Ophiopogon Polysaccharide Liposome Regulated the Immune Activity of Kupffer Cell through miR-4796
Abstract
:1. Introduction
2. Results
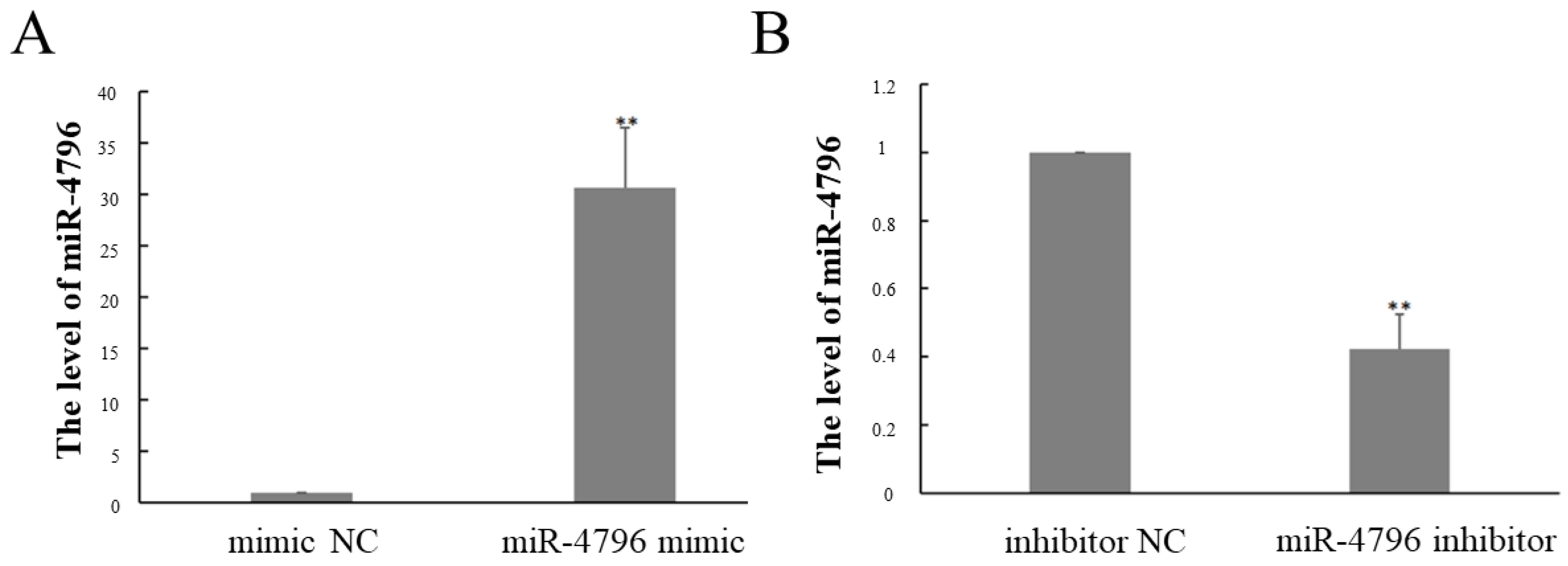
2.1. The Expression of miR-4796 after Transfected miR-4796 Mimic or Inhibitor
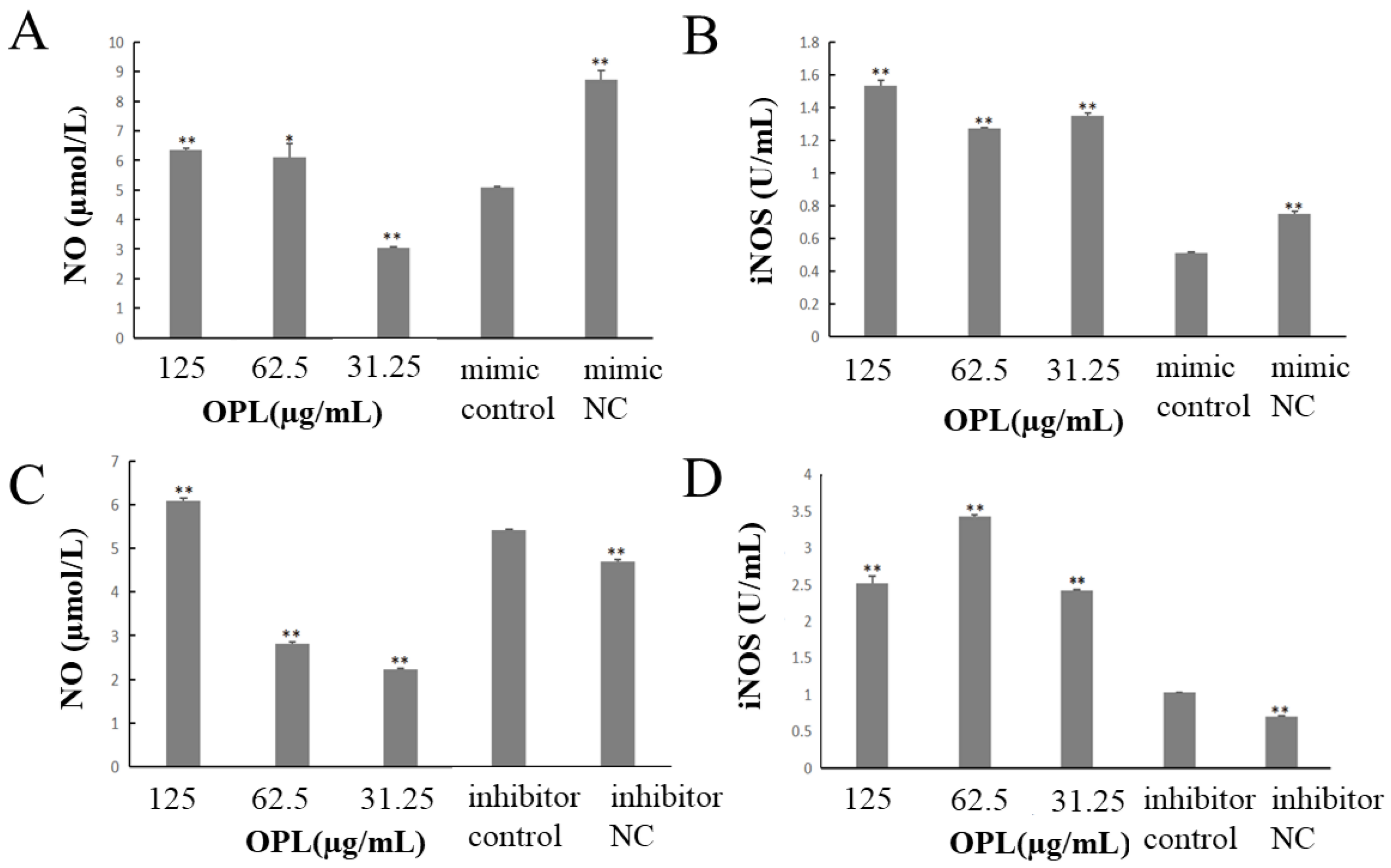
2.2. The Effect of OPL on the Secretion of NO and iNOS
2.3. The Effect of OPL on Cell Migration
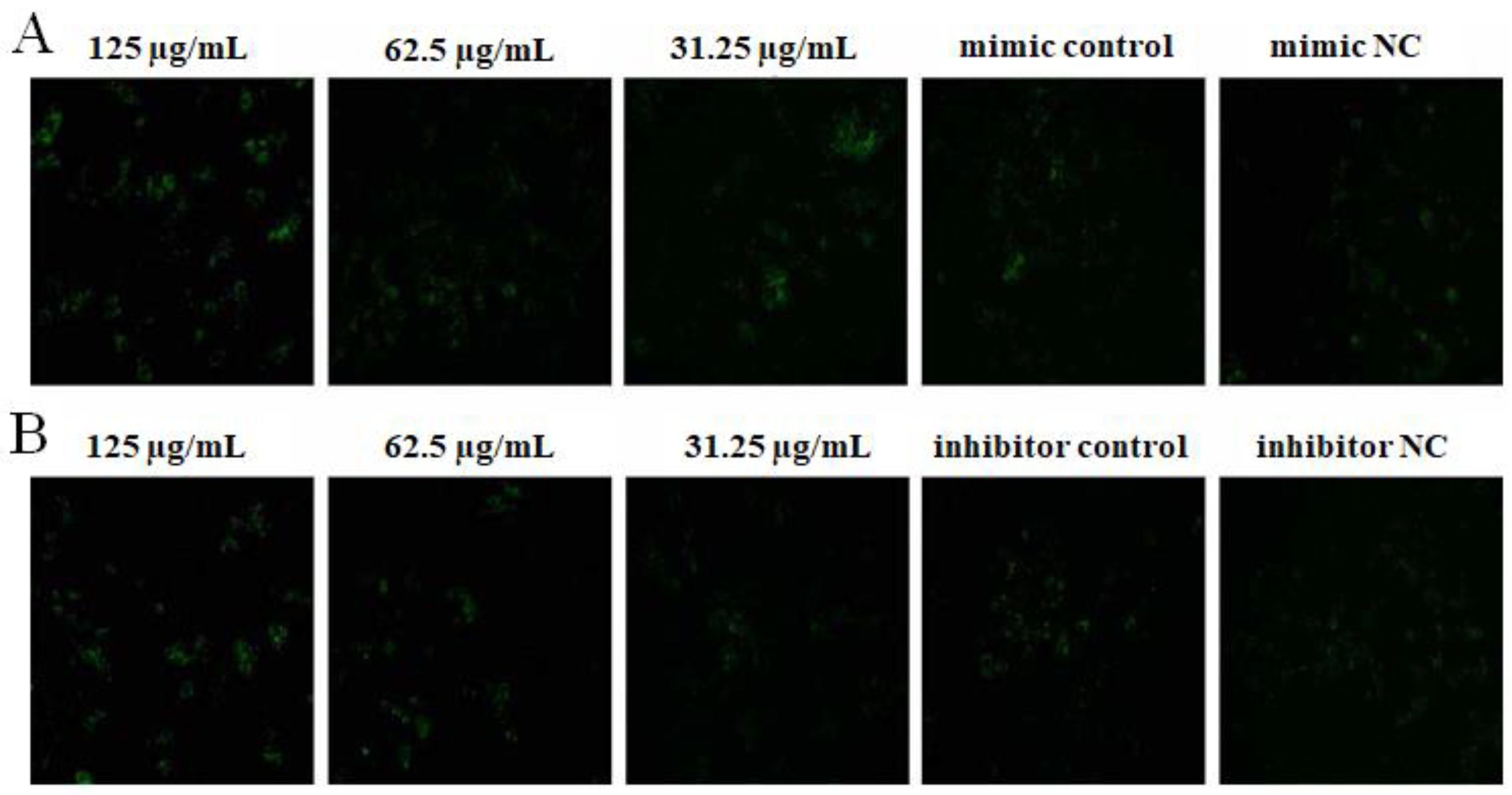
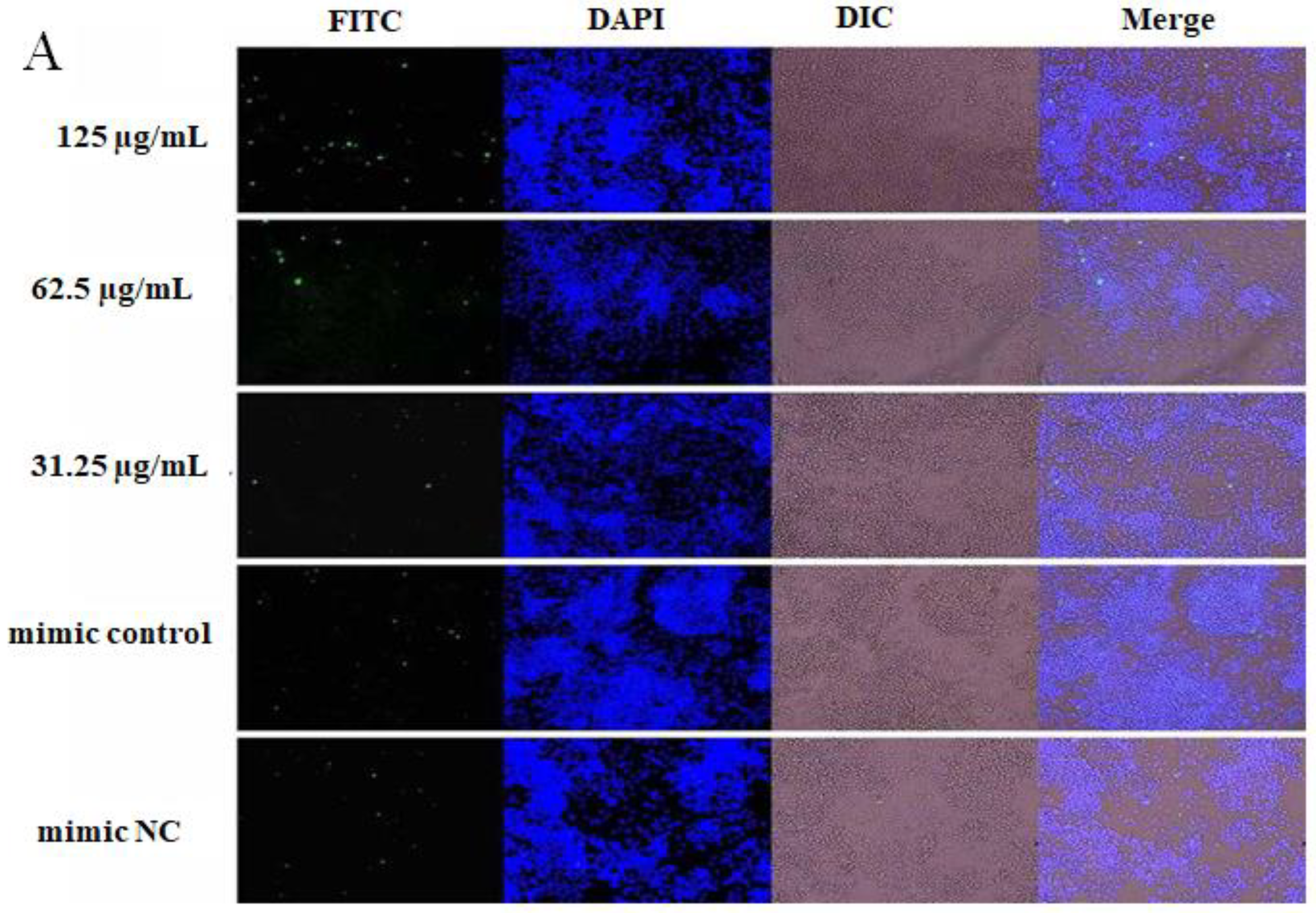
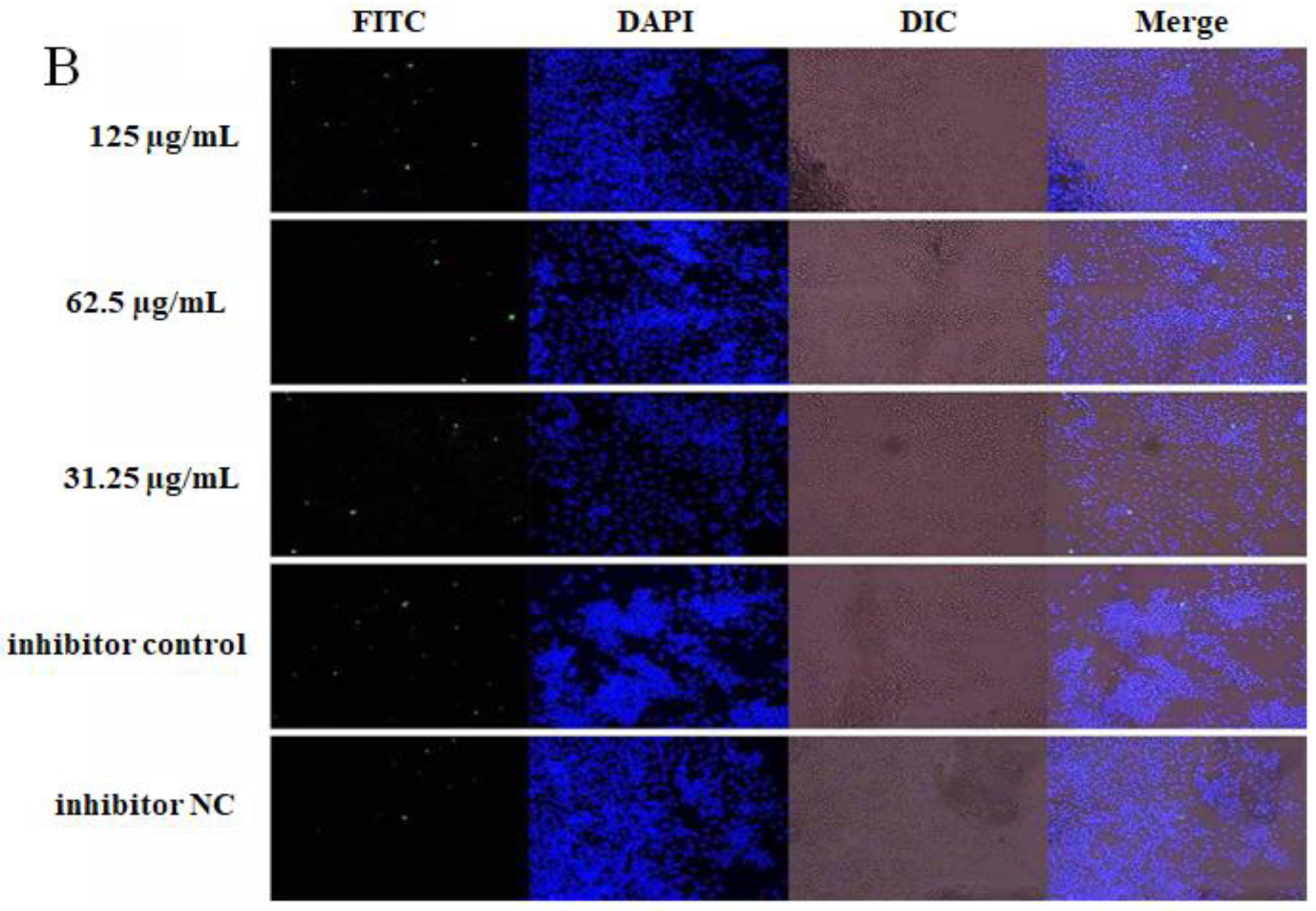
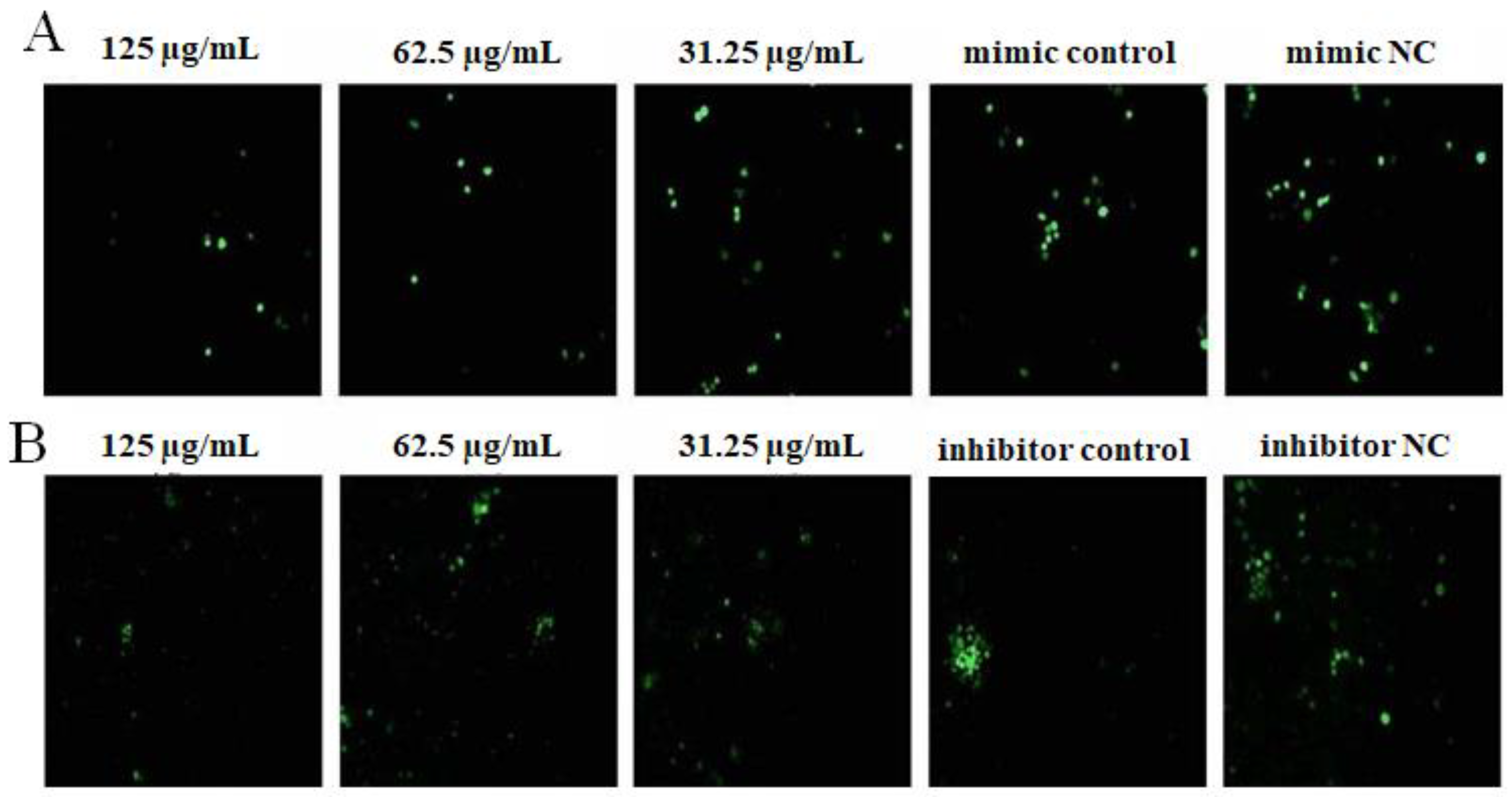
2.4. The Effect of OPL on the Phagocytosis of KCs for FITC-Dextran by Fluorescence Staining
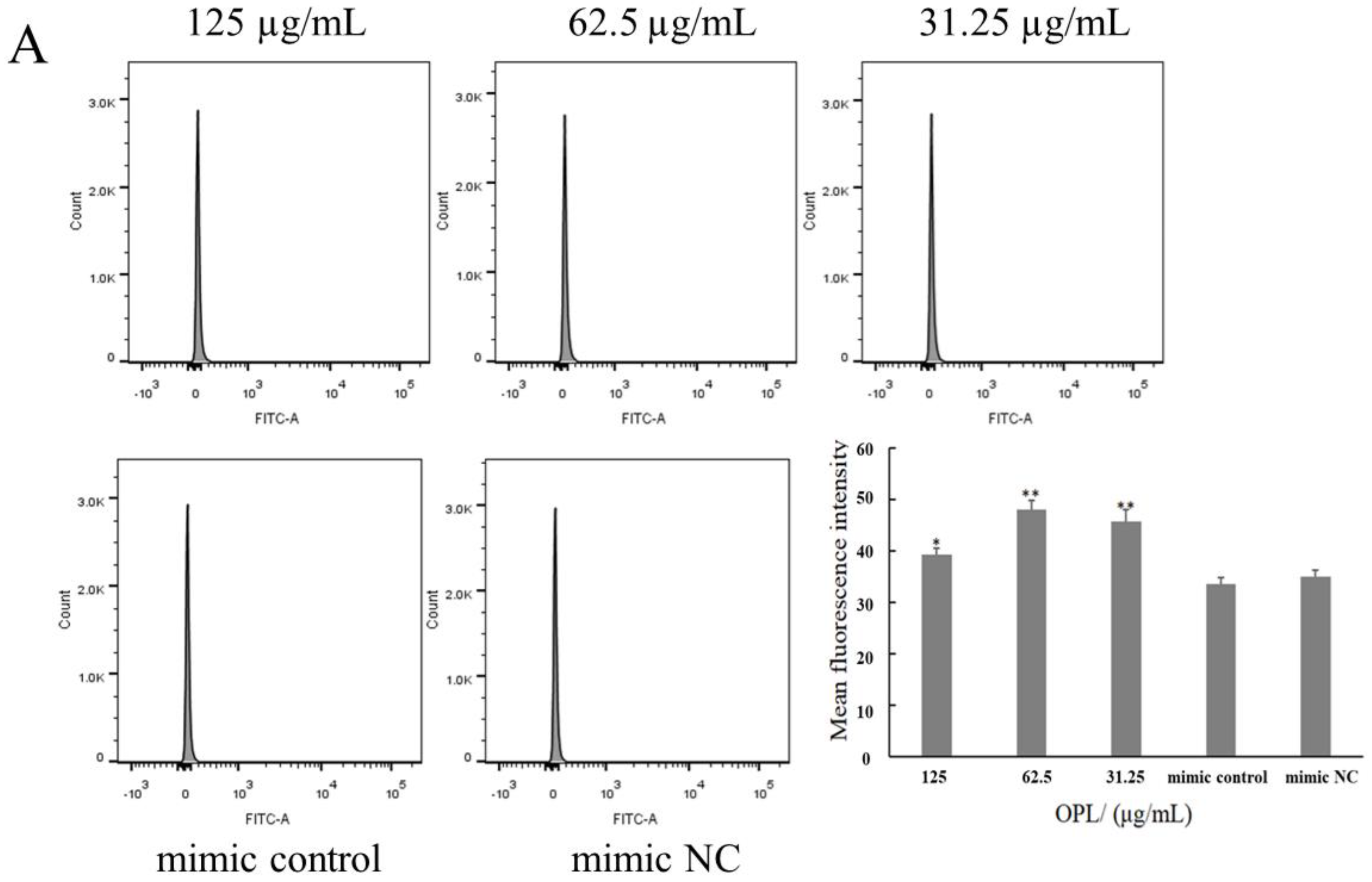
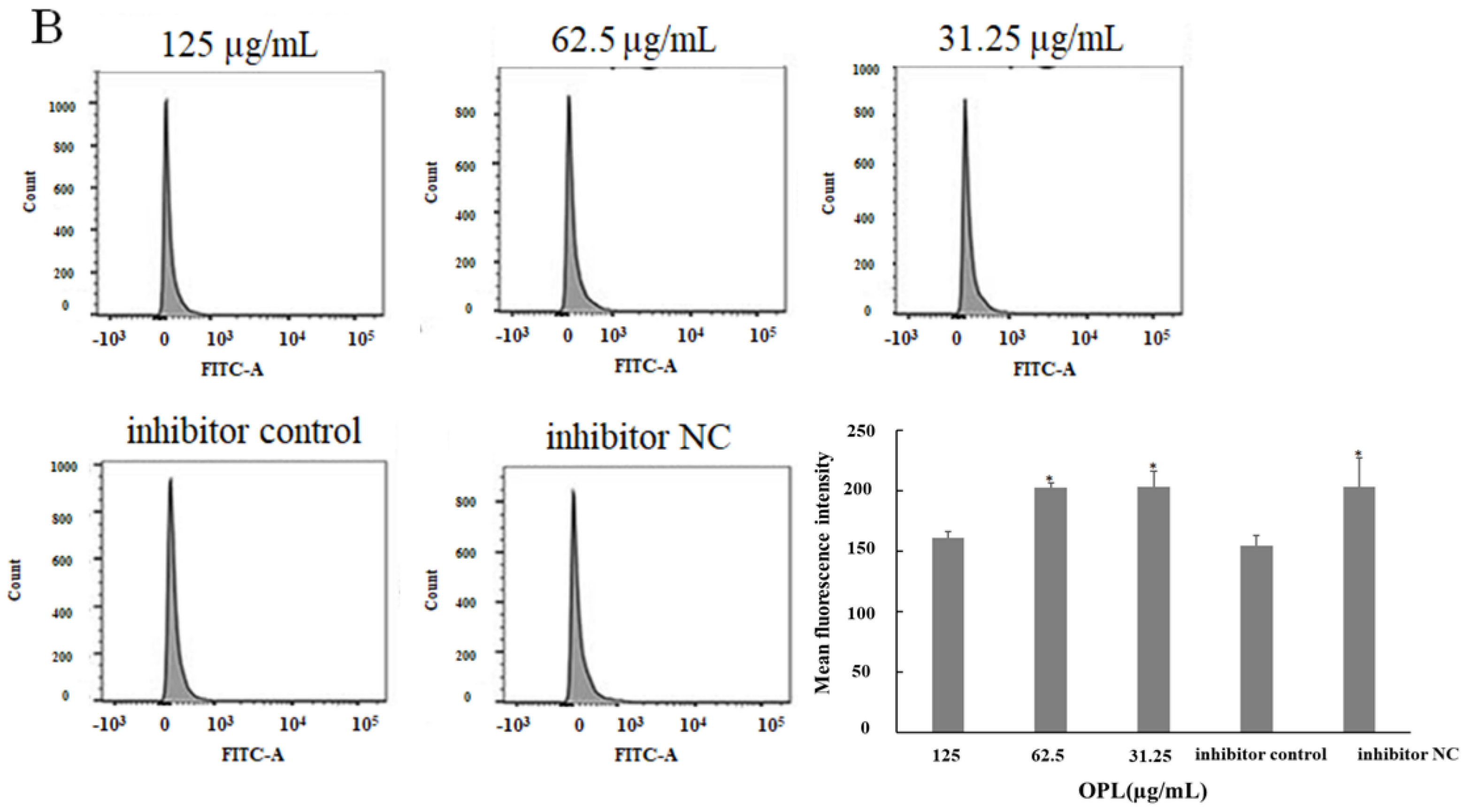
2.5. The Effect of OPL on the Phagocytosis of KCs for FITC-Dextran by Flow Cytometry
2.6. The Effect of OPL on the Phagocytosis of KCs for FITC-OVA
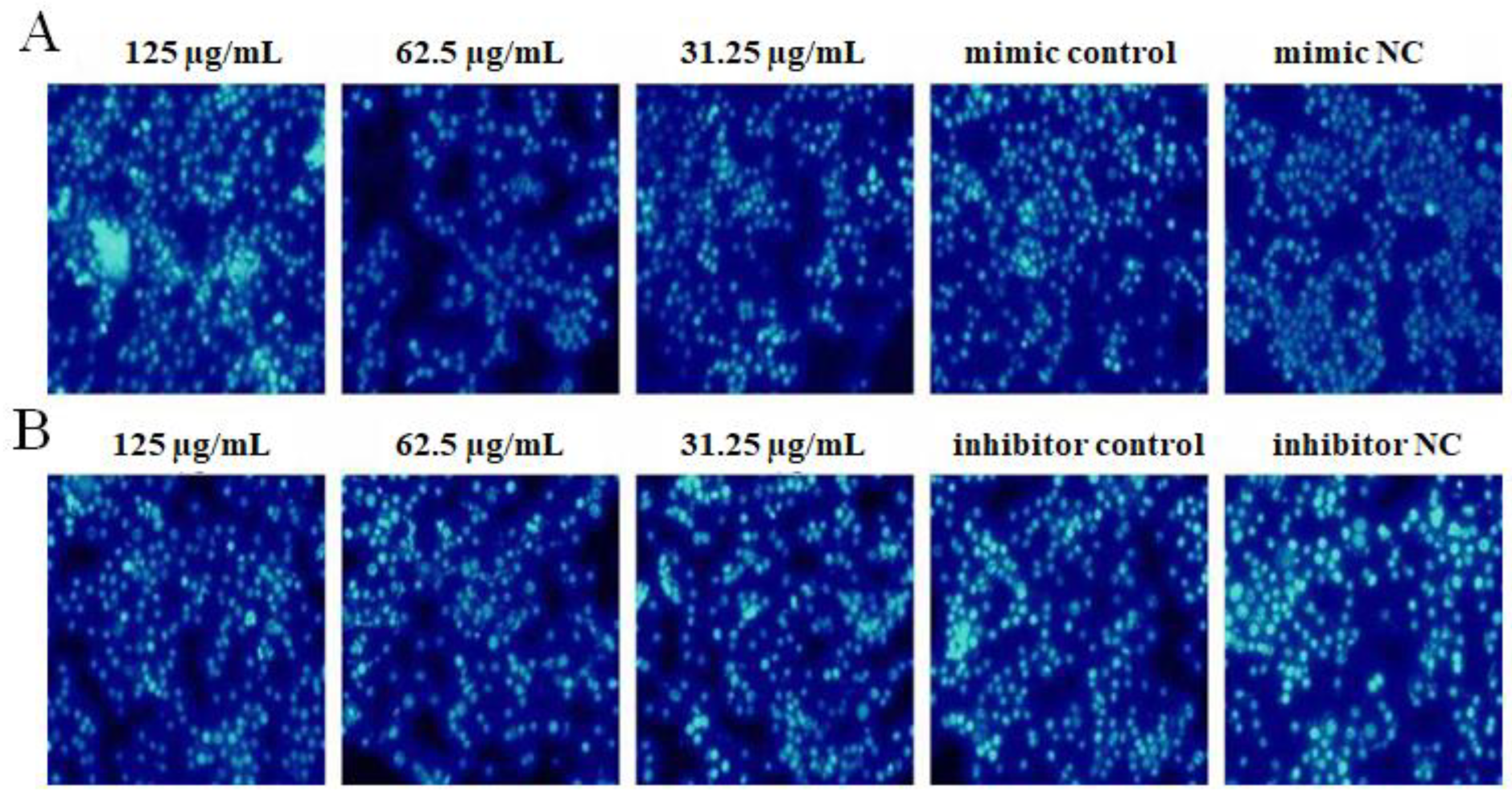
2.7. The Effect of OPL on the Cell Apoptosis
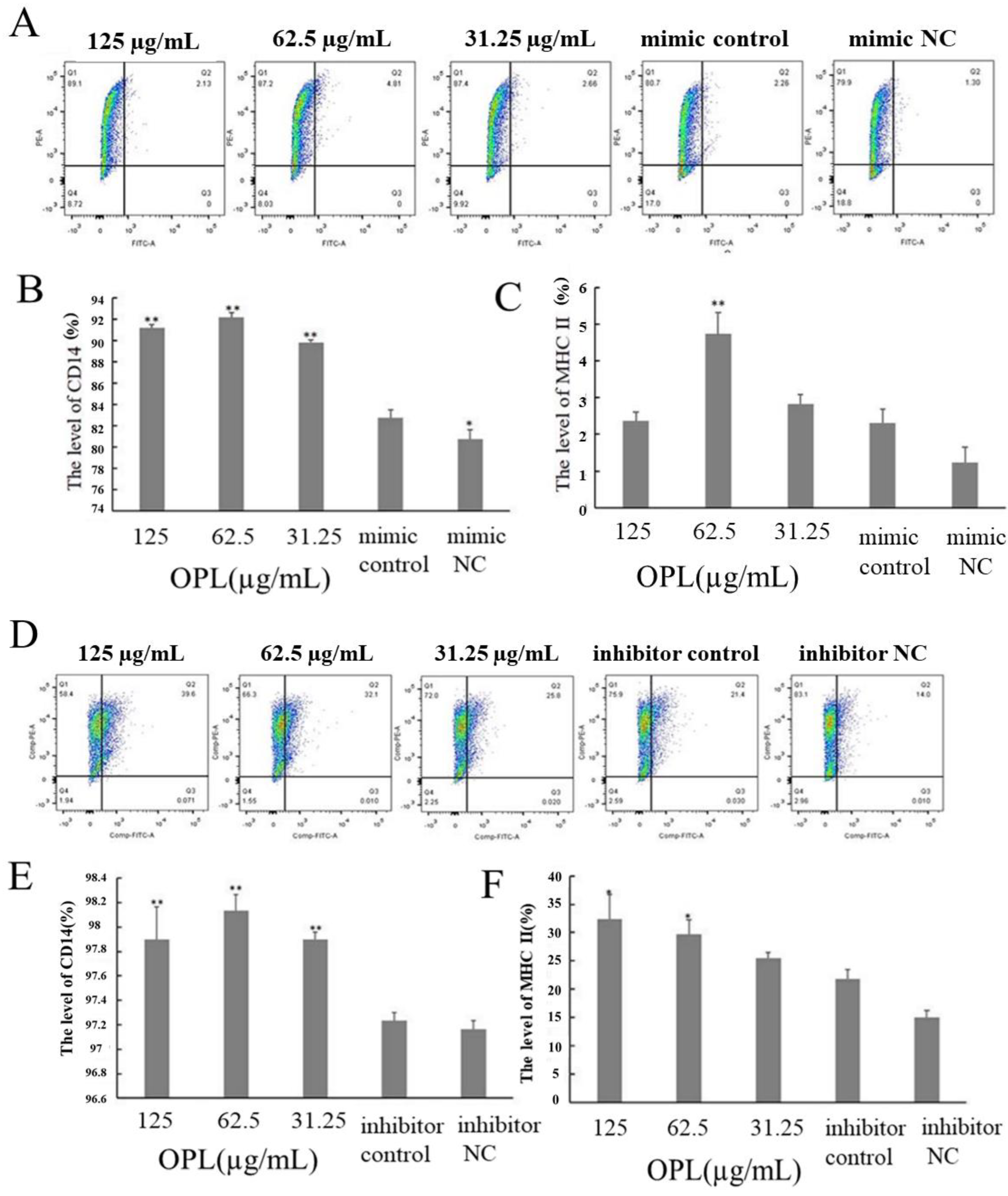
2.8. The Effect of OPL on the Expression of CD14 and MHCII
2.9. The Effect of OPL on the Secretion of ROS
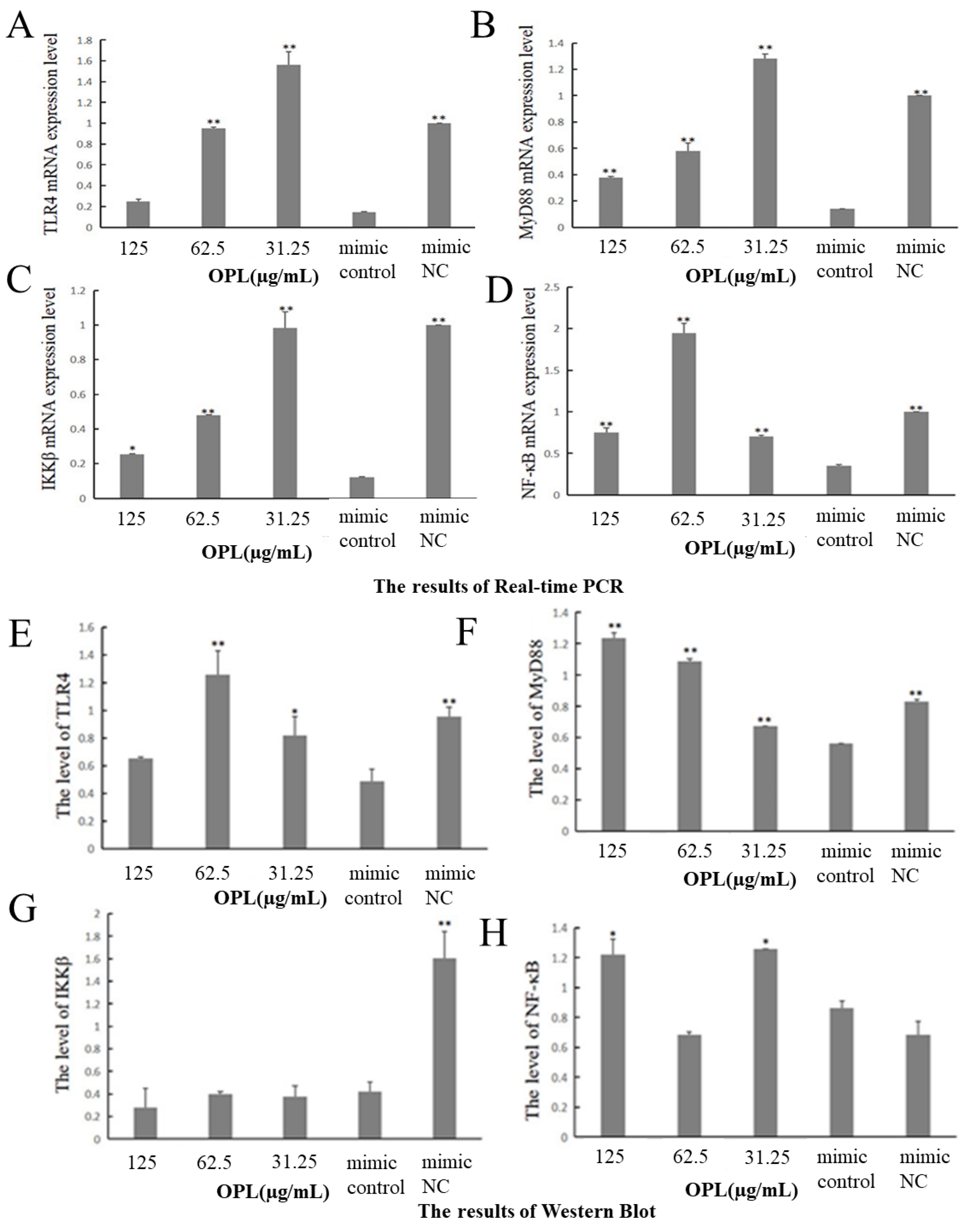
2.10. The Effect of OPL on the mRNA and Protein Expression of TLR4, MyD88, IKKβ and NF-κB
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Cell
4.1.2. Reagent
4.2. Methods
4.2.1. The Preparation of Ophiopogon Polysaccharide Liposomes
4.2.2. Effects of miR-4796 on the Biological Function of KCs Regulated by OPL
The Expression of miR-4796 after Transfected with miR-4796 Mimic or Inhibitor by Real-Time PCR Method
