Cell-Specific Response of NSIP- and IPF-Derived Fibroblasts to the Modification of the Elasticity, Biological Properties, and 3D Architecture of the Substrate
Abstract
:1. Introduction
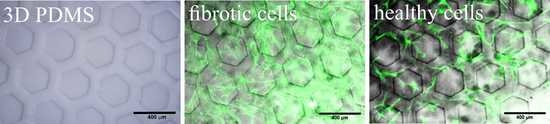
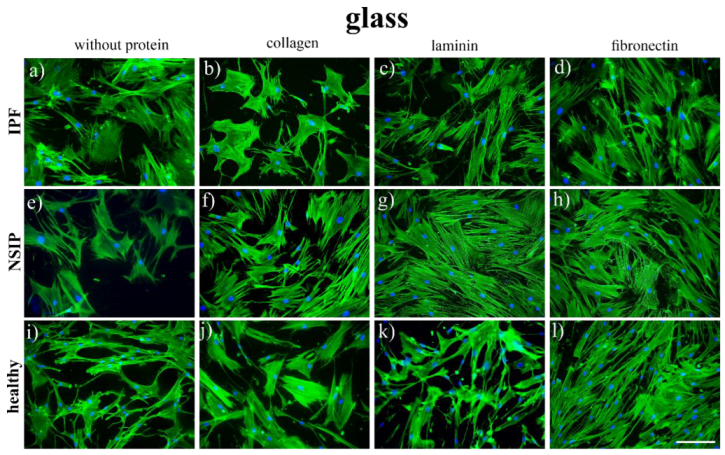
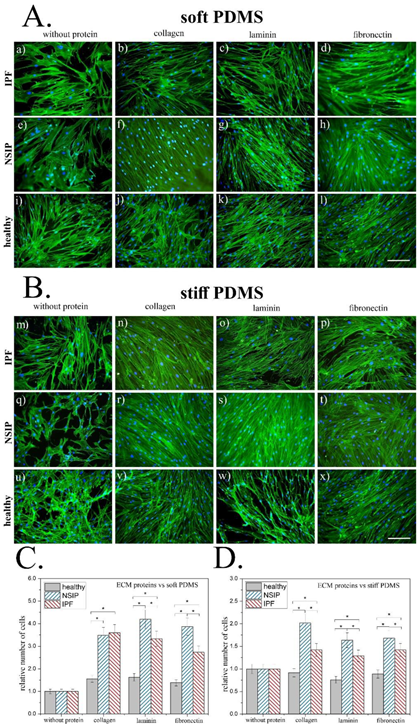
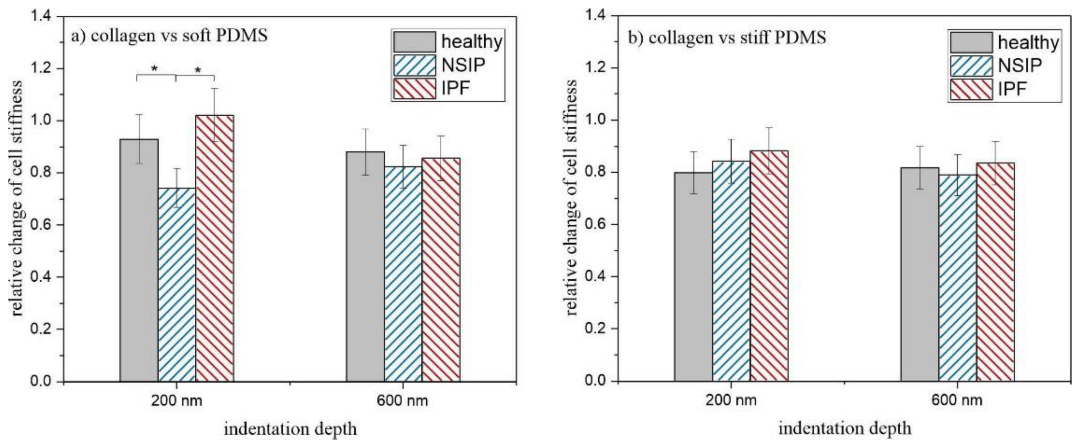
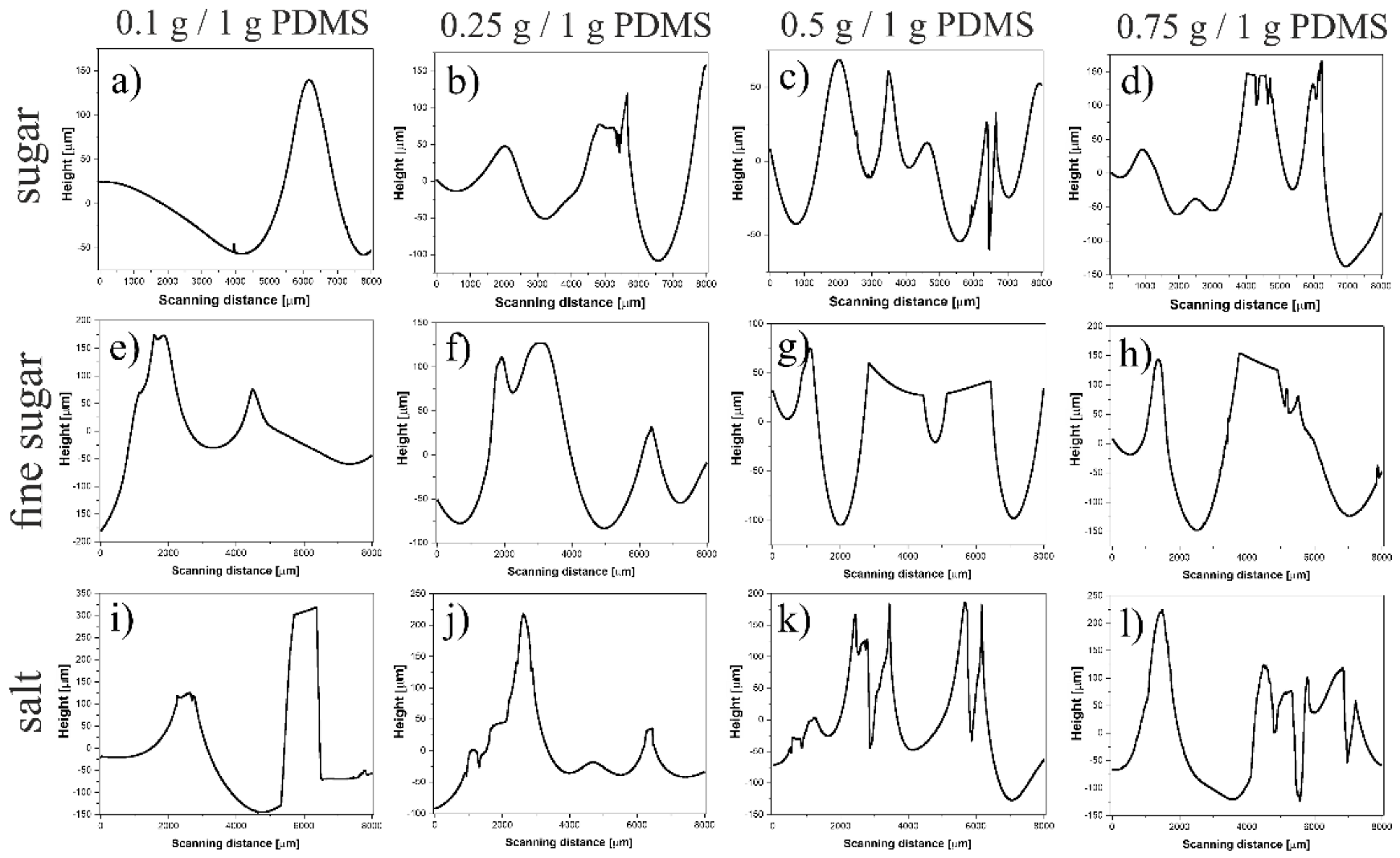
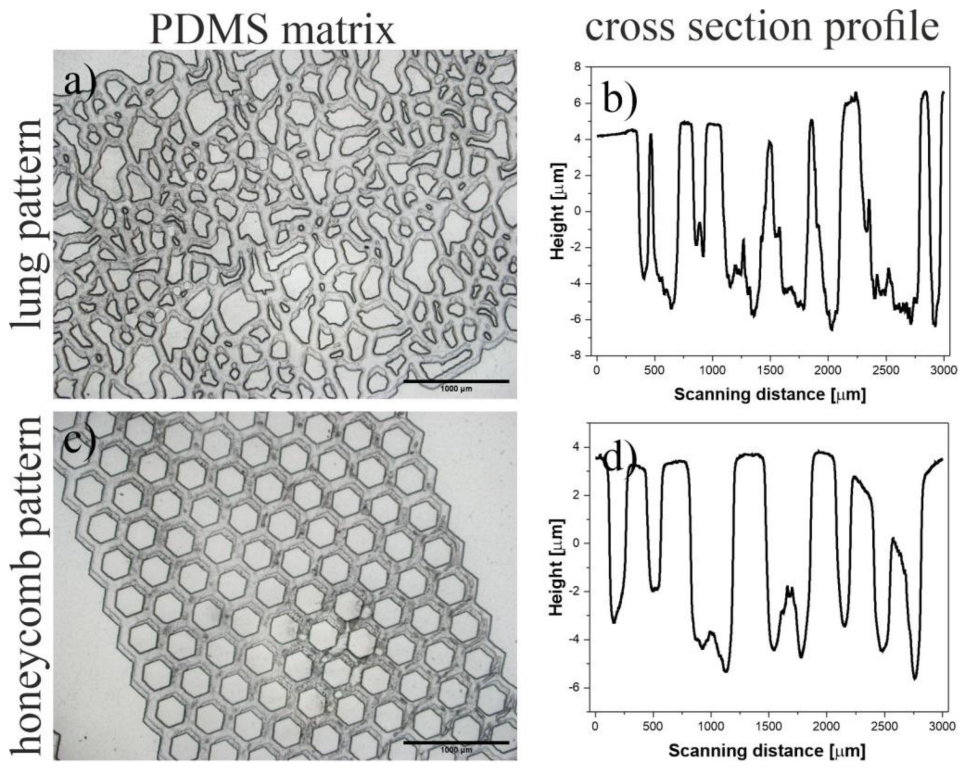
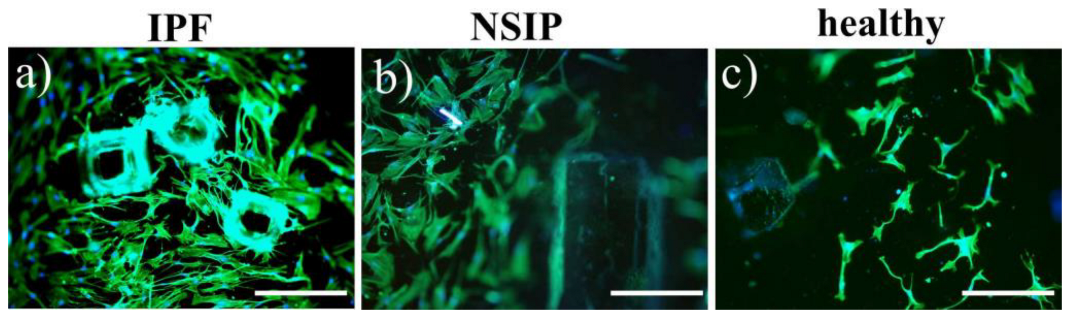
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of PDMS Substrates
4.2. Modification with ECM Proteins
4.3. 3D Patterning
4.4. Profilometry
4.5. Cell Culture
4.6. Fluorescence Microscopy
4.7. Force Spectroscopy
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Bois, R.; King, T.E. Challenges in pulmonary fibrosis 5: The NSIP/UIP debate. Thorax 2007, 62, 1008–1012. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Nagai, S. Idiopathic nonspecific interstitial pneumonia: An unrecognized autoimmune disease? Am. J. Respir. Crit. Care Med. 2007, 176, 632–633. [Google Scholar] [CrossRef]
- Glaspole, I.; Goh, N.S.L. Differentiating between IPF and NSIP. Chron. Respir. Dis. 2010, 7, 187–195. [Google Scholar] [CrossRef]
- Salonen, J.; Purokivi, M.; Bloigu, R.; Kaarteenaho, R. Prognosis and causes of death of patients with acute exacerbation of fibrosing interstitial lung diseases. BMJ Open Respir. Res. 2020, 7, e000563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schär, B. A brief overview of IPF and NSIP. Contin. Med. Educ. 2013, 31, 342–343. [Google Scholar]
- Ley, B.; Collard, H.R.; King, T.E. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Souza, C.A.; Müller, N.L.; Flint, J.; Wright, J.L.; Churg, A. Idiopathic pulmonary fibrosis: Spectrum of high-resolution CT findings. Am. J. Roentgenol. 2005, 185, 1531–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrisi, S.E.; Pavone, M.; Vancheri, A.; Vancheri, C. When to start and when to stop antifibrotic therapies. Eur. Respir. Rev. 2017, 26, 170053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, T.M.; Strek, M.E. Antifibrotic therapy for idiopathic pulmonary fibrosis. Respir. Res. 2019, 20, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathiyamoorthy, G.; Sehgal, S.; Ashton, R. Pirfenidone and Nintedanib for Treatment of Idiopathic Pulmonary Fibrosis. South Med. J. 2017, 110, 393–398. [Google Scholar] [CrossRef]
- Belhassen, M.; Dalon, F.; Nolin, M.; Van Ganse, E. Comparative outcomes in patients receiving pirfenidone or nintedanib for idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Fell, C.D.; Huggins, J.T.; Nunes, H.; Sussman, R.; Valenzuela, C.; Petzinger, U.; Stauffer, J.L.; Gilberg, F.; Bengus, M.; et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52, 1800230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Hunninghake, G.; King, T.E.; Lynch, D.A.; Colby, T.V.; Galvin, J.R.; Brown, K.K.; Man, P.C.; Cordier, J.F.; Du