Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis
Abstract
1. Introduction
2. Results
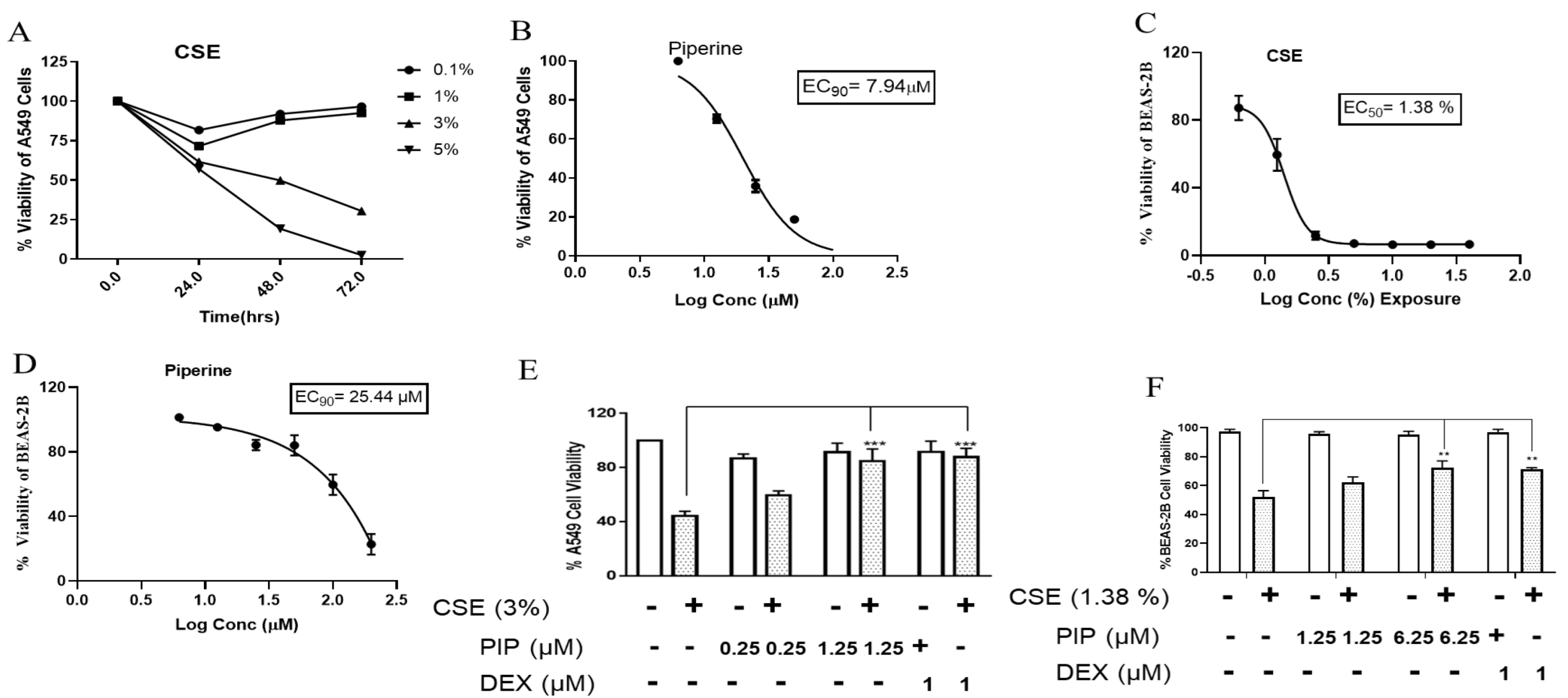
2.1. CSE Exposure Results in the Loss of Cell Viability in Lung Epithelial Cells
2.2. Piperine Protects the CSE-Induced Cell Death in Lung Epithelial Cells
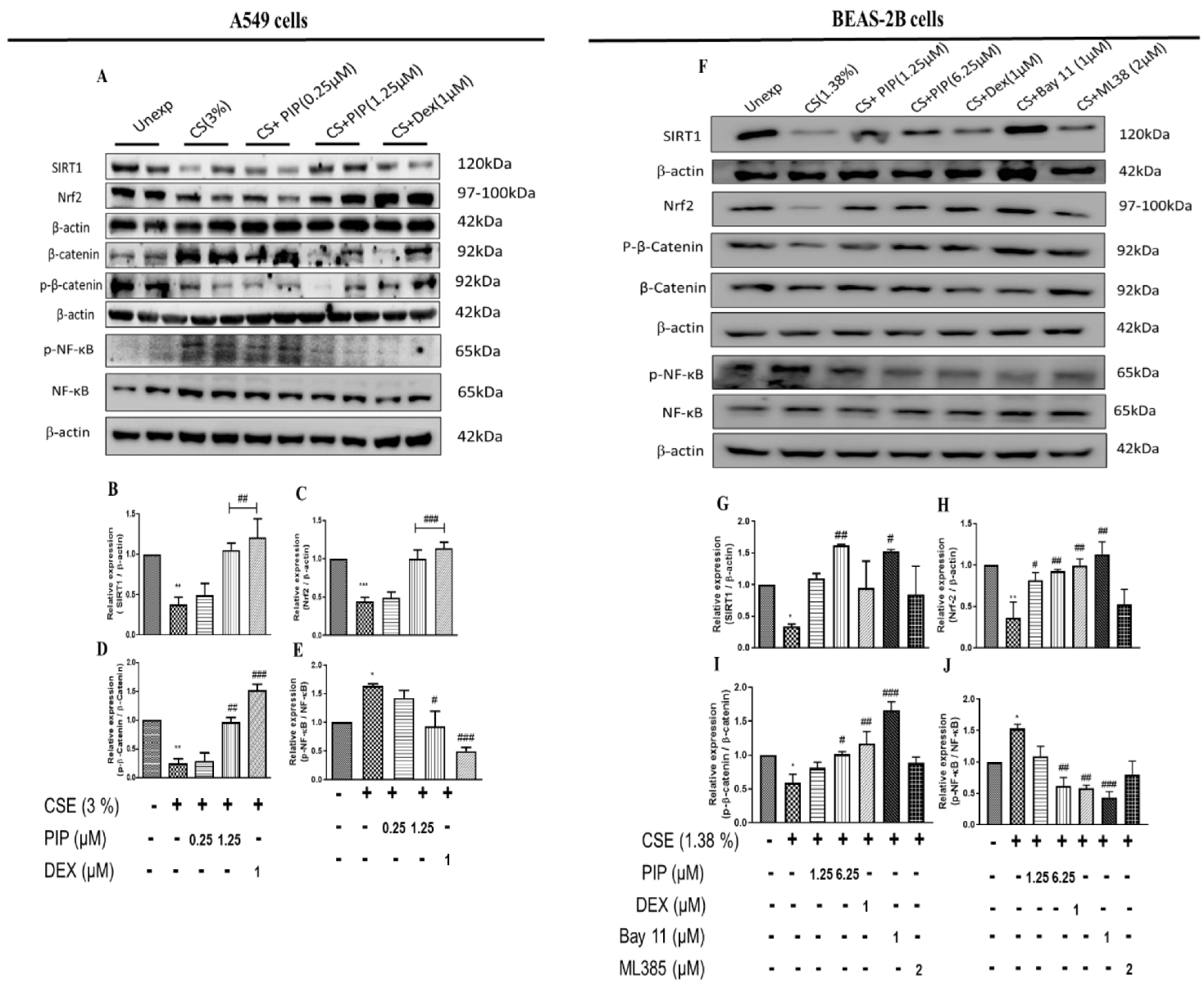
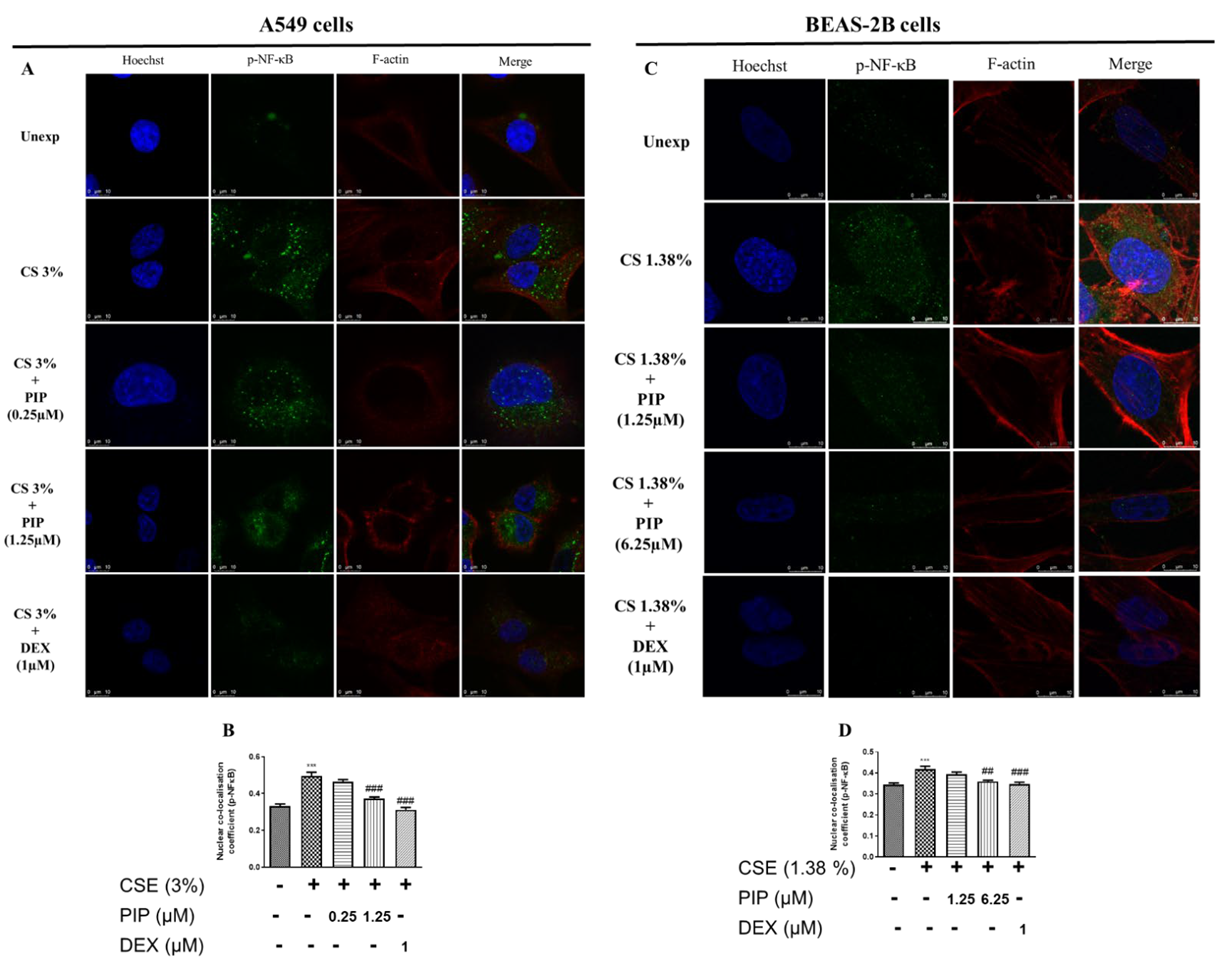
2.3. Piperine Curtails Oxidative Stress and Inflammation by Upregulating SIRT1, Nrf2 and Inhibiting NF-κB Translocation in Lung Epithelial Cells
2.4. Piperine Inhibits the Wnt Signaling in Lung Epithelial Cells
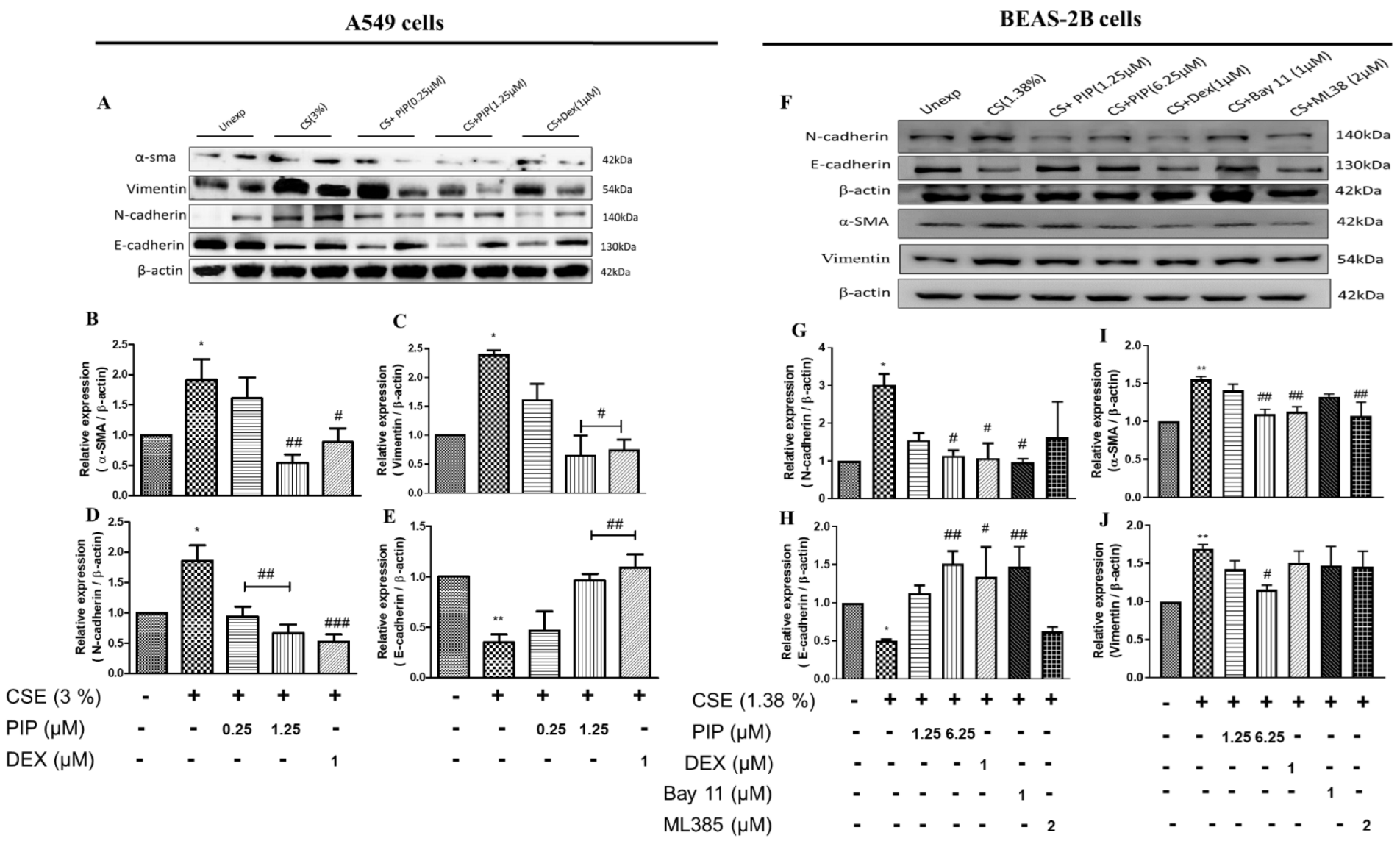
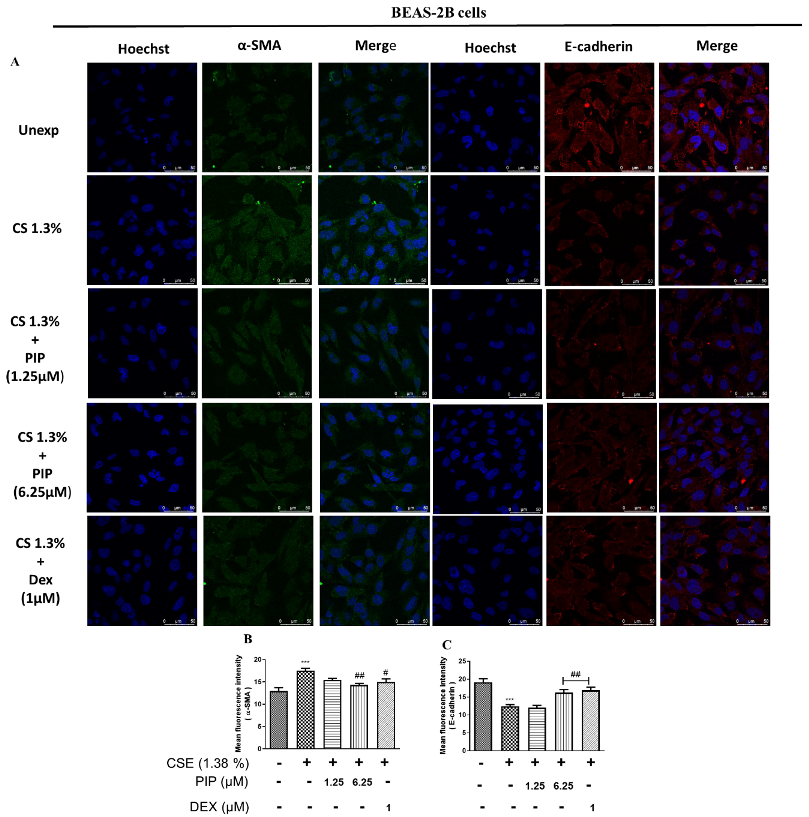
2.5. Piperine Diminishes Oxidative Stress, NF-κB, and Wnt Signaling Resulting in Inhibition of EMT in Lung Epithelial Cells
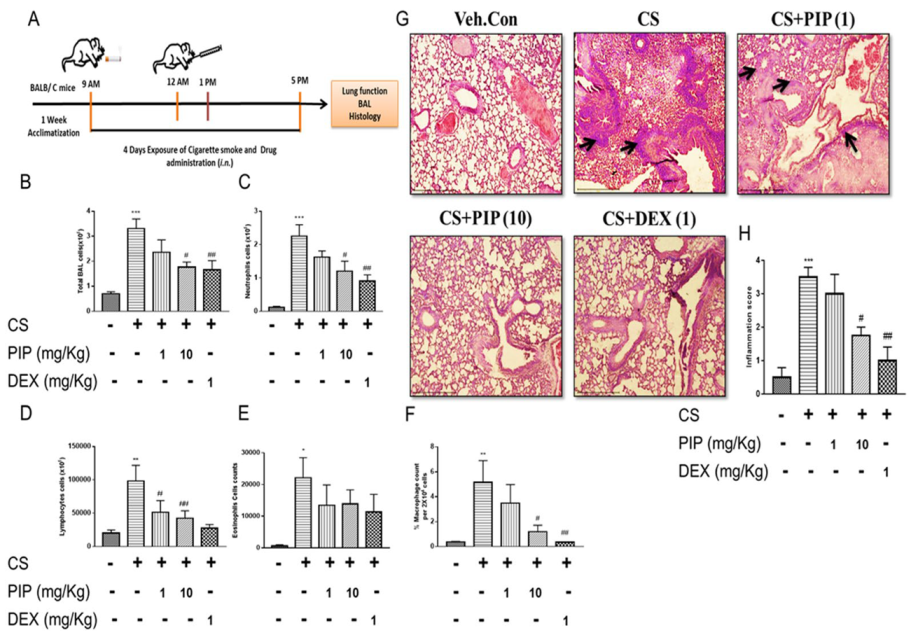
2.6. Piperine Restores the CS-Induced Altered Lung Mechanics in Mice
2.7. Piperine Inhibits The Release of Inflammatory Cytokines and Reduces Immune Cells Infiltration in Isolated BAL Fluid and Lung Tissues
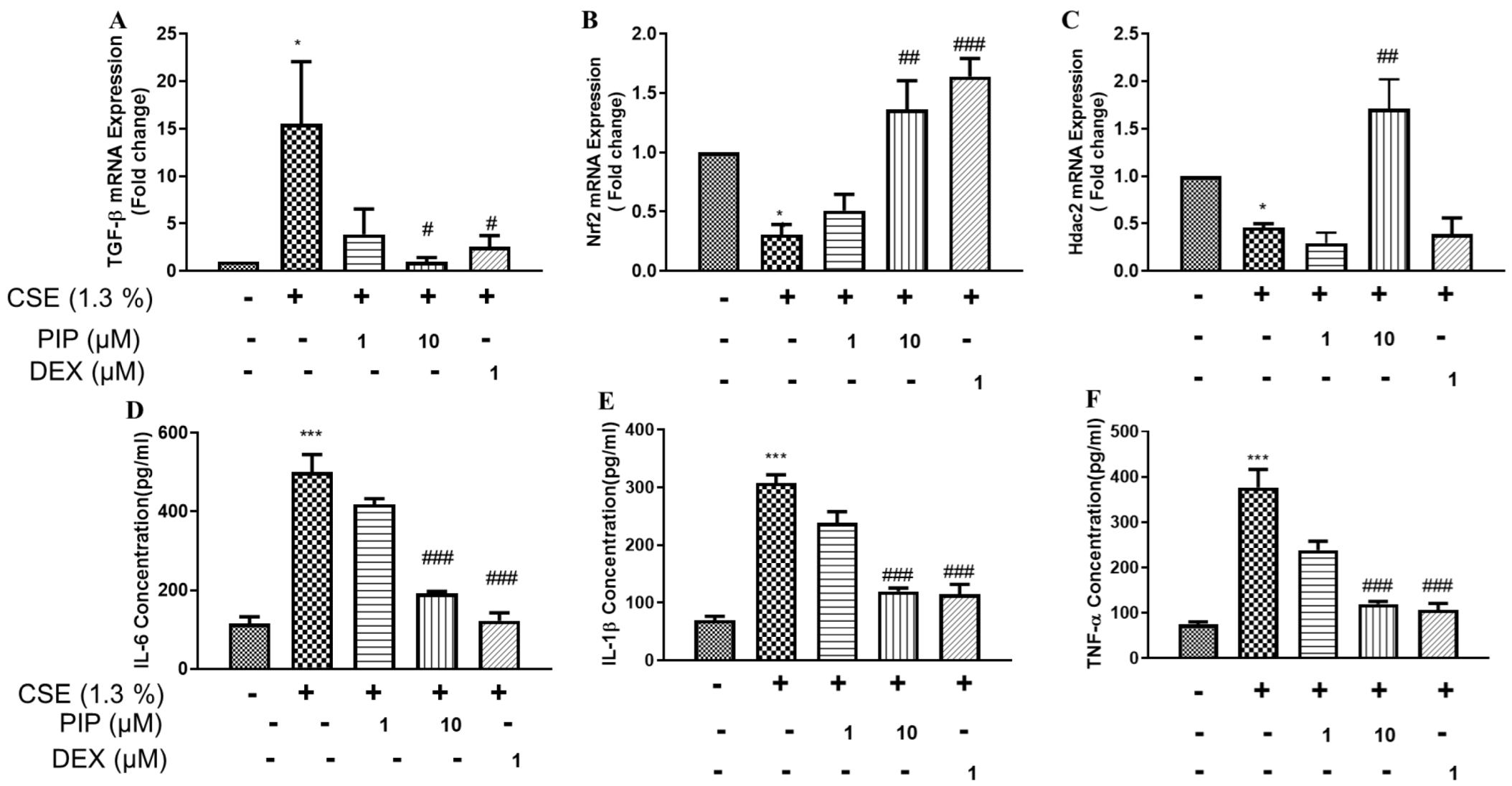
2.8. Piperine Curtails CS-Induced Oxidative Stress and Inflammatory Signaling in Isolated Lung Tissues
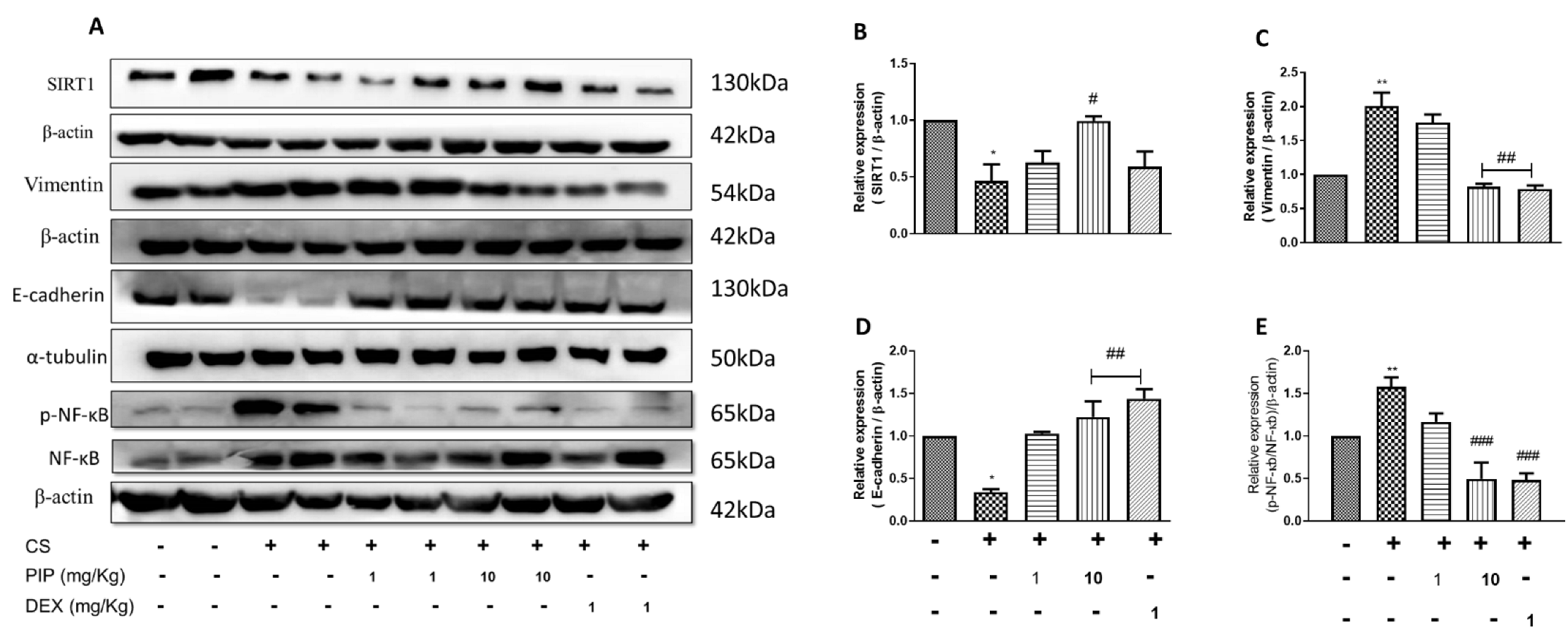
2.9. Piperine Inhibits EMT Changes and Modulates SIRT1 Signaling in Isolated Lung Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Aqueous Cigarette Smoke Extract (CSE)
4.3. Cell Viability Assay
4.4. Immunofluorescence
4.5. Cigarette Smoke Exposure in a Mouse Model
4.6. Assessment of Lung Function
4.7. Assessment of BAL Cytology
4.8. Measurement of Biochemical Parameters
4.9. RNA Isolation and Real-Time Polymerase Chain Reaction
4.10. Histopathological Analysis