miR-4796 Transfection and Treatment of OPL
Measurement of NO
Measurement of iNOS
Cell Scratch Test
Measurement of Phagocytosis of KCs on FITC-Dextran by Fluorescence Staining and Flow Cytometry
Measurement of Phagocytosis of KCs on FITC-OVA by Fluorescence Staining
Measurement of Apoptosis by Hoechst 33,258 Staining
Measuring the Expression Levels of CD14 and MHC II by Flow Cytometry
Measurement of ROS by Fluorescence Staining
4.2.3. The Function of miR-4796 in TLR4-NF-κB Signaling Pathway Regulated by OPL
Measuring the mRNA Expression of TLR4, IKKβ, MyD88 and NF-κB by Real-Time PCR
Measuring the Protein Expression of TLR4, IKKβ, MyD88 and NF-κB by Western Blot
4.2.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Veterinary Pharmacopoeia Committee. Veterinary Pharmacopoeia of the People’s Republic of China: The First Step of the 2015 Edition; China Medical Science and Technology Press: Beijing, China, 2015; pp. 225–226. [Google Scholar]
- Fan, M.M.; Zhang, J.Y.; Zhang, X.L. Research Progress on Chemical Components and Pharmacological Action of Radix Ophiopogonis. Inf. Tradit. Chin. Med. 2020, 37, 130–134. [Google Scholar]
- Zhu, N.; Lv, X.C.; Wang, Y.Y.; Li, J.L.; Liu, Y.M.; Lu, W.F.; Yang, L.C.; Zhao, J.; Wang, F.J.; Zhang, L.W. Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int. J. Biol. Macromol. 2016, 93 Pt A, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Huang, Q.; Xu, Z.Y.; Cai, W.R.; Qian, H.; Song, K. Study on immune activity of Ophiopogonis polysaccharide. J. Basic Chin. Med. 1998, 4, 44–46. [Google Scholar]
