Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma
Abstract
:1. Introduction
2. Results
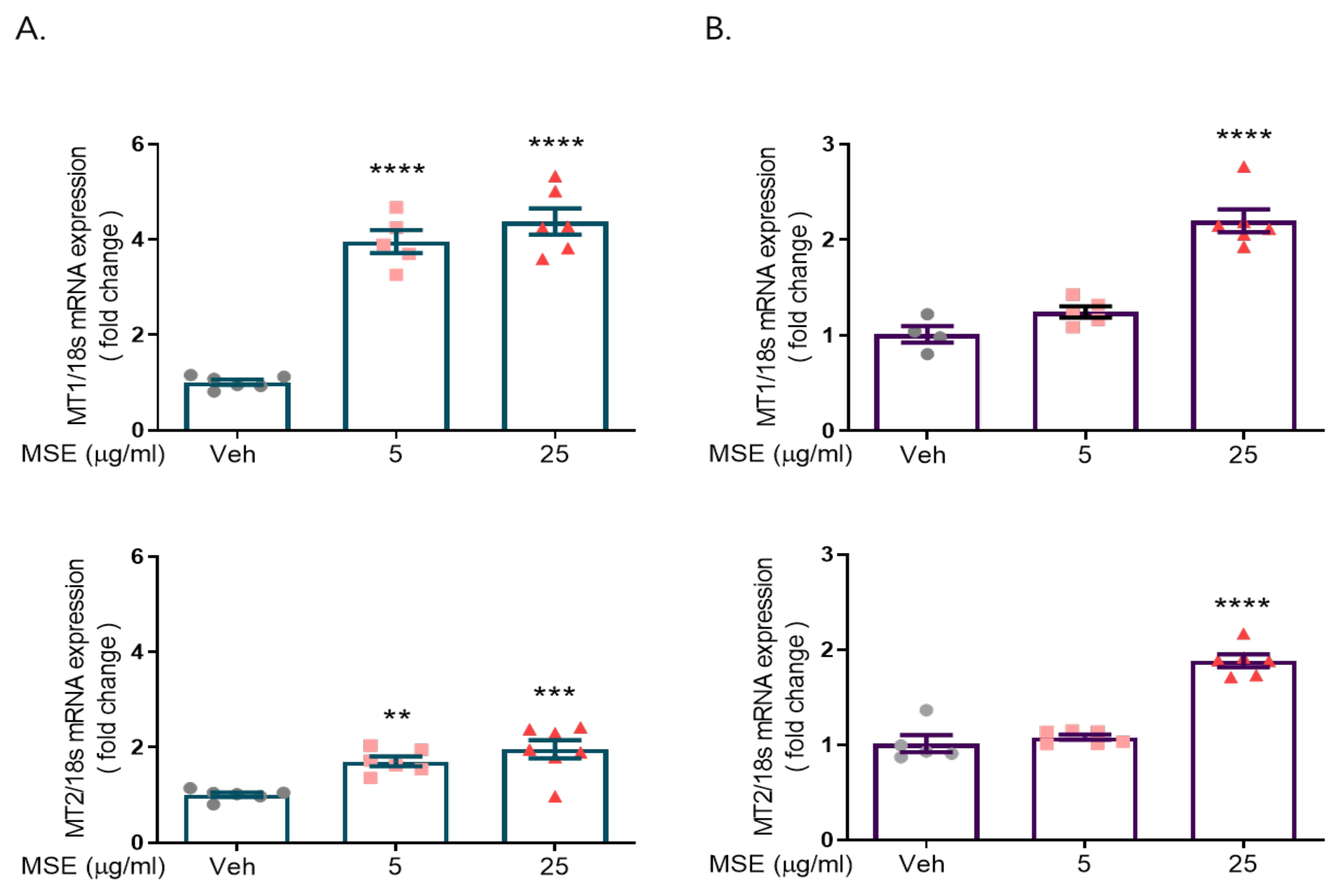
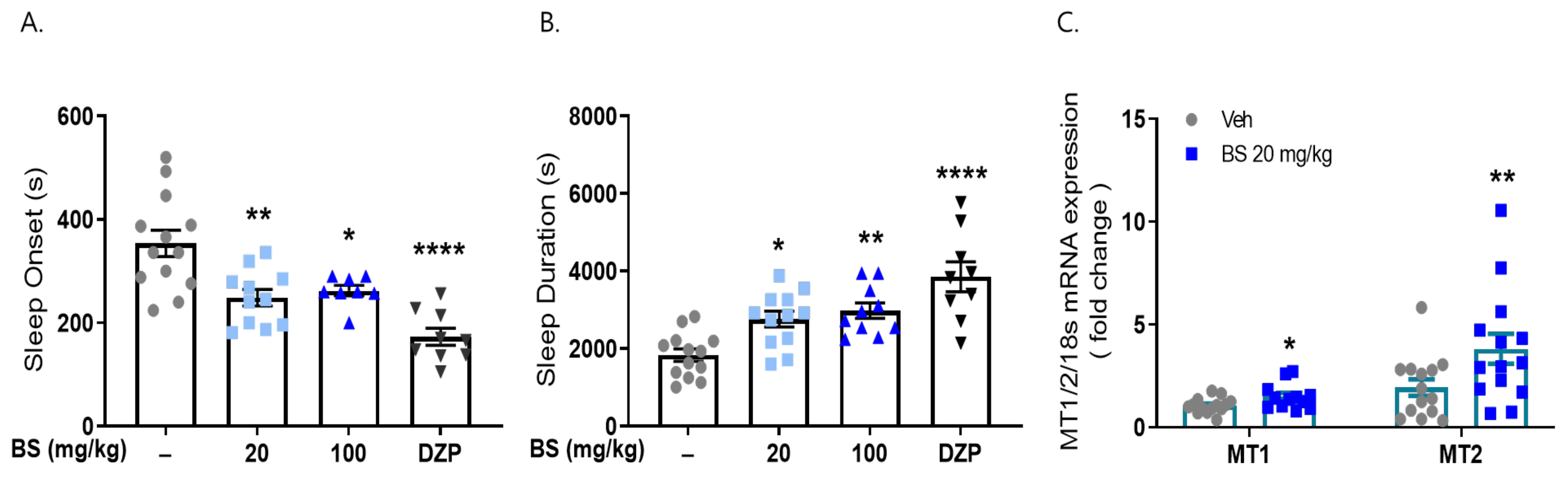
2.1. Maydis Stigma Extracts Increased mRNA Expression of Melatonin Receptors 1 and 2
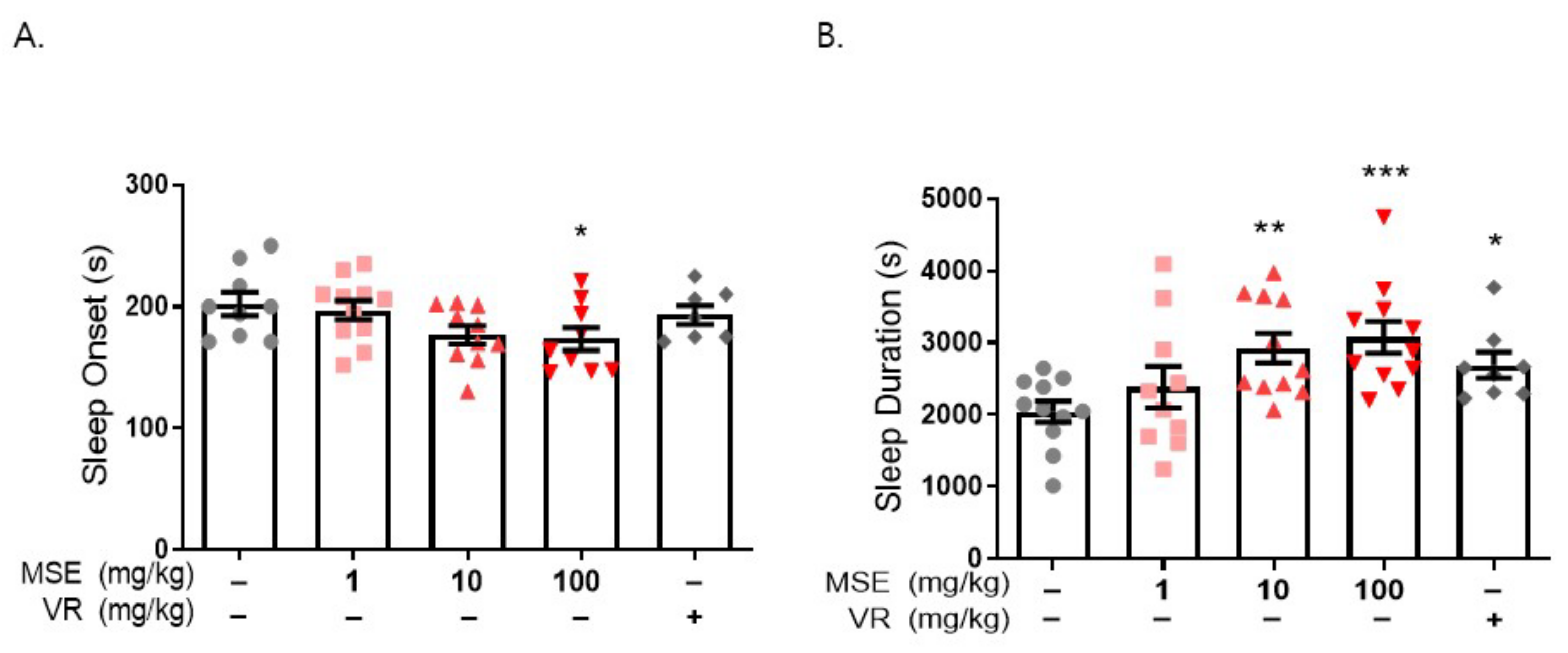
2.2. MS Extracts Reduced Sleep Onset Latency and Increased Sleep Duration
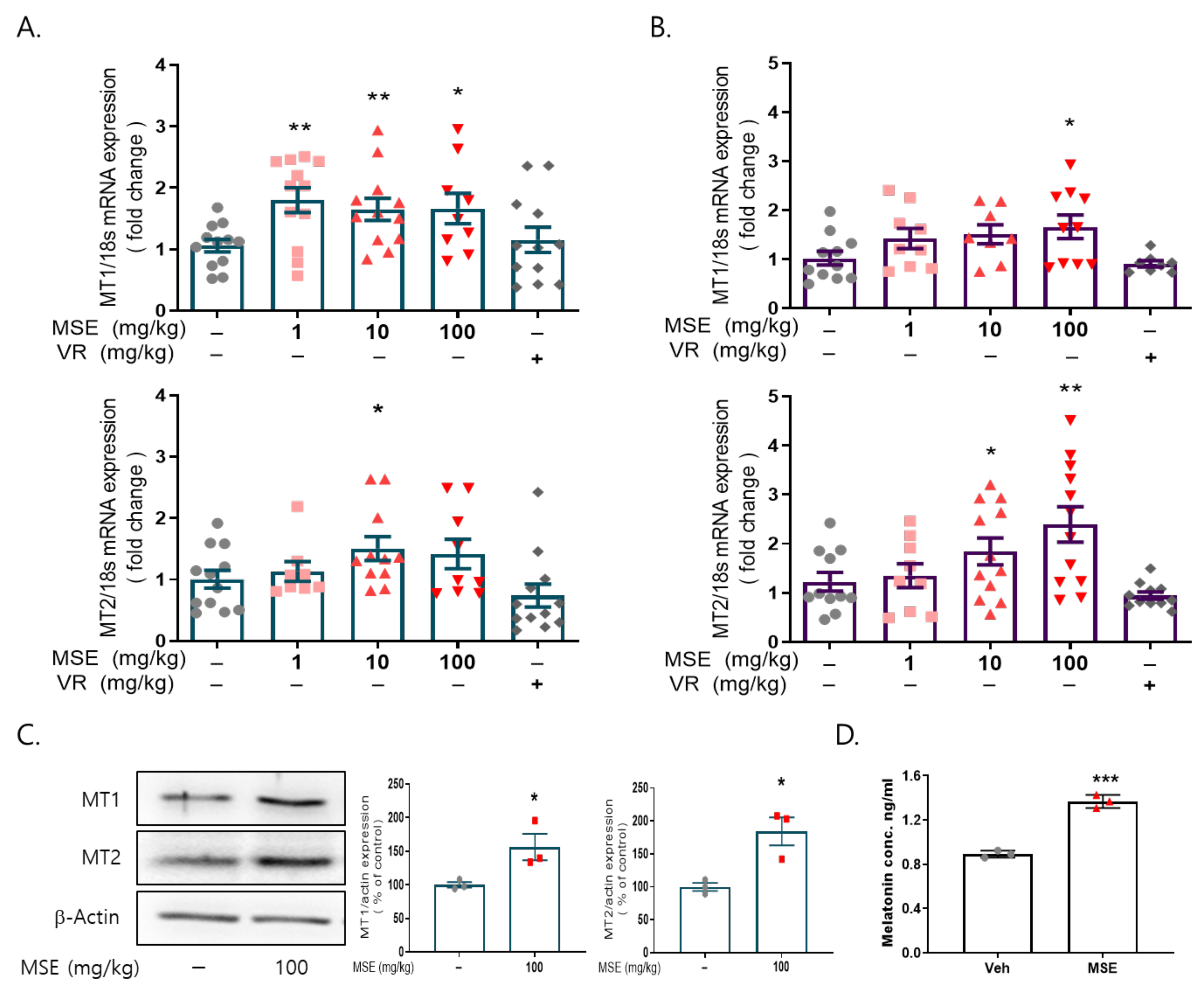
2.3. MS Extracts Increased the Expression of Circadian Clock Genes
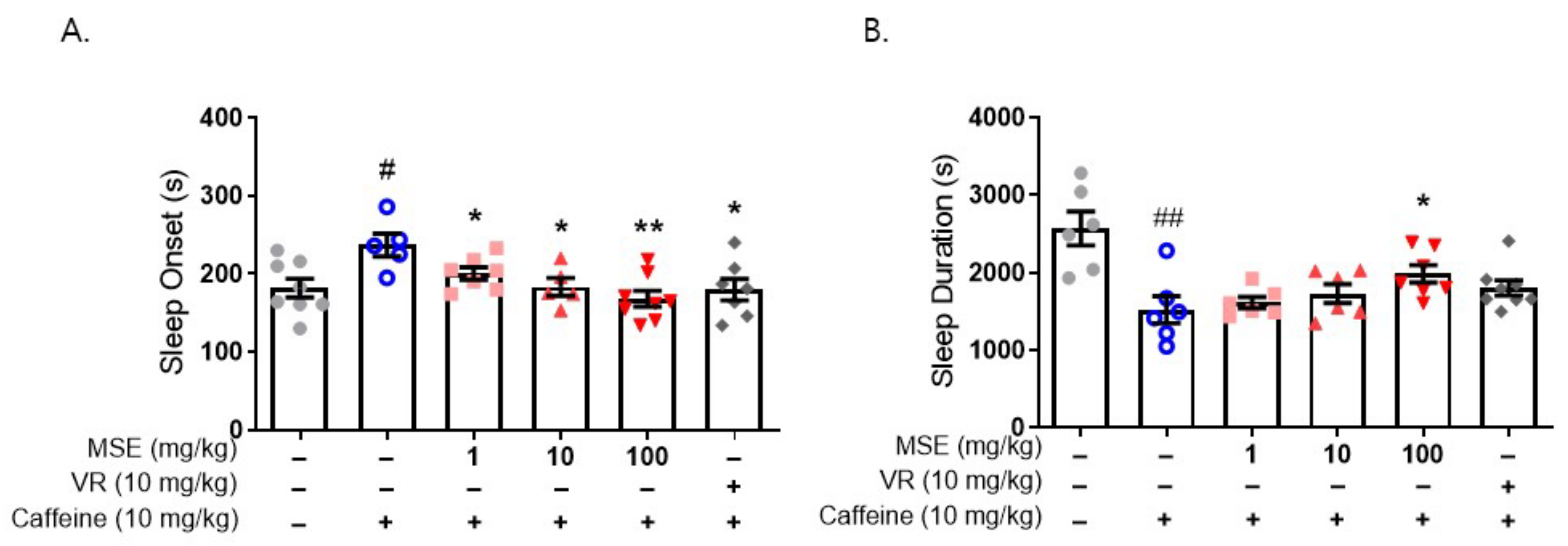
2.4. Butanol Fraction of MSE Reduced Sleep Onset Latency and Increased Sleep Duration
2.5. β-Sitosterol Had a Sleep-Promoting Effect
2.6. Effects of MS Extracts and β-Sitosterol on the EEG Waves
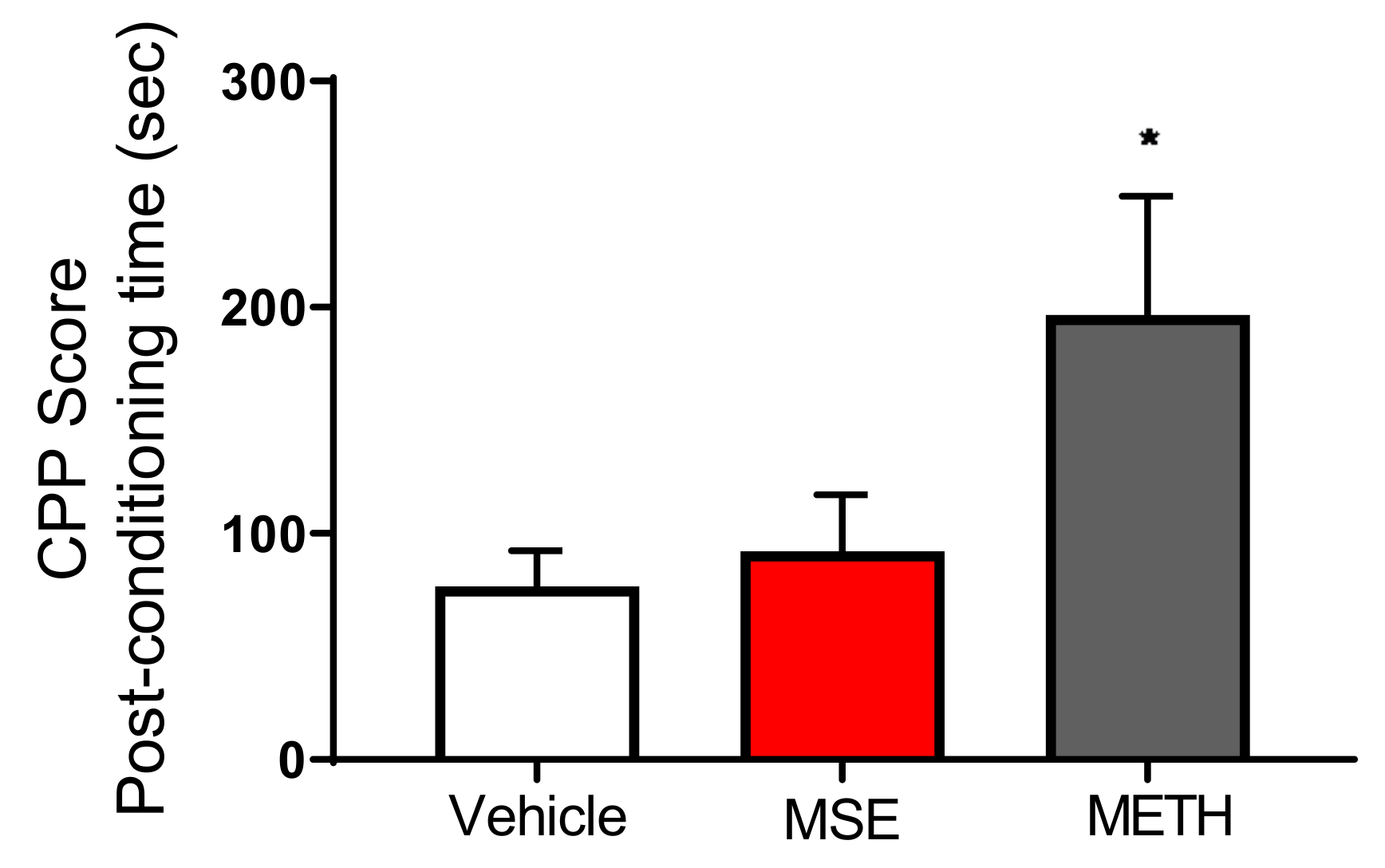
2.7. MS Extracts Do Not Cause Place Preference
2.8. MS Extracts Inhibited the H2O2-Induced Neuronal Cell Death in Rat Cortical Neurons
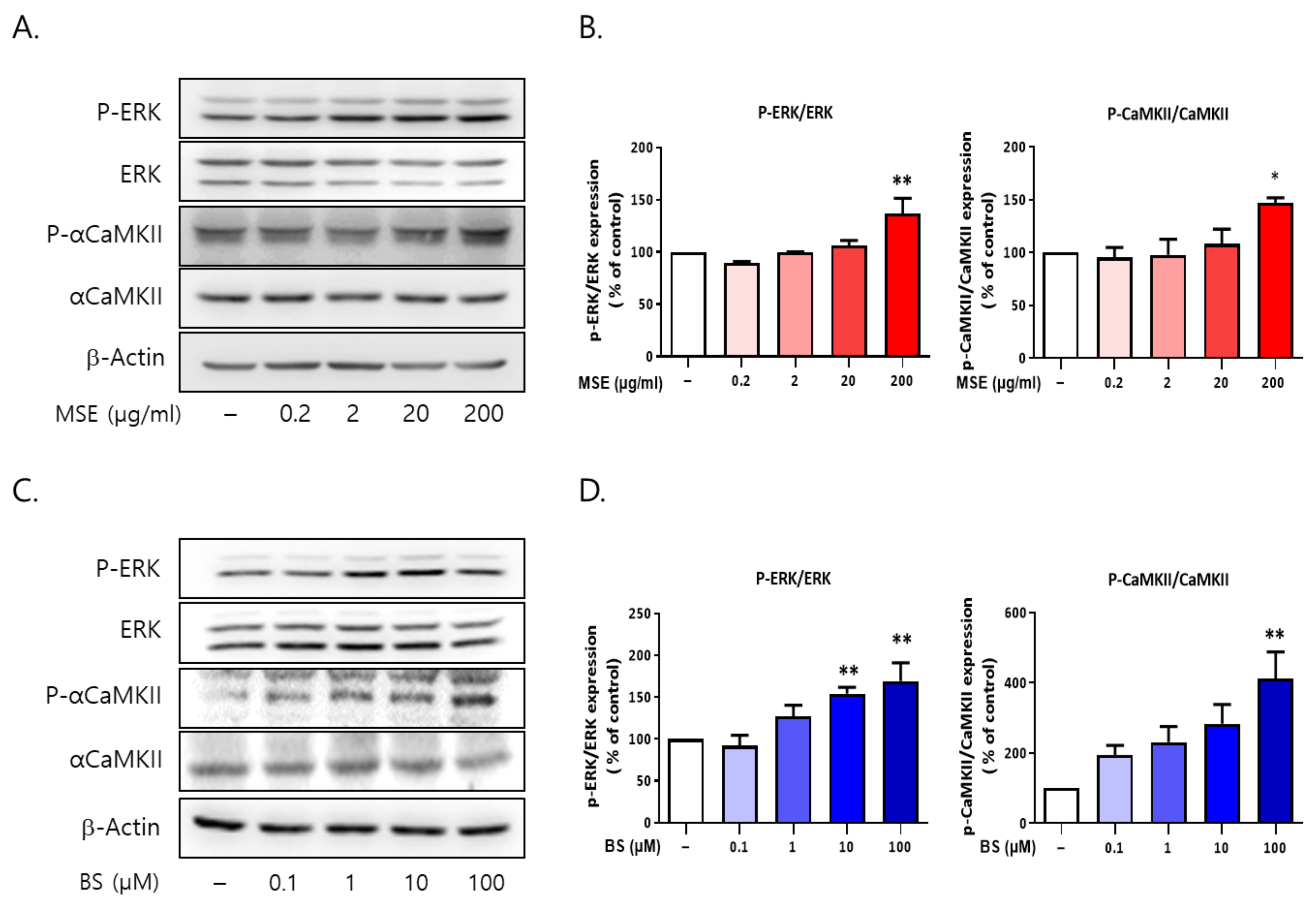
2.9. Both MS and BS Regulated the Phosphorylation of ERK and αCaMKII in Rat Primary Cultured Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Maydis Stigma Extract Preparation
4.2. Animals
4.3. Cell Culture and Drug Treatment
4.4. Pentobarbital-Induced Sleep Test
4.5. Electroencephalographic (EEG) Recording
4.6. Measurement of Melatonin Levels
4.7. Conditioned Place Preference (CPP) Test
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Western Blot Analysis
4.10. Measurement of Cell Viability
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troynikov, O.; Watson, C.G.; Nawaz, N. Sleep environments and sleep physiology: A review. J. Therm. Biol. 2018, 78, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist 2014, 20, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K. Sleep disturbance and neurological disease. Clin. Med. 2011, 11, 271–274. [Google Scholar] [CrossRef]