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Micro-RNAs: Crossroads between the exposure to environmental particulate pollution and the obstructive pulmonary disease. Int. J. Mol. Sci. 2020, 21, 7221. [Google Scholar] [CrossRef] [PubMed]
- WHO Report. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 8 June 2020).
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H. Global and regional estimates of COPD prevalence: Systematic review and meta–analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- Terzikhan, N.; Verhamme, K.M.; Hofman, A.; Stricker, B.H.; Brusselle, G.G.; Lahousse, L. Prevalence and incidence of COPD in smokers and non-smokers: The Rotterdam Study. Eur. J. Epidemiol. 2016, 31, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Papaioannou, A.I.; Papaporfyriou, A.; Baker, J.R.; Vuppusetty, C.; Loukides, S.; Barnes, P.J.; Ito, K. Decreased serum sirtuin-1 in COPD. Chest 2017, 152, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, Q.; Meng, Z.; Sun, L.; Zhu, L.; Liu, J.; Hu, J.; Ni, Z.; Wang, X. Suppression of Sirtuin-1 increases IL-6 expression by activation of the Akt pathway during allergic asthma. Cell. Physiol. Biochem. 2017, 43, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, P.; Guo, Q.; Li, S.; Zhang, L.; Zhao, M.; Xu, H.; Yang, Y.; Poon, W.; Fei, Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 2012, 237, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Z.; Li, X.; Zhang, X. SIRT1 overexpression protects non-small cell lung cancer cells against osteopontin-induced epithelial-mesenchymal transition by suppressing NF-κB signaling. OncoTargets Ther. 2018, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial–mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 2018, 247, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Murahidy, A.; Ito, M.; Adcock, I.; Barnes, P.; Ito, K. Reduction is histone deacetylase expression and activity in smoking asthmatics: A mechanism of steroid resistance. Proc. Am. Thorac. Soc. 2005, 2, A889. [Google Scholar]
- Grieger, J.A.; Wood, L.G.; Clifton, V.L. Antioxidant-rich dietary intervention for improving asthma control in pregnancies complicated by asthma: Study protocol for a randomized controlled trial. Trials 2014, 15, 108. [Google Scholar] [CrossRef]
- Knight, D.A.; Grainge, C.L.; Stick, S.M.; Kicic, A.; Schuliga, M. Epithelial mesenchymal transition in respiratory disease: Fact or fiction. Chest 2020, 157, 1591–1596. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Yuan, Y.-P.; Zhang, X.; Xu, S.-C.; Wang, S.-S.; Tang, Q.-Z. Piperine attenuates pathological cardiac fibrosis via PPAR-γ/AKT pathways. eBioMedicine 2017, 18, 179–187. [Google Scholar] [CrossRef]
- Sabina, E.P.; Nagar, S.; Rasool, M. A role of piperine on monosodium urate crystal-induced inflammation—An experimental model of gouty arthritis. Inflammation 2011, 34, 184–192. [Google Scholar] [CrossRef]
- Dhivya, V.; Priya, L.B.; Chirayil, H.T.; Sathiskumar, S.; Huang, C.-Y.; Padma, V.V. Piperine modulates isoproterenol induced myocardial ischemia through antioxidant and anti-dyslipidemic effect in male Wistar rats. Biomed. Pharmacother. 2017, 87, 705–713. [Google Scholar] [CrossRef]
- Bang, J.S.; Choi, H.M.; Sur, B.-J.; Lim, S.-J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Li, H.; Gu, L. Piperine ameliorates lipopolysaccharide-induced acute lung injury via modulating NF-κB signaling pathways. Inflammation 2016, 39, 303–308. [Google Scholar] [CrossRef]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J.d.N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine inhibits TGF-β signaling pathways and disrupts EMT-related events in human lung adenocarcinoma cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef]