- Li, M. Effect of Ophiopogon japonicas polysaccharide on immune and antioxidant function in training rats. Food Sci. Technol. 2014, 39, 182–186. [Google Scholar]
- Yang, G.; Yang, S.Q.; Xu, D.; Ren, S.J.; Shu, G. Effects of Ophiopogon japonicas polysaccharide on growth performance and immune function of Wugu male chicks with immunosuppression. Feed Ind. 2019, 40, 21–25. [Google Scholar]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Sang, W.J.; Nejati-koshk, K. Liposome: Classification, preparation, and applications. Nanoscale. Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fang, F.; Cao, K.K.; Wang, T.; Xu, H. Liposome preparation technology of lycium barbarum polysaccharide. Food Ferment. Ind. 2018, 44, 176–181. [Google Scholar]
- Zhang, Y.J.; Wu, F.D.; Xu, Z.G.; Ba, J.; Deng, H.; Yang, H. Preparation and Characterization of Astragalus polysaccharide Liposome. Chin. J. Vet. Med. 2019, 55, 72–75+127. [Google Scholar]
- Rao, M.; Peachman, K.K.; Alving, C.R. Liposome Formulations as Adjuvants for Vaccines. Curr. Top. Microbiol. Immunol. 2021, 433, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, L.; Li, E.T.; Li, Y.Y.; Lu, Y.; Wang, P.J.; Zhou, H.L.; Liu, J.G.; Hu, Y.L.; Wang, D.Y. Optimization of Glycyrrhiza polysaccharide liposome by response surface methodology and its immune activities. Int. J. Biol. Macromol. 2017, 102, 68–75. [Google Scholar] [CrossRef]
- Jia, R.; Yan, S.G.; Yan, Y. MicroRNA Mediates Post-transcriptional Regulation of Macrophage Polarization. Chin. J. Biochem. Mol. 2022, 38, 182–190. [Google Scholar] [CrossRef]