- Bishir, M.; Bhat, A.; Essa, M.M.; Ekpo, O.; Ihunwo, A.O.; Veeraraghavan, V.P.; Mohan, S.K.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020, 2020, 5764017. [Google Scholar] [CrossRef] [PubMed]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Konofal, E.; Lecendreux, M.; Cortese, S. Sleep and ADHD. Sleep Med. 2010, 11, 652–658. [Google Scholar] [CrossRef]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3 (Suppl. 5), S7–S10. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, S.; Hemavathy, D.; Prasad, S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J. Fam. Med. Prim. Care 2016, 5, 780–784. [Google Scholar] [CrossRef]
- Streatfeild, J.; Smith, J.; Mansfield, D.; Pezzullo, L.; Hillman, D. The social and economic cost of sleep disorders. Sleep 2021, 44, zsab132. [Google Scholar] [CrossRef]
- Mattila, T.; Hasala, H.; Kreivi, H.R.; Avellan-Hietanen, H.; Bachour, A.; Herse, F.; Leskela, R.L.; Toppila-Salmi, S.; Erhola, M.; Haahtela, T.; et al. Changes in the societal burden caused by sleep apnoea in Finland from 1996 to 2018: A national registry study. Lancet Reg. Health Eur. 2022, 16, 100338. [Google Scholar] [CrossRef]
- Saddichha, S. Diagnosis and treatment of chronic insomnia. Ann. Indian Acad. Neurol. 2010, 13, 94–102. [Google Scholar] [CrossRef]
- Ali, R.; Tariq, S.; Kareem, O.; Fayaz, F.; Aziz, T.; Meenu; Pottoo, F.H.; Siddiqui, N. Nutraceuticals for Sleep Disorders. Comb. Chem. High Throughput Screen 2021, 24, 1583–1592. [Google Scholar] [CrossRef]
- Su, L.Y.; Liu, Q.; Jiao, L.; Yao, Y.G. Molecular Mechanism of Neuroprotective Effect of Melatonin on Morphine Addiction and Analgesic Tolerance: An Update. Mol. Neurobiol. 2021, 58, 4628–4638. [Google Scholar] [CrossRef]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr. Pharm. Des. 2018, 24, 1563–1588. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [Green Version]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Velazquez, D.V.; Xavier, H.S.; Batista, J.E.; de Castro-Chaves, C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine 2005, 12, 363–369. [Google Scholar] [CrossRef] [PubMed]
- El-Ghorab, A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian corn silk (Zea mays L.). J. Agric. Food Chem. 2007, 55, 9124–9127. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.Z.; Yang, X.; Zhou, H.L.; Pan, S.X. Safety Evaluation, in Vitro and in Vivo Antioxidant Activity of the Flavonoid-Rich Extract from Maydis stigma. Molecules 2015, 20, 22102–22112. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiao, T.; Ruan, J.; Liu, W. Beneficial Effects of Corn Silk on Metabolic Syndrome. Curr. Pharm. Des. 2017, 23, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Yin, Y.; Yu, Z.; Liu, J.; Chen, F. Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int. J. Biol. Macromol. 2012, 50, 1133–1137. [Google Scholar] [CrossRef]
- Kan, A.; Orhan, I.; Coksari, G.; Sener, B. In-vitro neuroprotective properties of the Maydis stigma extracts from four corn varieties. Int. J. Food Sci. Nutr. 2012, 63, 1–4. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rakhshandeh, H.; Sadeghnia, H.R. Potentiating Effects of Lactuca sativa on Pentobarbital-Induced Sleep. Iran J. Pharm. Res. 2013, 12, 401–406. [Google Scholar]
- Hamedi, S.; Forouzanfar, F.; Rakhshandeh, H.; Arian, A. Hypnotic Effect of Portulaca oleracea on Pentobarbital-Induced Sleep in Mice. Curr. Drug Discov. Technol. 2019, 16, 198–203. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, J.N.; Kim, M.K.; Lee, J.; Ignarro, L.J.; Kim, H.J.; Shin, C.Y.; Han, S.H. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: A possible role in neuroprotection. J. Pineal Res. 2011, 50, 110–123. [Google Scholar] [CrossRef]
- Hong, S.I.; Kwon, S.H.; Hwang, J.Y.; Ma, S.X.; Seo, J.Y.; Ko, Y.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Quinpirole Increases Melatonin-Augmented Pentobarbital Sleep via Cortical ERK, p38 MAPK, and PKC in Mice. Biomol. Ther. 2016, 24, 115–122. [Google Scholar] [CrossRef]
- Ko, Y.H.; Shim, K.Y.; Lee, S.Y.; Jang, C.G. Evodiamine Reduces Caffeine-Induced Sleep Disturbances and Excitation in Mice. Biomol. Ther. 2018, 26, 432–438. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; Kim, M.; Lee, H.J.; Ryu, H.W.; Lee, Y.S.; Kim, H.J.; Cheong, J.H. Two newly-emerging substituted phenethylamines MAL and BOD induce differential psychopharmacological effects in rodents. J. Psychopharmacol. 2020, 34, 1056–1067. [Google Scholar] [CrossRef]
