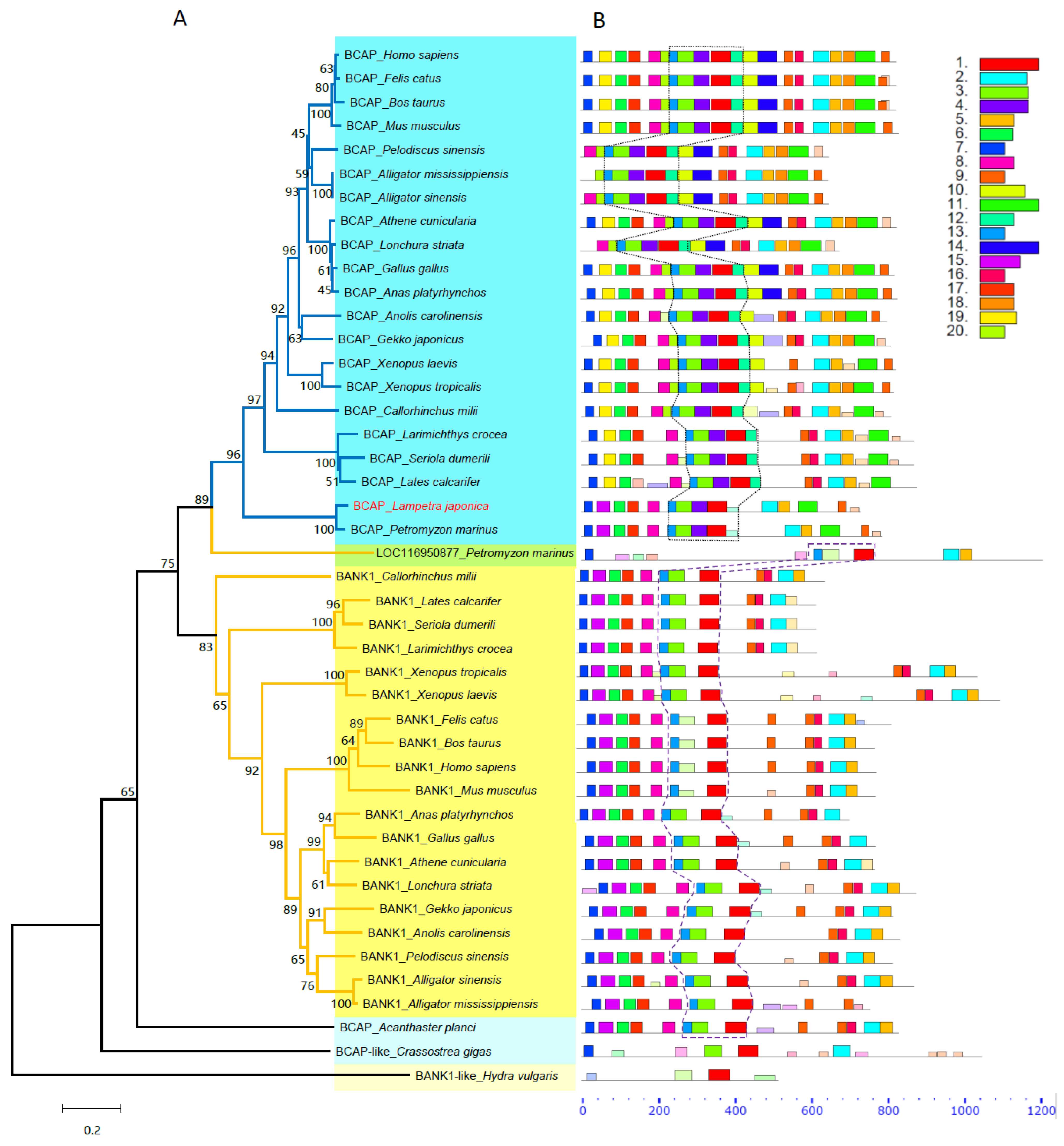
2.2. Phylogenetic and Conserved Motif Analyses of BCAP and BANK1 Families
2.3. Induced Expression of Recombinant Protein and Preparation of Its Rabbit Polyclonal Antibody
2.4. Functional Characterization of Lja-BCAP in Response to Mixed Bacteria Stimulation by RNAi Technique
2.5. Lja-BCAP Mainly Participates in the LPS-Mediated Immune Response of Lampreys
3. Discussion
4. Materials and Methods
4.1. Lamprey Immunization
4.2. PCR Amplification of the Target Gene
4.3. Homologous Sequence Comparison and Phylogenetic Analysis
4.4. Conserved Motif Analysis
4.5. Recombinant Expression of Truncated Lja-BCAP
4.6. Preparation and Titer Detection of Lja-BCAP Antibody
4.7. RNA Interference
4.8. Quantitative Real-Time PCR (qPCR) Detection
4.9. Western Blotting Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simeoni, L.; Kliche, S.; Lindquist, J. Adaptors and linkers in T and B cells. Curr. Opin. Immunol. 2004, 16, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Takeda, K.; Gotoh, K.; Takeshima, H.; Akira, S.; Kurosaki, T. Essential immunoregulatory role for BCAP in B cell development and function. J. Exp. Med. 2002, 195, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Aiba, Y.; Kameyama, M.; Yamazaki, T.; Tedder, T.F.; Kurosaki, T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood 2008, 111, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Maeda, A.; Iwamatsu, A.; Gotoh, K.; Kurosaki, T. BCAP: The tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity 2000, 13, 817–827. [Google Scholar] [CrossRef]
- Leevers, S.J.; Vanhaesebroeck, B.; Waterfield, M.D. Signalling through phosphoinositide 3-kinases: The lipids take centre stage. Curr. Opin. Cell. Biol. 1999, 11, 219–225. [Google Scholar] [CrossRef]
- Leu, C.M. Nck, a missing adaptor between the B-cell receptor complex and the BCAP/PI3K/Akt pathway. Cell. Mol. Immunol. 2014, 11, 120–122. [Google Scholar] [CrossRef]
- Castello, A.; Gaya, M.; Tucholski, J.; Oellerich, T.; Lu, K.H.; Tafuri, A.; Pawson, T.; Wienands, J.; Engelke, M.; Batista, F.D. Nck-mediated recruitment of BCAP to the BCR regulates the PI(3)K-Akt pathway in B cells. Nat. Immunol. 2013, 14, 966–975. [Google Scholar] [CrossRef]
- Baracho, G.V.; Miletic, A.V.; Omori, S.A.; Cato, M.H.; Rickert, R.C. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr. Opin. Immunol. 2011, 23, 178–183. [Google Scholar] [CrossRef]
- Lauenstein, J.U.; Udgata, A.; Bartram, A.; De Sutter, D.; Fisher, D.I.; Halabi, S.; Eyckerman, S.; Gay, N.J. Phosphorylation of the multifunctional signal transducer B-cell adaptor protein (BCAP) promotes recruitment of multiple SH2/SH3 proteins including GRB2. J. Biol. Chem. 2019, 294, 19852–19861. [Google Scholar] [CrossRef]
- Deason, K.; Troutman, T.D.; Jain, A.; Challa, D.; Mandraju, R.; Brewer, T.; Ward, S.; Pasare, C. BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation. J. Exp. Med. 2018, 215, 2413–2428. [Google Scholar] [CrossRef]