- Cheng, L.F.; Wu, N.P. Role of miR-155 in the regulation of lymphocyte immune function and the correlation with virus infection. Int. J. Epidemiol. Infect. Dis. 2016, 43, 47–50. [Google Scholar]
- Li, Y.; Qiu, L.G. Involvement of microRNA-155 in T cell immune responses. Int. J. Blood Transfus. Hematol. 2015, 38, 534–536. [Google Scholar]
- Yu, D.J.; Zhang, T.Q. Meng FR, Hu GX, Wang Q, Research Progress of immune Adjuvant. J. Econ. Anim. 2022, 26, 62–66. [Google Scholar] [CrossRef]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Park, C.; Park, C.; Park, S. IMIPMF: Inferring miRNA-disease interactions using probabilistic matrix factorization. J. Biomed. Inform. 2020, 102, 103358. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Hu, W.J.; Meng, K.; Yang, L.M.; Zhang, W.M.; Song, X.P.; Qu, X.H.; Zhang, Y.Y.; Ma, L.; Fan, Y.P. Activation of macrophages by the ophiopogon polysaccharide liposome from the root tuber of Ophiopogon japonicus. Int. J. Biol. Macromol. 2016, 91, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Song, X.P.; Gao, Y.Y.; Chen, Y.; Ma, L.; Zhang, W.M.; Hou, W.F.; Guo, C.; Tong, D.W. Preparation and optimization of ophiopogon polysaccharide liposome and its activity on Kupffer cells. Int. J. Pharm. 2014, 477, 421–430. [Google Scholar] [CrossRef]