Bois, R.M.; et al. Idiopathic nonspecific interstitial pneumonia: Report of an American Thoracic Society Project. Am. J. Respir. Crit. Care Med. 2008, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Takayanagi, N.; Sugiura, H.; Miyahara, Y.; Tokunaga, D.; Kawabata, Y.; Sugita, Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 2011, 37, 1411–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, K.R.; Andrei, A.C.; King, T.E.; Raghu, G.; Colby, T.V.; Wells, A.; Bassily, N.; Brown, K.; Du Bois, R.; Flint, A.; et al. Idiopathic interstitial pneumonia: Do community and academic physicians agree on diagnosis? Am. J. Respir. Crit. Care Med. 2007, 175, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis An Official ATS/ERS/JRS/ALAT Clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Furini, F.; Carnevale, A.; Casoni, G.L.; Guerrini, G.; Cavagna, L.; Govoni, M.; Sciré, C.A. The role of the multidisciplinary evaluation of interstitial lung diseases: Systematic literature review of the current evidence and future perspectives. Front. Med. 2019, 6, 246. [Google Scholar] [CrossRef] [Green Version]
- Aburto, M.; Herráez, I.; Iturbe, D.; Jiménez-Romero, A. Diagnosis of Idiopathic Pulmonary Fibrosis: Differential Diagnosis. Med. Sci. 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Westergren-Thorsson, G.; Hedström, U.; Nybom, A.; Tykesson, E.; Åhrman, E.; Hornfelt, M.; Maccarana, M.; van Kuppevelt, T.H.; Dellgren, G.; Wildt, M.; et al. Increased deposition of glycosaminoglycans and altered structure of heparan sulfate in idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2017, 83, 27–38. [Google Scholar] [CrossRef]
- Burgess, J.K.; Harmsen, M.C. Chronic lung diseases: Entangled in extracellular matrix. Eur. Respir. Rev. 2022, 31, 210202. [Google Scholar] [CrossRef]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef]
- Decaris, M.L.; Gatmaitan, M.; FlorCruz, S.; Luo, F.; Li, K.; Holmes, W.E.; Hellerstein, M.K.; Turner, S.M.; Emson, C.L. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol. Cell. Proteom. 2014, 13, 1741–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derya, M.; Yilmaz, I.; Aytekin, M. The role of extracellular matrix in lung diseases. Biol. Med. 2014, 6, 200. [Google Scholar] [CrossRef] [Green Version]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickelberg, O.; Köhler, E.; Reichenberger, F.; Bertschin, S.; Woodtli, T.; Erne, P.; Perruchoud, A.P.; Roth, M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 276, L814–L824. [Google Scholar] [CrossRef] [PubMed]