- Kim, N.; Nam, M.; Kang, M.S.; Lee, J.O.; Lee, Y.W.; Hwang, G.-S.; Kim, H.S. Piperine regulates UCP1 through the AMPK pathway by generating intracellular lactate production in muscle cells. Sci. Rep. 2017, 7, srep41066. [Google Scholar] [CrossRef]
- Moghadam, K.G.; Inançlı, H.M.; Bazazy, N.; Plinkert, P.K.; Efferth, T.; Sertel, S. Phytomedicine in otorhinolaryngology and pulmonology: Clinical trials with herbal remedies. Pharmaceuticals 2012, 5, 853–874. [Google Scholar] [CrossRef]
- Bhuyan, S.; Baruah, B. Medicinal plants used for the treatment of respiratory disorders: A study in Bongaigaon, North Eastern Himalayan Sub-region of India. Pharma Innov. 2015, 4, 108. [Google Scholar]
- Ahmed, A.; Thliveris, J.A.; Shaw, A.; Sowa, M.; Gilchrist, J.; Scott, J.E. Cigarette Smoke Induces Apoptosis by Activation of Caspase-3 in Isolated Fetal Rat Lung Type II Alveolar Ep-ithelial Cells in Vitro. Open J. Respir. Dis. 2013, 3, 4. [Google Scholar] [CrossRef]
- Jain, S.; Durugkar, S.; Saha, P.; Gokhale, S.B.; Naidu, V.; Sharma, P. Effects of intranasal azithromycin on features of cigarette smoke-induced lung inflammation. Eur. J. Pharmacol. 2022, 915, 174467. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, D.; Gupta, M.; Manek, G. Dexamethasone versus methylprednisolone in hospitalized COVID-19 patients: A systematic review and meta-analysis. Int. J. Crit. Care Emerg. Med. 2021, 7, 128. [Google Scholar]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Heijink, I.H.; de Bruin, H.G.; van den Berge, M.; Bennink, L.J.; Brandenburg, S.M.; Gosens, R.; van Oosterhout, A.J.; Postma, D.S. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax 2013, 68, 709–716. [Google Scholar] [CrossRef]