- Fan, Y.P.; Ma, X.; Zhang, J.; Ma, L.; Gao, Y.Y.; Zhang, W.M.; Song, X.P.; Hou, W.F.; Guo, C.; Tong, D.W. Ophiopogon polysaccharide liposome can enhance the non-specific and specific immune response in chickens. Carbohydr. Polym. 2015, 119, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug. Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Pan, S.H.; Wang, Y.P.; Zhang, Y. To investigate the effect of Emodin on fibroblast-like synoviocytes in rheumatoid arthritis based on TNF-α-HIF-1α-iNOS-NO signaling pathway. Lishizhen Med. Mater. Med. Res. 2020, 31, 817–820. [Google Scholar]
- Hou, G.Q.; Chen, H.J.; Yin, Y.H.; Pan, Y.H.; Zhang, X.H.; Jia, F. MEL Ameliorates Post-SAH Cerebral Vasospasm by Affecting the Expression of eNOS and HIF1α via H19/miR-138/eNOS/NO and H19/miR-675/HIF1α. Mol.-Nucl. Acids. 2020, 19, 523–532. [Google Scholar] [CrossRef]
- Pinto Bronson, I.; Cruz Nathan, D.; Lujan Oscar, R.; Propper Catherine, R.; Kellar Robert, S. In Vitro Scratch Assay to Demonstrate Effects of Arsenic on Skin Cell Migration. J. Vis. Exp. (JoVE) 2019, 144, e58838. [Google Scholar]
- Wang, B.; Cheng, Y.F.; Wu, J.; Li, G.L.; Wang, S.M. Effects of polysaccharides from roots of Radix Tetrastigma on proliferation, apoptosis, migration and invasion of HepG2 cells. J. Jinan Univ. 2020, 41, 444–454. [Google Scholar]
- Watanabe, S.; Hirose, M.; Ueno, T.; Kominami, E.; Namihisa, T. Integrity of the cytoskeletal system is important for phagocytosis by Kupffer cells. J. Liver 1990, 10, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.Y.; Xu, Y.; Yang, G. Study on the regulation of miR-34c on the polarization, crystal phagocytosis and migration of macrophages. Lab. Med. Clin. 2021, 18, 1993–1995+2001. [Google Scholar]