- Merl-Pham, J.; Basak, T.; Knüppel, L.; Ramanujam, D.; Athanason, M.; Behr, J.; Engelhardt, S.; Eickelberg, O.; Hauck, S.M.; Vanacore, R.; et al. Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biol. Plus 2019, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Karsdal, M.A.; Genovese, F.; Johnson, S.; Svensson, B.; Jacobsen, S.; Hägglund, P.; Leeming, D.J. The role of extracellular matrix quality in pulmonary fibrosis. Respiration 2014, 88, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Germanguz, I.; Aranda, E.; Xiong, J.; Kissel, N.; Nichols, A.; Gadee, E.; O’Neill, J. Fibrotic human lung extracellular matrix as a disease-specific substrate for 3D in-vitro models of pulmonary fibrosis. bioRxiv 2019, 833913. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, N.; Chen, H.; Fernandez, G.E.; Warburton, D.; Moats, R.; Mecham, R.P.; Krenitsky, D.; Pryhuber, G.S.; Shi, W. Spatial and temporal changes in extracellular elastin and laminin distribution during lung alveolar development. Sci. Rep. 2018, 8, 8334. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Rocco, P.R.M.; Negrini, D.; Passi, A. The extracellular matrix of the lung and its role in edema formation. An. Acad. Bras. Cienc. 2007, 79, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Henke, C.A.; Horowitz, J.C.; Noble, P.W.; Roman, J.; Sime, P.J.; Zhou, Y.; Wells, R.G.; White, E.S.; Tschumperlin, D.J. Matrix biology of idiopathic pulmonary fibrosis: A workshop report of the national heart, lung, and blood institute. Am. J. Pathol. 2014, 184, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, M.E.; Boeijen, F.R.; Emson, C.L.; Turner, S.M.; Zandieh-Doulabi, B.; Hanemaaijer, R.; Smit, T.H.; Stoop, R.; Everts, V. Extracellular matrix proteins: A positive feedback loop in lung fibrosis? Matrix Biol. 2014, 34, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.L.; Carruthers, A.M.; Mustelin, T.; Murray, L.A. Matrix regulation of idiopathic pulmonary fibrosis: The role of enzymes. Fibrogenes. Tissue Repair 2013, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- James, D.S.; Jambor, A.N.; Chang, H.-Y.; Alden, Z.; Tilbury, K.B.; Sandbo, N.K.; Campagnola, P.J. Probing ECM remodeling in idiopathic pulmonary fibrosis via second harmonic generation microscopy analysis of macro/supramolecular collagen structure. J. Biomed. Opt. 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.G.; Andriotis, O.G.; Roberts, J.J.W.; Lunn, K.; Tear, V.J.; Cao, L.; Ask, K.; Smart, D.E.; Bonfanti, A.; Johnson, P.; et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. eLife 2018, 7, e36354. [Google Scholar] [CrossRef]
- Kulkarni, T.; O’Reilly, P.; Antony, V.B.; Gaggar, A.; Thannickal, V.J. Matrix remodeling in pulmonary fibrosis and emphysema. Am. J. Respir. Cell Mol. Biol. 2016, 54, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muro, A.F.; Moretti, F.A.; Moore, B.B.; Yan, M.; Atrasz, R.G.; Wilke, C.A.; Flaherty, K.R.; Martinez, F.J.; Tsui, J.L.; Sheppard, D.; et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsitoura, E.; Trachalaki, A.; Vasarmidi, E.; Mastrodemou, S.; Margaritopoulos, G.A.; Kokosi, M.; Fanidis, D.; Galaris, A.; Aidinis, V.; Renzoni, E.; et al. Collagen 1a1 Expression by Airway Macrophages Increases In Fibrotic ILDs and Is Associated With FVC Decline and Increased Mortality. Front. Immunol. 2021, 12, 645548. [Google Scholar] [CrossRef] [PubMed]