- Chen, X.; Yan, H.; Chen, Y.; Li, G.; Bin, Y.; Zhou, X. Moderate oxidative stress promotes epithelial–mesenchymal transition in the lens epithelial cells via the TGF-β/Smad and Wnt/β-catenin pathways. Mol. Cell. Biochem. 2021, 476, 1631–1642. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19 (accessed on 8 June 2022).
- Nosetti, L.; Agosti, M.; Franchini, M.; Milan, V.; Piacentini, G.; Zaffanello, M. Long-Term Pulmonary Damage From SARS-CoV-2 in an Infant With Brief Unexplained Resolved Events: A Case Report. Front. Med. 2021, 880, 646837. [Google Scholar] [CrossRef]
- Zakeri, A.; Jadhav, A.P.; Sullenger, B.A.; Nimjee, S.M. Ischemic stroke in COVID-19-positive patients: An overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J. NeuroInterv. Surg. 2021, 13, 202–206. [Google Scholar] [CrossRef]
- Comer, B.S.; Ba, M.; Singer, C.A.; Gerthoffer, W.T. Epigenetic targets for novel therapies of lung diseases. Pharmacol. Ther. 2015, 147, 91–110. [Google Scholar] [CrossRef]
- Barnes, P.J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 2019, 116, 105598. [Google Scholar] [CrossRef]
- Bartis, D.; Mise, N.; Mahida, R.Y.; Eickelberg, O.; Thickett, D.R. Epithelial–mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax 2014, 69, 760–765. [Google Scholar] [CrossRef]
- Sohal, S.S.; Reid, D.; Soltani, A.; Weston, S.; Muller, H.K.; Wood-Baker, R.; Walters, E.H. Changes in airway histone deacetylase2 in smokers and COPD with inhaled corticosteroids: A randomized controlled trial. PLoS ONE 2013, 8, e64833. [Google Scholar] [CrossRef]
