Nicotinamide Mononucleotide Protects against Retinal Dysfunction in a Murine Model of Carotid Artery Occlusion
Abstract
1. Introduction
2. Results
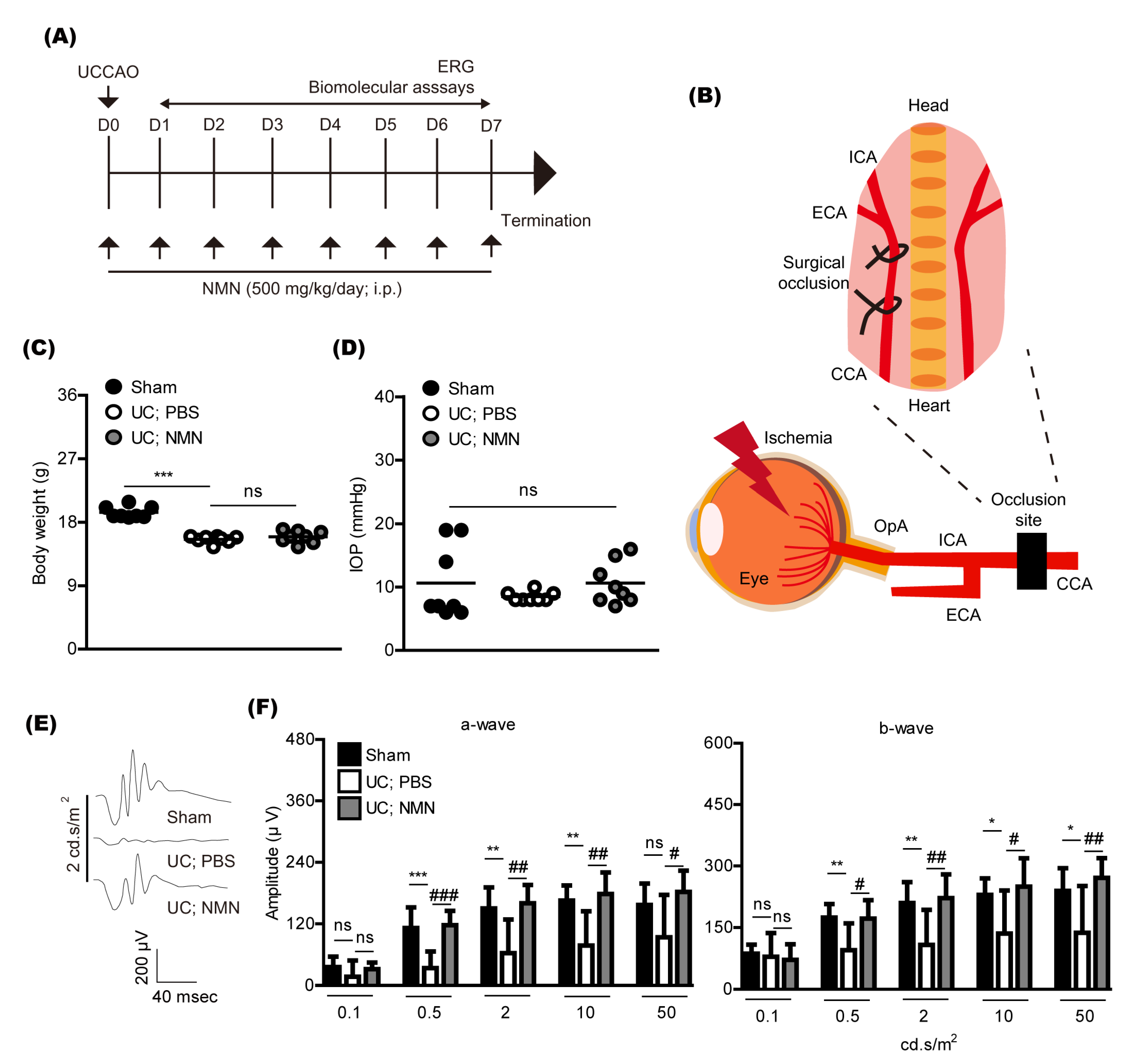
2.1. Consecutive Treatment of NMN Protects against Retinal Dysfunction in a Mouse Model of Unilateral Common Carotid Artery Occlusion
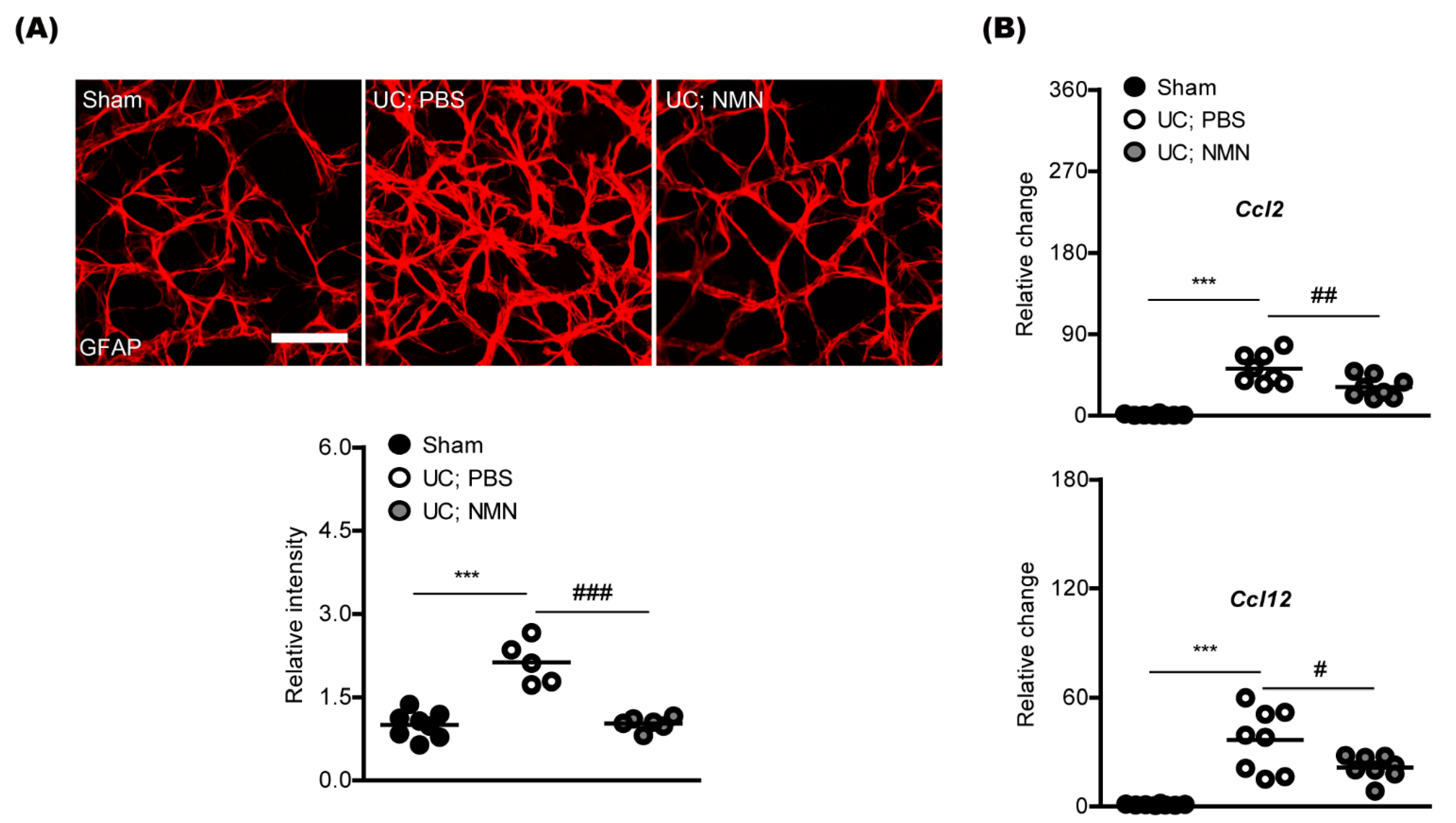
2.2. Consecutive Treatment of NMN Reduces Pathological Gliosis in a Mouse Model of Unilateral Common Carotid Artery Occlusion
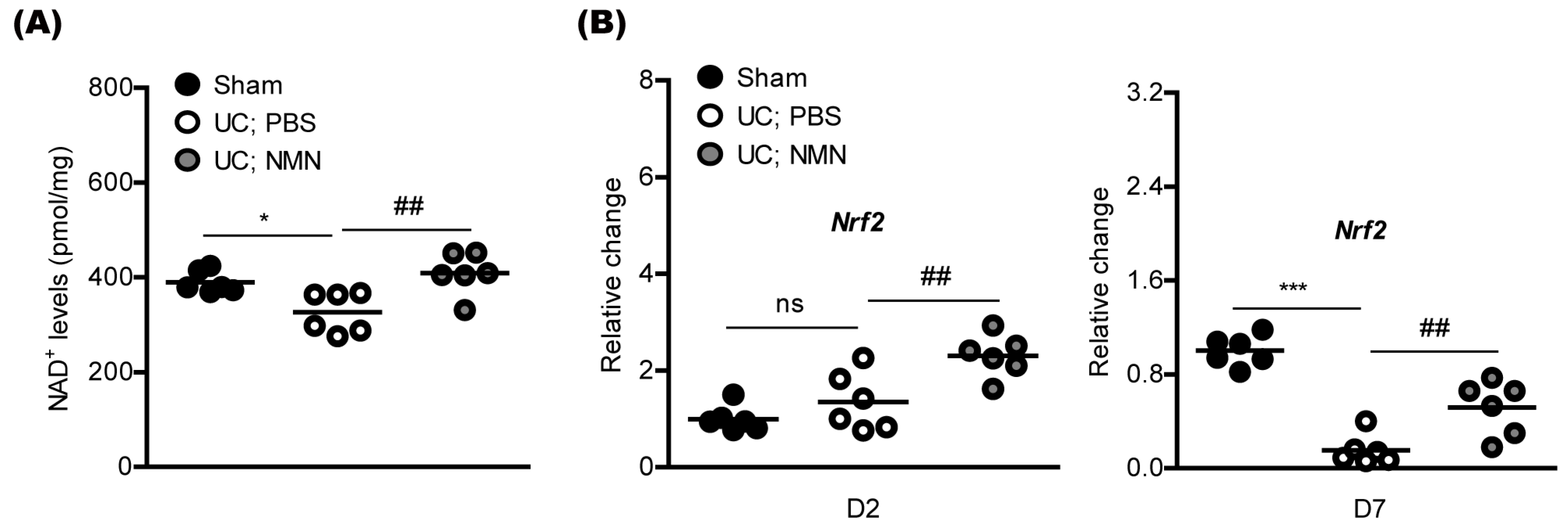
2.3. Consecutive Treatment of NMN Preserves Redox Balance and Activates an Antioxidant Pathway in a Mouse Model of Unilateral Common Carotid Artery Occlusion
2.4. Consecutive Treatment of NMN Does Not Affect Retinal Thickness in a Mouse Model of Unilateral Common Carotid Artery Occlusion
3. Discussion
4. Materials and Methods
4.1. Animal, Unilateral Common Carotid Artery Occlusion, and NMN Treatment
4.2. Electroretinography (ERG) and Optical Coherence Tomography (OCT)
4.3. Immunohistochemistry (IHC)
4.4. Quantitative PCR (qPCR)
4.5. Nicotinamide Adenine Dinucleotide (NAD+) Assay
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilares-Morgado, R.; Nunes, H.M.M.; Dos Reis, R.S.; Barbosa-Breda, J. Management of ocular arterial ischemic diseases: A review. Graefe’s Arch. Clin. Exp. 2022. [Google Scholar] [CrossRef] [PubMed]
- Long, C.P.; Chan, A.X.; Bakhoum, C.Y.; Toomey, C.B.; Madala, S.; Garg, A.K.; Freeman, W.R.; Goldbaum, M.H.; DeMaria, A.N.; Bakhoum, M.F. Prevalence of subclinical retinal ischemia in patients with cardiovascular disease-a hypothesis driven study. EClinicalMedicine 2021, 33, 100775. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Yang, L.; Negishi, K.; Kurihara, T. Ocular Ischemic Syndrome and Its Related Experimental Models. Int. J. Mol. Sci. 2022, 23, 5249. [Google Scholar] [CrossRef]