- Upagupta, C.; Shimbori, C.; Alsilmi, R.; Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 180033. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Cho, S.J.; Cho, W.K.; Park, J.W.; Lee, J.H.; Choi, A.M.; Rosas, I.O.; Zheng, M.; Peltz, G.; Lee, C.G.; et al. Laminin α1 is a genetic modifier of TGF-β1-stimulated pulmonary fibrosis. JCI Insight 2018, 3, e99574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.D.; Mao, Y.; Ying, Y.G. The involvement of the laminin-integrin α7β1 signaling pathway in mechanical ventilation-induced pulmonary fibrosis. J. Thorac. Dis. 2017, 9, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Morales-Nebreda, L.I.; Rogel, M.R.; Eisenberg, J.L.; Hamill, K.J.; Soberanes, S.; Nigdelioglu, R.; Chi, M.; Cho, T.; Radigan, K.A.; Ridge, K.M.; et al. Lung-specific loss of a3 laminin worsens bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Gu, H.; Weng, D.; Zhou, Y.; Li, Q.; Zhang, F.; Zhang, Y.; Shen, L.; Hu, Y.; Li, H. Association of serum levels of laminin, type IV collagen, procollagen III N-terminal peptide, and hyaluronic acid with the progression of interstitial lung disease. Medicine 2017, 96, e6617. [Google Scholar] [CrossRef]
- Korfei, M.; von der Beck, D.; Henneke, I.; Markart, P.; Ruppert, C.; Mahavadi, P.; Ghanim, B.; Klepetko, W.; Fink, L.; Meiners, S.; et al. Comparative proteome analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia (NSIP) and organ donors. J. Proteom. 2013, 85, 109–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, E.R.; Kairalla, R.A.; De Carvalho, C.R.R.; Capelozzi, V.L. Abnormal deposition of collagen/elastic vascular fibres and prognostic significance in idiopathic interstitial pneumonias. Thorax 2007, 62, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.J.; Chang, W.A.; Liao, S.H.; Chang, K.F.; Sheu, C.C.; Kuo, P.L. The effects of epigallocatechin gallate (EGCG) on pulmonary fibroblasts of idiopathic pulmonary fibrosis (Ipf)—A next-generation sequencing and bioinformatic approach. Int. J. Mol. Sci. 2019, 20, 1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicens-Zygmunt, V.; Estany, S.; Colom, A.; Montes-Worboys, A.; Machahua, C.; Sanabria, A.J.; Llatjos, R.; Escobar, I.; Manresa, F.; Dorca, J.; et al. Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir. Res. 2015, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrini, J.L.; Chaudhry, S.; Sarrazy, V.; Koehler, A.; Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012, 4, 410–421. [Google Scholar] [CrossRef]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haak, A.J.; Tan, Q.; Tschumperlin, D.J. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol. 2018, 73, 64–76. [Google Scholar] [CrossRef]