- De la Pena, J.B.; Lee, H.C.; de la Pena, I.C.; Woo, T.S.; Yoon, S.Y.; Lee, H.L.; Han, J.S.; Lee, J.I.; Cho, Y.J.; Shin, C.Y.; et al. Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, Zoletil(R): Difference between acute and repeated exposure. Behav. Brain Res. 2012, 233, 434–442. [Google Scholar] [CrossRef]
- Von Gall, C.; Weaver, D.R.; Moek, J.; Jilg, A.; Stehle, J.H.; Korf, H.W. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann. N. Y. Acad. Sci. 2005, 1040, 508–511. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal. Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Liu, W.L.; Wu, B.F.; Shang, J.H.; Zhao, Y.L.; Huang, A.X. Moringa oleifera Lam Seed Oil Augments Pentobarbital-Induced Sleeping Behaviors in Mice via GABAergic Systems. J. Agric. Food Chem. 2020, 68, 3149–3162. [Google Scholar] [CrossRef]
- Aguirre-Hernandez, E.; Rosas-Acevedo, H.; Soto-Hernandez, M.; Martinez, A.L.; Moreno, J.; Gonzalez-Trujano, M.E. Bioactivity-guided isolation of beta-sitosterol and some fatty acids as active compounds in the anxiolytic and sedative effects of Tilia americana var. mexicana. Planta Med. 2007, 73, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. The effect of beta-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. RSC Adv. 2018, 8, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Manber, R.; Armitage, R. Sex, steroids, and sleep: A review. Sleep 1999, 22, 540–555. [Google Scholar]
- Koehl, M.; Battle, S.; Meerlo, P. Sex differences in sleep: The response to sleep deprivation and restraint stress in mice. Sleep 2006, 29, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsey, A.; de Lecea, L.; Jennings, K.J. Neurobiological and Hormonal Mechanisms Regulating Women’s Sleep. Front Neurosci. 2020, 14, 625397. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Phytochemical screening, total phenolic content and phytotoxic activity of corn (Zea mays) extracts against some indicator species. Nat. Prod. Res. 2018, 32, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Kim, S.L.; Choi, J.W.; Park, Y.I. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014, 109, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Okada, Y.; Okuyama, T. The favorable effect of style of Zea mays L. on streptozotocin induced diabetic nephropathy. Biol. Pharm. Bull. 2005, 28, 919–920. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Q.; Xu, T.; Bu, X.M.; Liu, B.Y. Anti-inflammation effects of corn silk in a rat model of carrageenin-induced pleurisy. Inflammation 2012, 35, 822–827. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, G.; Comai, S. Differential Function of Melatonin MT1 and MT2 Receptors in REM and NREM Sleep. Front. Endocrinol. 2019, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Tosini, G.; Owino, S.; Guillaume, J.L.; Jockers, R. Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. BioEssays 2014, 36, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Doghramji, K. Melatonin and its receptors: A new class of sleep-promoting agents. J. Clin. Sleep Med. 2007, 3 (Suppl. 5), S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W.; Gillette, M.U. Melatonin, sleep, and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004, 5, 523–532. [Google Scholar] [CrossRef]
- Cheng, X.P.; Sun, H.; Ye, Z.Y.; Zhou, J.N. Melatonin modulates the GABAergic response in cultured rat hippocampal neurons. J. Pharmacol. Sci. 2012, 119, 177–185. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [Green Version]
- Dubocovich, M.L.; Rivera-Bermudez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef] [Green Version]
- Weissova, K.; Skrabalova, J.; Skalova, K.; Cervena, K.; Bendova, Z.; Miletinova, E.; Koprivova, J.; Sonka, K.; Dudysova, D.; Bartos, A.; et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018, 52, 1–6. [Google Scholar] [CrossRef]
- Agez, L.; Laurent, V.; Pevet, P.; Masson-Pevet, M.; Gauer, F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 2007, 144, 522–530. [Google Scholar] [CrossRef]
- Lewandowska, K.; Malkiewicz, M.A.; Sieminski, M.; Cubala, W.J.; Winklewski, P.J.; Medrzycka-Dabrowska, W.A. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit—A clinical review. Sleep Med. 2020, 69, 127–134. [Google Scholar] [CrossRef]
- Laudon, M.; Frydman-Marom, A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int. J. Mol. Sci. 2014, 15, 15924–15950. [Google Scholar] [CrossRef] [Green Version]