- Vestergaard, N.; Cehofski, L.J.; Honoré, B.; Aasbjerg, K.; Vorum, H. Animal Models Used to Simulate Retinal Artery Occlusion: A Comprehensive Review. Transl. Vis. Sci. Technol. 2019, 8, 23. [Google Scholar] [CrossRef]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Nadeeshani, H.; Li, J.; Ying, T.; Zhang, B.; Lu, J. Nicotinamide mononucleotide (NMN) as an anti-aging health product-Promises and safety concerns. J. Adv. Res. 2022, 37, 267–278. [Google Scholar] [CrossRef]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of altered NAD metabolism in metabolic disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front. Cell Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Amorim, J.A.; Moustafa, G.A.; Lee, J.J.; Yu, Z.; Ishihara, K.; Iesato, Y.; Barbisan, P.; Ueta, T.; Togka, K.A.; et al. Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging 2020, 12, 24504–24521. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Miwa, Y.; Shinojima, A.; Ban, N.; Yamaguchi, S.; Nishioka, K.; Negishi, K.; Yoshino, J.; Kurihara, T. Nicotinamide Mononucleotide Prevents Retinal Dysfunction in a Mouse Model of Retinal Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 11228. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Kubota, S.; Ban, N.; Yoshida, M.; Santeford, A.; Sene, A.; Nakamura, R.; Zapata, N.; Kubota, M.; Tsubota, K.; et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016, 17, 69–85. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Lee, D.; Nakai, A.; Miwa, Y.; Tomita, Y.; Serizawa, N.; Katada, Y.; Hatanaka, Y.; Tsubota, K.; Negishi, K.; Kurihara, T. Retinal Degeneration in a Murine Model of Retinal Ischemia by Unilateral Common Carotid Artery Occlusion. BioMed Res. Int. 2021, 2021, 7727648. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, H.; Miwa, Y.; Shinojima, A.; Katada, Y.; Tsubota, K.; Kurihara, T. Retinal dysfunction induced in a mouse model of unilateral common carotid artery occlusion. PeerJ 2021, 9, e11665. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, H.; Yoon, K.Y.; Chang, Y.Y.; Song, H.B. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp. Eye Res. 2020, 201, 108275. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Tomita, Y.; Miwa, Y.; Jeong, H.; Mori, K.; Tsubota, K.; Kurihara, T. Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia. Pharmaceuticals 2021, 14, 223. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Jeong, H.; Miwa, Y.; Tsubota, K.; Negishi, K.; Kurihara, T. Pemafibrate Prevents Retinal Dysfunction in a Mouse Model of Unilateral Common Carotid Artery Occlusion. Int. J. Mol. Sci. 2021, 22, 9408. [Google Scholar] [CrossRef] [PubMed]
- Shade, C. The Science Behind NMN-A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integr. Med. 2020, 19, 12–14. [Google Scholar]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Nagahisa, T.; Yamaguchi, S.; Kosugi, S.; Homma, K.; Miyashita, K.; Irie, J.; Yoshino, J.; Itoh, H. Intestinal Epithelial NAD+ Biosynthesis Regulates GLP-1 Production and Postprandial Glucose Metabolism in Mice. Endocrinology 2022, 163, bqac023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dilxat, T.; Shi, Q.; Qiu, T.; Lin, J. The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene 2022, 822, 146348. [Google Scholar] [CrossRef]
- Luo, C.; Ding, W.; Yang, C.; Zhang, W.; Liu, X.; Deng, H. Nicotinamide Mononucleotide Administration Restores Redox Homeostasis via the Sirt3-Nrf2 Axis and Protects Aged Mice from Oxidative Stress-Induced Liver Injury. J. Proteome Res. 2022, 21, 1759–1770. [Google Scholar] [CrossRef]
- Pu, Q.; Guo, X.X.; Hu, J.J.; Li, A.L.; Li, G.G.; Li, X.Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. 2022, 147, 112659. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Kong, Y.Y.; Li, G.Q.; Guan, Y.F.; Wang, P.; Miao, C.Y. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci. Rep. 2017, 7, 717. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Sheng, R.; Qin, Z.H. Pharmacology and Potential Implications of Nicotinamide Adenine Dinucleotide Precursors. Aging Dis. 2021, 12, 1879–1897. [Google Scholar] [CrossRef]