- Hinz, B. Mechanical aspects of lung fibrosis: A spotlight on the myofibroblast. Proc. Am. Thorac. Soc. 2012, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Marinković, A.; Liu, F.; Tschumperlin, D.J. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am. J. Respir. Cell Mol. Biol. 2013, 48, 422–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarelli, A.V.; Tonelli, R.; Marchioni, A.; Bruzzi, G.; Gozzi, F.; Andrisani, D.; Castaniere, I.; Manicardi, L.; Moretti, A.; Tabbì, L.; et al. Fibrotic idiopathic interstitial lung disease: The molecular and cellular key players. Int. J. Mol. Sci. 2021, 22, 8952. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Venkatesan, N.; Tanaka, R.; Ludwig, M.S. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis: Temporal aspects. Am. J. Respir. Crit. Care Med. 2000, 162, 1569–1576. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [Green Version]
- Orzechowska, B.; Awsiuk, K.; Wnuk, D.; Pabijan, J.; Stachura, T.; Soja, J.; Sładek, K.; Raczkowska, J. Discrimination between NSIP-and IPF-Derived Fibroblasts Based on Multi-Parameter Characterization of Their Growth, Morphology and Physic-Chemical Properties. Int. J. Mol. Sci. 2022, 23, 2162. [Google Scholar] [CrossRef]
- Miki, H.; Mio, T.; Nagai, S.; Hoshino, Y.; Nagao, T.; Kitaichi, M.; Izumi, T. Fibroblast Contractility. Am. J. Respir. Crit. Care Med. 2000, 162, 2259–2264. [Google Scholar] [CrossRef]
- Parker, M.W.; Rossi, D.; Peterson, M.; Smith, K.; Sikström, K.; White, E.S.; Connett, J.E.; Henke, C.A.; Larsson, O.; Bitterman, P.B. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 2014, 124, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 70062. [Google Scholar] [CrossRef] [Green Version]
- Belloli, E.A.; Beckford, R.; Hadley, R.; Flaherty, K.R. Idiopathic non-specific interstitial pneumonia. Respirology 2016, 21, 259–268. [Google Scholar] [CrossRef]
- Park, I.N.; Jegal, Y.; Kim, D.S.; Do, K.H.; Yoo, B.; Shim, T.S.; Lim, C.M.; Lee, S.D.; Koh, Y.; Kim, W.S.; et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur. Respir. J. 2009, 33, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, P.; Cavanagh, B.L.; Ahearne, M. Effect of substrate topography on the regulation of human corneal stromal cells. Colloids Surf. B Biointerfaces 2020, 190, 110971. [Google Scholar] [CrossRef]
- Guo, Z.; Genlong, J.; Huang, Z.; Li, H.; Ge, Y.; Wu, Z.; Yu, P.; Li, Z. Synergetic effect of growth factor and topography on fibroblast proliferation. Biomed. Phys. Eng. Express 2020, 6, 65036. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudo, M.; Trichet, L.; Le Digabel, J.; Richert, A.; Hersen, P.; Ladoux, B. Substrate topography induces a crossover from 2D to 3D behavior in fibroblast migration. Biophys. J. 2009, 97, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Pacha-Olivenza, M.