- Kasper, S.; Hajak, G. The efficacy of agomelatine in previously-treated depressed patients. Eur. Neuropsychopharmacol. 2013, 23, 814–821. [Google Scholar] [CrossRef]
- Hardeland, R. Tasimelteon, a melatonin agonist for the treatment of insomnia and circadian rhythm sleep disorders. Curr. Opin. Investig. Drugs 2009, 10, 691–701. [Google Scholar] [PubMed]
- Liu, C.; Weaver, D.R.; Jin, X.; Shearman, L.P.; Pieschl, R.L.; Gribkoff, V.K.; Reppert, S.M. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 1997, 19, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; von Gall, C.; Pieschl, R.L.; Gribkoff, V.K.; Stehle, J.H.; Reppert, S.M.; Weaver, D.R. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol. Cell. Biol. 2003, 23, 1054–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubocovich, M.L.; Yun, K.; Al-Ghoul, W.M.; Benloucif, S.; Masana, M.I. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998, 12, 1211–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. 3), 34–42. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Ghorbani, A.; Rakhshandeh, H. Hypnotic Effect of Ocimum basilicum on Pentobarbital-Induced Sleep in Mice. Iran Red. Crescent. Med. J. 2016, 18, e24261. [Google Scholar] [CrossRef] [Green Version]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Karim, S.M.R.; Sharmin, T.; Morshed, M.M. Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Akk, G.; Covey, D.F.; Evers, A.S.; Steinbach, J.H.; Zorumski, C.F.; Mennerick, S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol. Ther. 2007, 116, 35–57. [Google Scholar] [CrossRef] [Green Version]
- Carver, C.M.; Reddy, D.S. Neurosteroid Structure-Activity Relationships for Functional Activation of Extrasynaptic deltaGABA(A) Receptors. J Pharmacol. Exp. Ther. 2016, 357, 188–204. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain. Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, M.H.; Jang, C.; Kwon, J.W.; Park, J.W. The effect of alpha rhythm sleep on EEG activity and individuals’ attention. J. Phys. Ther. Sci. 2013, 25, 1515–1518. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Pachinger, T.; Ripper, B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci. Lett. 1997, 238, 9–12. [Google Scholar] [CrossRef]