- Palmer, R.D.; Elnashar, M.M.; Vaccarezza, M. Precursor comparisons for the upregulation of nicotinamide adenine dinucleotide. Novel approaches for better aging. Aging Med. 2021, 4, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M.A.; Park, J.H.; Liao, Y.J. Optical coherence tomography study of retinal changes in normal aging and after ischemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.K.; Stanford, M.P.; Shariati, M.A.; Dalal, R.; Liao, Y.J. Optical coherence tomography study of experimental anterior ischemic optic neuropathy and histologic confirmation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5981–5988. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.; Brachert, M.; Albrecht, P.; Ringelstein, M.; Finis, D.; Geerling, G.; Aktas, O.; Guthoff, R. Alterations of the outer retina in non-arteritic anterior ischaemic optic neuropathy detected using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2017, 45, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Kim, Y.C.; Kang, K.T. Clinical Significance of Choroidal Thickness in Eyes with Ocular Ischemic Syndrome. Korean J. Ophthalmol. KJO 2022, 36, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.; Yao, X.; van Hemert, J.; Sagong, M. Clinical significance of metabolic quantification for retinal nonperfusion in diabetic retinopathy. Sci. Rep. 2022, 12, 9342. [Google Scholar] [CrossRef] [PubMed]
- Singh, C. Metabolism and Vascular Retinopathies: Current Perspectives and Future Directions. Diagnostics 2022, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Barroso, I.; McCarthy, M.I. The Genetic Basis of Metabolic Disease. Cell 2019, 177, 146–161. [Google Scholar] [CrossRef]
- Yilmaz, B.S.; Gurung, S.; Perocheau, D.; Counsell, J.; Baruteau, J. Gene therapy for inherited metabolic diseases. J. Mother Child 2020, 24, 53–64. [Google Scholar] [CrossRef]
- Miwa, Y.; Tsubota, K.; Kurihara, T. Effect of midazolam, medetomidine, and butorphanol tartrate combination anesthetic on electroretinograms of mice. Mol. Vis. 2019, 25, 645–653. [Google Scholar]