Á.; Tejero, R.; Fernández-Calderón, M.C.; Anitua, E.; Troya, M.; González-Martín, M.L. Relevance of Topographic Parameters on the Adhesion and Proliferation of Human Gingival Fibroblasts and Oral Bacterial Strains. Biomed. Res. Int. 2019, 2019, 8456342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziano, A.; d’Aquino, R.; Cusella-De Angelis, M.G.; Laino, G.; Piattelli, A.; Pacifici, M.; de Rosa, A.; Papaccio, G. Concave Pit-Containing Scaffold Surfaces Improve Stem Cell-Derived Osteoblast Performance and Lead to Significant Bone Tissue Formation. PLoS ONE 2007, 2, e496. [Google Scholar] [CrossRef] [Green Version]
- Cun, X.; Hosta-Rigau, L. Topography: A biophysical approach to direct the fate of mesenchymal stem cells in tissue engineering applications. Nanomaterials 2020, 10, 2070. [Google Scholar] [CrossRef]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Geiser, T.; et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Groll, J.; Schmid, O. Real-Time Measurement of Cell Mechanics as a Clinically Relevant Readout of an In Vitro Lung Fibrosis Model Established on a Bioinspired Basement Membrane. Adv. Mater. 2022, 34, 2205083. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef]
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface 2014, 11, 20140687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoica, I.; Barzic, A.I.; Butnaru, M.; Doroftei, F.; Hulubei, C. Surface topography effect on fibroblasts population on epiclon-based polyimide films. J. Adhes. Sci. Technol. 2015, 29, 2190–2207. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Reinmann, A.; Bruinink, A. The effect of topographic characteristics on cell migration velocity. Biomaterials 2006, 27, 5230–5241. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the Micro- and nanoscale to control cell function. Angew. Chem. -Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elter, P.; Weihe, T.; Lange, R.; Gimsa, J.; Beck, U. The influence of topographic microstructures on the initial adhesion of L929 fibroblasts studied by single-cell force spectroscopy. Eur. Biophys. J. 2011, 40, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Berry, C.C.; Campbell, G.; Spadiccino, A.; Robertson, M.; Curtis, A.S.G. The influence of microscale topography on fibroblast attachment and motility. Biomaterials 2004, 25, 5781–5788. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [Green Version]
- Yuen, P.K.; Su, H.; Goral, V.N.; Fink, K.A. Three-dimensional interconnected microporous poly(dimethylsiloxane) microfluidic devices. Lab Chip 2011, 11, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012, 41, 79–87. [Google Scholar] [CrossRef]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If Cell Mechanics Can Be Described by Elastic Modulus: Study of Different Models and Probes Used in Indentation Experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Xu, G.K.; Wang, G.F. Are elastic moduli of biological cells depth dependent or not? Another explanation using a contact mechanics model with surface tension. Soft Matter 2018, 14, 7534–7541. [Google Scholar] [CrossRef] [PubMed]