- Roth, C.; Achermann, P.; Borbely, A.A. Alpha activity in the human REM sleep EEG: Topography and effect of REM sleep deprivation. Clin. Neurophysiol. 1999, 110, 632–635. [Google Scholar] [CrossRef]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N. Adverse Effects of Hypnotic Medications. J. Clin. Sleep Med. 2017, 13, 839. [Google Scholar] [CrossRef] [Green Version]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef]
- Tatsuki, F.; Sunagawa, G.A.; Shi, S.; Susaki, E.A.; Yukinaga, H.; Perrin, D.; Sumiyama, K.; Ukai-Tadenuma, M.; Fujishima, H.; Ohno, R.; et al. Involvement of Ca2+-Dependent Hyperpolarization in Sleep Duration in Mammals. Neuron 2016, 90, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, T.; Vietri, J. Residual Effects of Sleep Medications Are Commonly Reported and Associated with Impaired Patient-Reported Outcomes among Insomnia Patients in the United States. Sleep Disord. 2015, 2015, 607148. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dong, J.W.; Zhao, J.H.; Tang, L.N.; Zhang, J.J. Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence. Curr. Neuropharmacol. 2014, 12, 289–302. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, N.; Grewal, M.S.; Gupta, A.K. Differential antioxidative response of tolerant and sensitive maize (Zea mays L.) genotypes to drought stress at reproductive stage. Indian J. Biochem. Biophys. 2013, 50, 150–158. [Google Scholar]
- Witt-Enderby, P.A.; MacKenzie, R.S.; McKeon, R.M.; Carroll, E.A.; Bordt, S.L.; Melan, M.A. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil. Cytoskelet. 2000, 46, 28–42. [Google Scholar] [CrossRef]
- Leonard, A.S.; Lim, I.A.; Hemsworth, D.E.; Horne, M.C.; Hell, J.W. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Oliver, M.D. Cellular and Molecular Mechanisms of REM Sleep Homeostatic Drive: A Plausible Component for Behavioral Plasticity. Front. Neural Circuits 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Desarnaud, F.; Macone, B.W.; Datta, S. Activation of extracellular signal-regulated kinase signaling in the pedunculopontine tegmental cells is involved in the maintenance of sleep in rats. J. Neurochem. 