| Name | Direction | Sequence (5’ → 3’) | Accession Number |
|---|---|---|---|
| Hprt | Forward | TCAGTCAACGGGGGACATAAA | NM_013556.2 |
| Reverse | GGGGCTGTACTGCTTAACCAG | ||
| Nrf2 | Forward | TAGATGACCATGAGTCGCTTGC | NM_010902.4 |
| Reverse | GCCAAACTTGCTCCATGTCC | ||
| Ccl2 | Forward | CCCAATGAGTAGGCTGGAGA | NM_011333.3 |
| Reverse | TCTGGACCCATTCCTTCTTG | ||
| Ccl12 | Forward | GCTACAGGAGAATCACAAGCAGC | NM_011331.3 |
| Reverse | ACGTCTTATCCAAGTGGTTTATGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Tomita, Y.; Miwa, Y.; Jeong, H.; Shinojima, A.; Ban, N.; Yamaguchi, S.; Nishioka, K.; Negishi, K.; Yoshino, J.; et al. Nicotinamide Mononucleotide Protects against Retinal Dysfunction in a Murine Model of Carotid Artery Occlusion. Int. J. Mol. Sci. 2022, 23, 14711. https://doi.org/10.3390/ijms232314711
Lee D, Tomita Y, Miwa Y, Jeong H, Shinojima A, Ban N, Yamaguchi S, Nishioka K, Negishi K, Yoshino J, et al. Nicotinamide Mononucleotide Protects against Retinal Dysfunction in a Murine Model of Carotid Artery Occlusion. International Journal of Molecular Sciences. 2022; 23(23):14711. https://doi.org/10.3390/ijms232314711
Chicago/Turabian StyleLee, Deokho, Yohei Tomita, Yukihiro Miwa, Heonuk Jeong, Ari Shinojima, Norimitsu Ban, Shintaro Yamaguchi, Ken Nishioka, Kazuno Negishi, Jun Yoshino, and et al. 2022. "Nicotinamide Mononucleotide Protects against Retinal Dysfunction in a Murine Model of Carotid Artery Occlusion" International Journal of Molecular Sciences 23, no. 23: 14711. https://doi.org/10.3390/ijms232314711
APA StyleLee, D., Tomita, Y., Miwa, Y., Jeong, H., Shinojima, A., Ban, N., Yamaguchi, S., Nishioka, K., Negishi, K., Yoshino, J., & Kurihara, T. (2022). Nicotinamide Mononucleotide Protects against Retinal Dysfunction in a Murine Model of Carotid Artery Occlusion. International Journal of Molecular Sciences, 23(23), 14711. https://doi.org/10.3390/ijms232314711