- Michalik, M.; Pierzchalska, M.; Legutko, A.; Ura, M.; Ostaszewska, A.; Soja, J.; Sanak, M. Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture. Med. Sci. Monit. 2009, 15, 194–201. [Google Scholar]
- Michalik, M.; Wójcik-Pszczoła, K.; Paw, M.; Wnuk, D.; Koczurkiewicz, P.; Sanak, M.; Pękala, E.; Madeja, Z. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell. Mol. Life Sci. 2018, 75, 3943–3961. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.H.; Lee, S.K.; Sammut, D.; Davies, D.E.; Howarth, P.H. Preliminary study of the cellular characteristics of primary bronchial fibroblasts in patients with asthma: Expression of α-smooth muscle actin, fibronectin containing extra type III domain a, and smoothelin. J. Investig. Allergol. Clin. Immunol. 2012, 22, 20–27. [Google Scholar]
- Holm Nielsen, S.; Willumsen, N.; Leeming, D.J.; Daniels, S.J.; Brix, S.; Karsdal, M.A.; Genovese, F.; Nielsen, M.J. Serological Assessment of Activated Fibroblasts by alpha-Smooth Muscle Actin (α-SMA): A Noninvasive Biomarker of Activated Fibroblasts in Lung Disorders. Transl. Oncol. 2019, 12, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Hung, C. Origin of Myofibroblasts in Lung Fibrosis. Curr. Tissue Microenviron. Rep. 2020, 1, 155–162. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Xu, H.; Zhang, Z.; Xu, C. Effect of topography parameters on cellular morphology during guided cell migration on a graded micropillar surface. Acta Bioeng. Biomech. 2021, 23, 147–157. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter 2016, 28, 183001. [Google Scholar] [CrossRef]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zennaro, C.; Rastaldi, M.P.; Bakeine, G.J.; Delfino, R.; Tonon, F.; Farra, R.; Grassi, G.; Artero, M.; Tormen, M.; Carraro, M. Ananoporous surface is essential for glomerular podocyte differentiation in three-dimensional culture. Int. J. Nanomed. 2016, 11, 4957–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Jiang, Z.; Zhou, L.; Zhao, K.; Ma, X.; Cheng, G. Hydrophilic cell-derived extracellular matrix as a niche to promote adhesion and differentiation of neural progenitor cells. RSC Adv. 2017, 7, 45587–45594. [Google Scholar] [CrossRef]
- Gabasa, M.; Duch, P.; Jorba, I.; Giménez, A.; Lugo, R.; Pavelescu, I.; Rodríguez-Pascual, F.; Molina-Molina, M.; Xaubet, A.; Pereda, J.; et al. Epithelial contribution to the profibrotic stiff microenvironment and myofibroblast population in lung fibrosis. Mol. Biol. Cell 2017, 28, 3741–3755. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Fiore, V.F.; Sulchek, T.A.; Barker, T.H. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J. Pathol. 2013, 229, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Riehle, M.O.; Wilkinson, C.D.W.; Curtis, A.S.G. Investigating filopodia sensing using arrays of defined nano-pits down to 35 nm diameter in size. Int. J. Biochem. Cell Biol. 2004, 36, 2005–2015. [Google Scholar] [CrossRef]