- Singh, M.; Ni, M.; Sullivan, J.; Hamerman, J.; Campbell, D. B cell adaptor for PI3-kinase (BCAP) modulates CD8+ effector and memory T cell differentiation. J. Exp. Med. 2018, 215, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Luff, D.; Wojdyla, K.; Oxley, D.; Chessa, T.; Hudson, K.; Hawkins, P.; Stephens, L.; Barry, S.; Okkenhaug, K. PI3Kδ forms distinct multiprotein complexes at the TCR signalosome in naïve and differentiated CD4+ T cells. Front. Immunol. 2021, 12, 631271. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Jiang, M.; Qi, L.; Yang, D.; Xiao, W.; Fang, F. BCAP regulates dendritic cell maturation through the dual-regulation of NF-κB and PI3K/AKT signaling during infection. Front. Immunol. 2020, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Irizarry-Caro, R.; McDaniel, M.; Overcast, G.; Jain, V.; Troutman, T.; Pasare, C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl. Acad. Sci. USA 2020, 117, 30628–30638. [Google Scholar] [CrossRef]
- Carpentier, S.; Ni, M.; Duggan, J.; James, R.; Cookson, B.; Hamerman, J. The signaling adaptor BCAP inhibits NLRP3 and NLRC4 inflammasome activation in macrophages through interactions with Flightless-1. Sci. Signal. 2019, 12, eaau0615. [Google Scholar] [CrossRef]
- Pancer, Z.; Amemiya, C.T.; Ehrhardt, G.R.; Ceitlin, J.; Gartland, G.L.; Cooper, M.D. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature 2004, 430, 174–180. [Google Scholar] [CrossRef]
- Alder, M.N.; Rogozin, I.B.; Iyer, L.M.; Glazko, G.V.; Cooper, M.D.; Pancer, Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science 2005, 310, 1892–1893. [Google Scholar] [CrossRef]
- Pancer, Z.; Cooper, M.D. The evolution of adaptive imminity. Annu. Rev. Immunol. 2006, 24, 497–518. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Im, S.; Kim, S.; Lazarte, J.; Jung, J.; Gong, T.; Kim, Y.; Lee, J.; Kim, H.; et al. Generation and characterization of hagfish variable lymphocyte receptor B against glycoprotein of viral hemorrhagic septicemia virus (VHSV). Mol. Immunol. 2018, 99, 30–38. [Google Scholar] [CrossRef]
- Collins, B.; Nakahara, H.; Acharya, S.; Cooper, M.; Herrin, B.; Wilson, I. Crystal structure of an anti-idiotype variable lymphocyte receptor. Acta Crystallogr. F Struct. Biol. Commun. 2017, 73, 682–687. [Google Scholar] [CrossRef]
- Gunn, R.; Herrin, B.; Acharya, S.; Cooper, M.; Wilson, I. VLR recognition of TLR5 expands the molecular characterization of protein antigen binding by non-Ig-based antibodies. J. Mol. Biol. 2018, 430, 1350–1367. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, J.; Sutoh, Y.; Fugo, K.; Otsuka, N.; Iwabuchi, K.; Kasahara, M. Identification of a third variable lymphocyte receptor in the lamprey. Proc. Natl. Acad. Sci. USA 2010, 107, 14304–14308. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Li, J.; Holland, S.J.; Iyer, L.M.; Hirano, M.; Schorpp, M.; Aravind, L.; Cooper, M.D.; Boehm, T. Genomic donor cassette sharing during VLRA and VLRC assembly in jawless vertebrates. Proc. Natl. Acad. Sci. USA 2014, 111, 14828–14833. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Hirano, M.; Herrin, B.R.; Li, J.; Yu, C.; Sadlonova, A.; Cooper, M.D. Dual nature of the adaptive immune system in lampreys. Nature 2009, 459, 796. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Im, S.P.; Lee, J.S.; Lazarte, J.M.S.; Kim, S.W.; Jung, J.W.; Kim, J.Y.; Kim, Y.R.; Lee, S.; Kim, G.J.; et al. Globular-shaped variable lymphocyte receptors B antibody multimerized by a hydrophobic clustering in hagfish. Sci. Rep. 2018, 8, 10801. [Google Scholar] [CrossRef]
- Holland, S.J.; Berghuis, L.M.; King, J.J.; Iyer, L.M.; Sikora, K.; Fifield, H.; Peter, S.; Quinlan, E.M.; Sugahara, F.; Shingate, P.; et al. Expansions, diversification, and interindividual copy number variations of AID/APOBEC family cytidine deaminase genes in lampreys. Proc. Natl. Acad. Sci. USA 2018, 115, E3211–E3220. [Google Scholar] [CrossRef]
- Ritzmann, S.E.; Daniels, J.C.; Sakai, H.; Beathard, G.A. The lymphocyte in immunobiology. Ann. Allergy 1973, 31, 109–125. [Google Scholar] [PubMed]
- Cannon, J.P.; Haire, R.N.; Rast, J.P.; Litman, G.W. The phylogenetic origins of the antigen binding receptors and somatic diversification mechanisms. Immunol. Rev. 2004, 200, 12–22. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, J.; Chen, L.; Liu, X.; Su, P.; Han, Y.; Feng, B.; Li, Q. A novel BTK-like protein involved in immune response in Lethenteron japonicum. Immunol. Lett. 2012, 146, 57–63. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Shi, B.; Xiao, R.; Gou, M.; Wang, H.; Li, Q. Identification and characterisation of the immune response properties of Lampetra japonica BLNK. Sci. Rep. 2016, 6, 25308. [Google Scholar] [CrossRef]
- Liu, C.; Su, P.; Li, R.; Zhang, Q.; Zhu, T.; Liu, X.; Li, Q. Molecular cloning, expression pattern, and molecular evolution of the spleen tyrosine kinase in lamprey, Lampetra japonica. Dev. Genes. Evol. 2015, 225, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Y.; Han, Y.; Su, P.; Gou, M.; Pang, Y.; Li, Q.; Liu, X. A novel Vav3 homolog identified in lamprey, Lampetra japonica, with roles in lipopolysaccharide-mediated immune response. Int. J. Mol. Sci. 2017, 18, 2035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Luo, K.; Liu, X.; Dong, S.; Han, Y.; Gou, M.; Su, P.; Li, Q.; Zhu, T. A novel Lyn-like protein tyrosine kinase identified in lamprey and its role in immune response. Acta. Biochim. Biophys. Sin. (Shanghai) 2020, 52, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Uinuk-Ool, T.; Mayer, W.E.; Sato, A.; Dongak, R.; Cooper, M.D.; Klein, J. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 14356–14361. [Google Scholar] [CrossRef]
- Hirano, M.; Guo, P.; McCurley, N.; Schorpp, M.; Das, S.; Boehm, T.; Cooper, M.D. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 2013, 501, 435–438. [Google Scholar] [CrossRef]