- Atto, B.; Eapen, M.S.; Sharma, P.; Frey, U.; Ammit, A.J.; Markos, J.; Chia, C.; Larby, J.; Haug, G.; Weber, H.C. New therapeutic targets for the prevention of infectious acute exacerbations of COPD: Role of epithelial adhesion molecules and inflammatory pathways. Clin. Sci. 2019, 133, 1663–1703. [Google Scholar] [CrossRef]
- Gross, N.J.; Barnes, P.J. New therapies for asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017, 195, 159–166. [Google Scholar] [CrossRef]
- Meghwal, M.; Goswami, T. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rashid, S.; Arafah, A.; Qamar, W.; Alsaffar, R.M.; Ahmad, A.; Almatroudi, N.M.; Alqahtani, S.M.; Rashid, S.M.; Ahmad, S.B. Piperine regulates Nrf-2/Keap-1 signalling and exhibits anticancer effect in experimental colon carcinogenesis in Wistar rats. Biology 2020, 9, 302. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Sun, J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene 2019, 710, 91–97. [Google Scholar] [CrossRef]
- Jensen, E.; Dahl, R.; Steffensen, F. Bronchial reactivity to cigarette smoke in smokers: Repeatability, relationship to methacholine reactivity, smoking and atopy. Eur. Respir. J. 1998, 11, 670–676. [Google Scholar] [CrossRef]
- Jeffery, P.K. Structural and inflammatory changes in COPD: A comparison with asthma. Thorax 1998, 53, 129. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.-L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 13392. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, Z.; Meyer, J.M.; Ji, A.; van der Westhuyzen, D.R. Macrophage SR-BI regulates LPS-induced pro-inflammatory signaling in mice and isolated macrophages. J. Lipid Res. 2012, 53, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Van der Vaart, H.; Postma, D.S.; Timens, W.; Hylkema, M.N.; Willemse, B.W.; Boezen, H.M.; Vonk, J.M.; De Reus, D.M.; Kauffman, H.F.; Ten Hacken, N.H. Acute effects of cigarette smoking on inflammation in healthy intermittent smokers. Respir. Res. 2005, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Adcock, I.M.; Brown, C.R.; Kwon, O.; Barnes, P.J. Oxidative stress induces NFκB DNA binding and inducible NOS mRNA in human epithelial cells. Biochem. Biophys. Res. Commun. 1994, 199, 1518–1524. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Vézina, F.-A.; Cantin, A.M. Antioxidants and chronic obstructive pulmonary disease. Chronic Obstr. Pulm. Dis. J. COPD Found. 2018, 5, 277. [Google Scholar] [CrossRef]
- Milara, J.; Peiró, T.; Serrano, A.; Cortijo, J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax 2013, 68, 410–420. [Google Scholar] [CrossRef]
- Haswell, L.E.; Hewitt, K.; Thorne, D.; Richter, A.; Gaça, M.D. Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: A potential model of goblet cell hyperplasia. Toxicol. In Vitr. 2010, 24, 981–987. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Walters, E.H.; Shukla, S.D.; Weston, S.; Muller, H.K.; Ward, C.; Sohal, S.S. β-catenin, Twist and Snail: Transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci. Rep. 2017, 7, 10832. [Google Scholar] [CrossRef]
- Guan, R.; Wang, J.; Cai, Z.; Li, Z.; Wang, L.; Li, Y.; Xu, J.; Li, D.; Yao, H.; Liu, W. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 2020, 28, 101356. [Google Scholar] [CrossRef]
- Yao, H.; Chung, S.; Hwang, J.-w.; Rajendrasozhan, S.; Sundar, I.K.; Dean, D.A.; McBurney, M.W.; Guarente, L.; Gu, W.; Rönty, M. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Investig. 2012, 122, 2032–2045. [Google Scholar] [CrossRef]