- Ito, Y.; Awano, N.; Inomata, M.; Kuse, N.; Tone, M.; Takada, K.; Fujimoto, K.; Muto, Y.; Kumasaka, T.; Izumo, T. An autopsy case of idiopathic pulmonary fibrosis with remarkable honeycomb cyst expansion. Respir. Med. Case Rep. 2022, 36, 101588. [Google Scholar] [CrossRef]
- Hochhegger, B.; Marchiori, E.; Zanon, M.; Rubin, A.S.; Fragomeni, R.; Altmayer, S.; Carvalho, C.R.R.; Baldi, B.G. Imaging in idiopathic pulmonary fibrosis: Diagnosis and mimics. Clinics 2019, 74, e225. [Google Scholar] [CrossRef]
- Wellman, T.J.; Mondonedo, J.R.; Davis, G.S.; Bates, J.H.T.; Suki, B. Topographic Distribution of Idiopathic Pulmonary Fibrosis: A hybrid physics- and agent-based model. Physiol. Meas. 2019, 39, 064007. [Google Scholar] [CrossRef]
- Raczkowska, J.; Rysz, J.; Budkowski, A.; Lekki, J.; Lekka, M.; Bernasik, A.; Kowalski, K.; Czuba, P. Surface patterns in solvent-cast polymer blend films analyzed with an integral-geometry approach. Macromolecules 2003, 36, 2419–2427. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Starodubtseva, M.N.; Yegorenkov, N.I.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska, N.; Orzechowska, B.; Awsiuk, K.; Rysz, J.; Tymetska, S.; Raczkowska, J. Cell-Specific Response of NSIP- and IPF-Derived Fibroblasts to the Modification of the Elasticity, Biological Properties, and 3D Architecture of the Substrate. Int. J. Mol. Sci. 2022, 23, 14714. https://doi.org/10.3390/ijms232314714
Janiszewska N, Orzechowska B, Awsiuk K, Rysz J, Tymetska S, Raczkowska J. Cell-Specific Response of NSIP- and IPF-Derived Fibroblasts to the Modification of the Elasticity, Biological Properties, and 3D Architecture of the Substrate. International Journal of Molecular Sciences. 2022; 23(23):14714. https://doi.org/10.3390/ijms232314714
Chicago/Turabian StyleJaniszewska, Natalia, Barbara Orzechowska, Kamil Awsiuk, Jakub Rysz, Svitlana Tymetska, and Joanna Raczkowska. 2022. "Cell-Specific Response of NSIP- and IPF-Derived Fibroblasts to the Modification of the Elasticity, Biological Properties, and 3D Architecture of the Substrate" International Journal of Molecular Sciences 23, no. 23: 14714. https://doi.org/10.3390/ijms232314714
APA StyleJaniszewska, N., Orzechowska, B., Awsiuk, K., Rysz, J., Tymetska, S., & Raczkowska, J. (2022). Cell-Specific Response of NSIP- and IPF-Derived Fibroblasts to the Modification of the Elasticity, Biological Properties, and 3D Architecture of the Substrate. International Journal of Molecular Sciences, 23(23), 14714. https://doi.org/10.3390/ijms232314714