- Harwood, N.E.; Batista, F.D. Early events in B cell activation. Annu. Rev. Immunol. 2010, 28, 185–210. [Google Scholar] [CrossRef]
- Choudhuri, K.; Wiseman, D.; Brown, M.; Gould, K.; van der Merwe, P. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature 2005, 436, 578–582. [Google Scholar] [CrossRef]
- Tinti, M.; Pio Nardozza, A.; Ferrari, E.; Sacco, F.; Corallino, S.; Castagnoli, L.; Cesareni, G. The 4G10, pY20 and p-TYR-100 antibody specificity: Profiling by peptide microarrays. New Biotechnol. 2012, 29, 571–577. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Pang, Y.; Han, Y.; Li, J.; Wang, Z.; Liu, X.; Li, H.; Hua, Y.; Jiang, H.; et al. Chromosome-level genome assembly of Lethenteron reissneri provides insights into lamprey evolution. Mol. Ecol. Resour. 2021, 21, 448–463. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic. Acids. Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.; Harborth, J.; Lendecke, W.; Yalcin, A.; Weber, K.; Tusch, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Symbol | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Lja-BCAP-546 | CCGCAUCAUGUGUGGGCAATT | UUGCCCACACAUGAUGCGGTT |
| Scrambled-546 | CCAAGCCUGGUGAUGGACUTT | AGUCCAUCACCAGGUUUGGTT |
| Lja-BCAP-891 | GCUGCUCGCAGAUUCCUUATT | UAAGGAAUCUGCGAGCAGCTT |
| Scembled-891 | CGAGACUCCGCAUAUUUCGTT | CGAAAUAUGCGGAGUCUCGTT |
| Lja-BCAP-1148 | GCAAAUUCAUGGACGACUATT | UAGUCGUCCAUGAAUUUGCTT |
| Scembled-1148 | CCAAGGAGGAUUUACCAUATT | UAUGGUAAAUCCUCCUUGGTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, M.; Liu, X.; Wei, L.; Li, J.; Gou, M.; Zhu, T.; Han, Y.; Liu, X. Molecular Characterization of a B Cell Adaptor for Phosphoinositide 3-Kinase Homolog in Lamprey (Lampetra japonica) and Its Function in the Immune Response. Int. J. Mol. Sci. 2022, 23, 14449. https://doi.org/10.3390/ijms232214449
Chai M, Liu X, Wei L, Li J, Gou M, Zhu T, Han Y, Liu X. Molecular Characterization of a B Cell Adaptor for Phosphoinositide 3-Kinase Homolog in Lamprey (Lampetra japonica) and Its Function in the Immune Response. International Journal of Molecular Sciences. 2022; 23(22):14449. https://doi.org/10.3390/ijms232214449
Chicago/Turabian StyleChai, Mengqi, Xiujia Liu, Lin Wei, Jun Li, Meng Gou, Ting Zhu, Yinglun Han, and Xin Liu. 2022. "Molecular Characterization of a B Cell Adaptor for Phosphoinositide 3-Kinase Homolog in Lamprey (Lampetra japonica) and Its Function in the Immune Response" International Journal of Molecular Sciences 23, no. 22: 14449. https://doi.org/10.3390/ijms232214449
APA StyleChai, M., Liu, X., Wei, L., Li, J., Gou, M., Zhu, T., Han, Y., & Liu, X. (2022). Molecular Characterization of a B Cell Adaptor for Phosphoinositide 3-Kinase Homolog in Lamprey (Lampetra japonica) and Its Function in the Immune Response. International Journal of Molecular Sciences, 23(22), 14449. https://doi.org/10.3390/ijms232214449