- Li, Q.J.; Zhang, Z.W.; Li, H.; Pan, X.Y.; Chen, S.S.; Cui, Z.Y.; Ma, J.; Zhou, Z.X.; Xing, B. Lycium barbarum polysaccharides protects H9c2 cells from hypoxia-induced injury by down-regulation of miR-122. Biomed. Pharmacother. 2019, 110, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.B.; Zhao, B. Astragalus polysaccharide protects hypoxia-induced injury by up-regulation of miR-138 in rat neural stem cells. Biomed. Pharmacother. 2018, 102, 295–301. [Google Scholar] [CrossRef]
- Zhang, S.; Hang, F.H.; Wang, B.Q.; Gao, W.; Wu, L.N.; Li, Y.; Li, W.Y. The expressions and correlations of IL-10, CD14 and CD68 in laryngal squamous cell carcinoma. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 839–843. [Google Scholar]
- Lu, Z.J.; Yu, W.F.; Sun, W.M.; Wang, Z.M. Antigen presenting action and MHC limitation of Kupffer cells in immune response of halothane hepatitis. Acad. J. Nav. Med. Univ. 2002, 23, 617–619. [Google Scholar]
- Li, M.; Wang, J.; Fang, Y.; Gong, S.T.; Li, M.Y.; Wu, M.H.; Lai, X.M.; Zeng, G.C.; Wang, Y.; Yang, K.; et al. MicroRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci. Rep. 2016, 6, 23351. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends. Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.L.; Zheng, G.Q.; You, L.J.; Wen, L.R.; Li, C.; Xiong, F.X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods. 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Jin, W.W.; Dong, L.N.; Wei, M. MiR-155 exerts antimicrobial effects by regulating macrophage polarization and the secretion of ROS/NO. Int. J. Immunol. 2020, 43, 263–269. [Google Scholar]