2011, 116, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumoulin, M.C.; Aton, S.J.; Watson, A.J.; Renouard, L.; Coleman, T.; Frank, M.G. Extracellular signal-regulated kinase (ERK) activity during sleep consolidates cortical plasticity in vivo. Cereb. Cortex 2015, 25, 507–515. [Google Scholar] [CrossRef]




| Sense | Antisense | |
|---|---|---|
| MT1 | TTGTGGCGAGTTTAGCTGTG | GACACTCAGGCCCATTAGGA |
| MT2 | ATTCCTGCACCTTCATCCAG | CCTGGAGCACCAGTATCCAT |
| Per1 | ATGACATACCAGGTGCCGTC | GTCCTCTGAGAACCGTGGC |
| Per2 | TCCAGGCACCCGGAATAGTA | CACAACAGGCATGGTGAAGC |
| Cry1 | ATGTCCCGAGTTGTAGCAGC | TGAGAGCAATTTCCACCGCT |
| Cry2 | TCGAGATACCGGGGACTCTG | GAAGCTGGGCCACTGGATAG |
| Bmal1 | CCGTGGACCAAGGAAGTTGA | CTGTTAGCTGCGGGAAGGTT |
| Clock | AGCGATGTCTCAAGCTGCAA | CCTCTATCATCCGTGTCCGC |
| 18S rRNA | CATTAAATCAGTTATGGTTCCTTTGG | TCGGCATGTATTAGCTCTAGAATTACC |
| Gapdh | GTG AAG GTC GGT GTG AAC GGA TTT | CAC AGT CTT CTG AGT GGC AGT GAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.-E.; Mabunga, D.F.; Kim, H.J.; Han, S.-H.; Kim, H.Y.; Shin, C.Y.; Kwon, K.J. Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma. Int. J. Mol. Sci. 2022, 23, 14612. https://doi.org/10.3390/ijms232314612
Kim R-E, Mabunga DF, Kim HJ, Han S-H, Kim HY, Shin CY, Kwon KJ. Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma. International Journal of Molecular Sciences. 2022; 23(23):14612. https://doi.org/10.3390/ijms232314612
Chicago/Turabian StyleKim, Ryeong-Eun, Darine Froy Mabunga, Hee Jin Kim, Seol-Heui Han, Hahn Young Kim, Chan Young Shin, and Kyoung Ja Kwon. 2022. "Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma" International Journal of Molecular Sciences 23, no. 23: 14612. https://doi.org/10.3390/ijms232314612
APA StyleKim, R.-E., Mabunga, D. F., Kim, H. J., Han, S.-H., Kim, H. Y., Shin, C. Y., & Kwon, K. J. (2022). Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma. International Journal of Molecular Sciences, 23(23), 14612. https://doi.org/10.3390/ijms232314612