- Rong, L.; Wu, J.; Wang, W.; Zhao, R.; Xu, X.; Hu, D. Sirt 1 activator attenuates the bleomycin-induced lung fibrosis in mice via inhibiting epithelial-to-mesenchymal transition (EMT). Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2144–2150. [Google Scholar]
- Liao, W.; Lim, A.Y.; Tan, W.D.; Abisheganaden, J.; Wong, W.F. Restoration of HDAC2 and Nrf2 by andrographolide overcomes corticosteroid resistance in chronic obstructive pulmonary disease. Br. J. Pharmacol. 2020, 177, 3662–3673. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, P.; Durugkar, S.; Jain, S.; Shantanu, P.A.; Panda, S.R.; Jala, A.; Gokhale, S.; Sharma, P.; Naidu, V.G.M. Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis. Int. J. Mol. Sci. 2022, 23, 14722. https://doi.org/10.3390/ijms232314722
Saha P, Durugkar S, Jain S, Shantanu PA, Panda SR, Jala A, Gokhale S, Sharma P, Naidu VGM. Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis. International Journal of Molecular Sciences. 2022; 23(23):14722. https://doi.org/10.3390/ijms232314722
Chicago/Turabian StyleSaha, Pritam, Sneha Durugkar, Siddhi Jain, P. A. Shantanu, Samir R. Panda, Aishwarya Jala, Sharad Gokhale, Pawan Sharma, and V. G. M. Naidu. 2022. "Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis" International Journal of Molecular Sciences 23, no. 23: 14722. https://doi.org/10.3390/ijms232314722
APA StyleSaha, P., Durugkar, S., Jain, S., Shantanu, P. A., Panda, S. R., Jala, A., Gokhale, S., Sharma, P., & Naidu, V. G. M. (2022). Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis. International Journal of Molecular Sciences, 23(23), 14722. https://doi.org/10.3390/ijms232314722