- Zhang, X.J.; Xue, H.; Zhou, P.; Liu, L.; Yu, J.; Dai, P.F.; Qu, M.Q. Angelica polysaccharide alleviates oxidative response damage in HaCaT cells through up-regulation of miR-126. Exp. Mol. Pathol. 2019, 110, 104281. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, Y. Lycium barbarum polysaccharides alleviate hydrogen peroxide-induced injury by up-regulation of miR-4295 in human trabecular meshwork cells. Exp. Molpathol. 2019, 106, 109–115. [Google Scholar] [CrossRef]
- Yang, F.F.; Shen, X.M.; Huang, X.X.; Zhang, Y. Interactions between Salmonella and host macrophages—Dissecting NF-κB signaling pathway responses. Microb. Pathog. 2021, 154, 104846. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Lin, R.Y.; Hou, X.N.; Wu, J.; Zhao, W.B.; Ma, H.H.; Fan, Z.Y.; Li, S.J.; Zhu, Y.; Zhang, D.Y. Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae Miquel on RAW 264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 164, 4329–4338. [Google Scholar] [CrossRef]
- Ma, Y.J.; Lu, C.W.; Lu, D. Expressions of IKKβ and NF-κB p65 in esophageal cancer and precancerous lesions. Chin. J. Gastroenterol. Hepatol. 2009, 18, 512–514. [Google Scholar]
- Dong, Y.; Zhang, F.; Li, X.X. Effects of Astragalus polysaccharides on ventricular remodeling and miRNA-21 in rats with acute myocardial infarction. J. Hainan Med. Univ. 2021, 27, 572–578. [Google Scholar] [CrossRef]
- Li, R.Y.; Xiang, X.H.; Zhang, B.; Zhang, Q.; Xia, S.H. Oxymatrine participates in regulation of TLR4/NF-κB inflammatory response pathway via miR-211-5p in PSCs. Drug Eval. Res. 2018, 41, 540–546. [Google Scholar]
- Dai, S.L.; Li, X.Q.; Wu, J.; Yue, C.W.; Li, Y.J. CNE2 stem-like cells of nasopharyngeal carcinoma have stem cell characteristics and high level of autophagy. Chin. J. Tissue Eng. Res. 2019, 23, 2703–2708. [Google Scholar]
- Jin, H.; Liu, Z.J.; Yan, C.L.; Zhang, Q.J.; Liu, F.L.; Wu, Z.W.; Jin, L.M. The Influence of Zhengan Xifeng Decoction on the Expressions of mRNA and protein of Cholecystokinin in spontaneously hypertensive rats. Pharmacol. Clin. Chin. Mater. Med. 2018, 34, 7–11. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Pan, X.; Duan, X.; Ke, L.; Song, X.; Zhang, W.; Ma, W.; Liu, Y.; Fan, Y. Ophiopogon Polysaccharide Liposome Regulated the Immune Activity of Kupffer Cell through miR-4796. Int. J. Mol. Sci. 2022, 23, 14659. https://doi.org/10.3390/ijms232314659
Cui J, Pan X, Duan X, Ke L, Song X, Zhang W, Ma W, Liu Y, Fan Y. Ophiopogon Polysaccharide Liposome Regulated the Immune Activity of Kupffer Cell through miR-4796. International Journal of Molecular Sciences. 2022; 23(23):14659. https://doi.org/10.3390/ijms232314659
Chicago/Turabian StyleCui, Jing, Xingxue Pan, Xueqin Duan, Liting Ke, Xiaoping Song, Weimin Zhang, Wuren Ma, Yingqiu Liu, and Yunpeng Fan. 2022. "Ophiopogon Polysaccharide Liposome Regulated the Immune Activity of Kupffer Cell through miR-4796" International Journal of Molecular Sciences 23, no. 23: 14659. https://doi.org/10.3390/ijms232314659




