Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Synthesis of Iron-Based Composite Nanomaterials
3.2. Characterization of Iron-Based Composite Nanomaterials by Microscopy
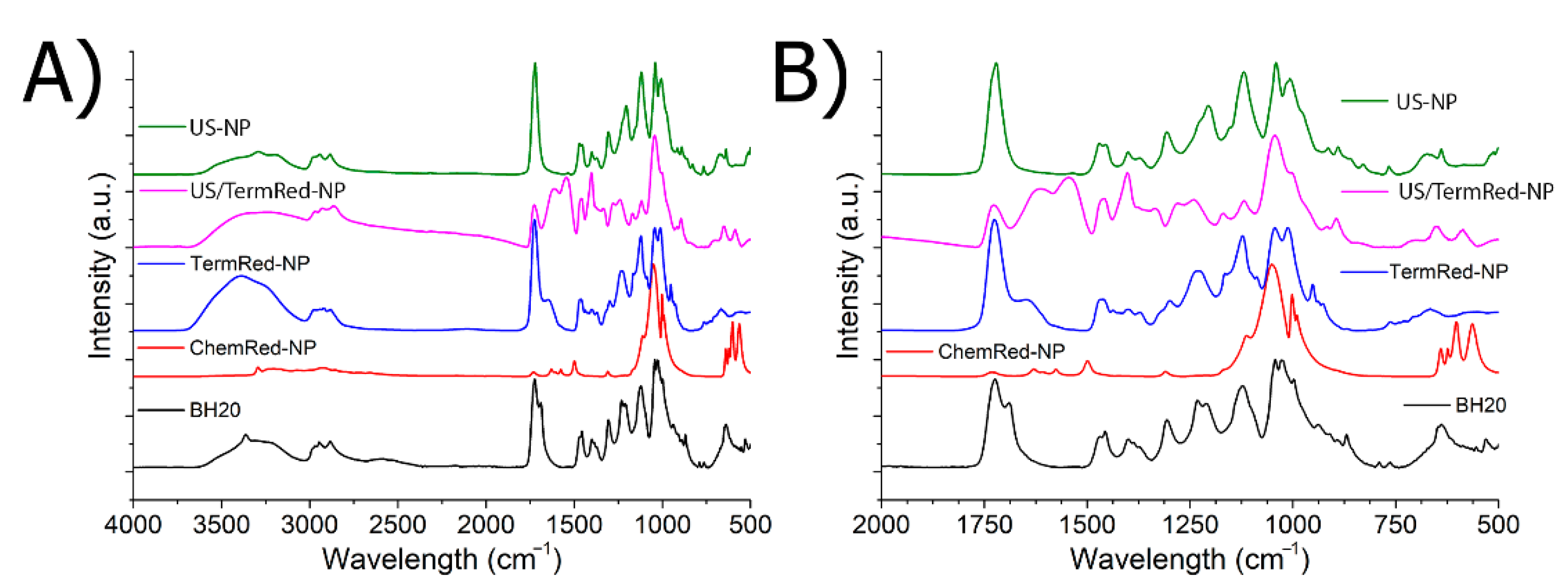
3.3. Stabilization and Composition of Iron-Based Nanomaterials
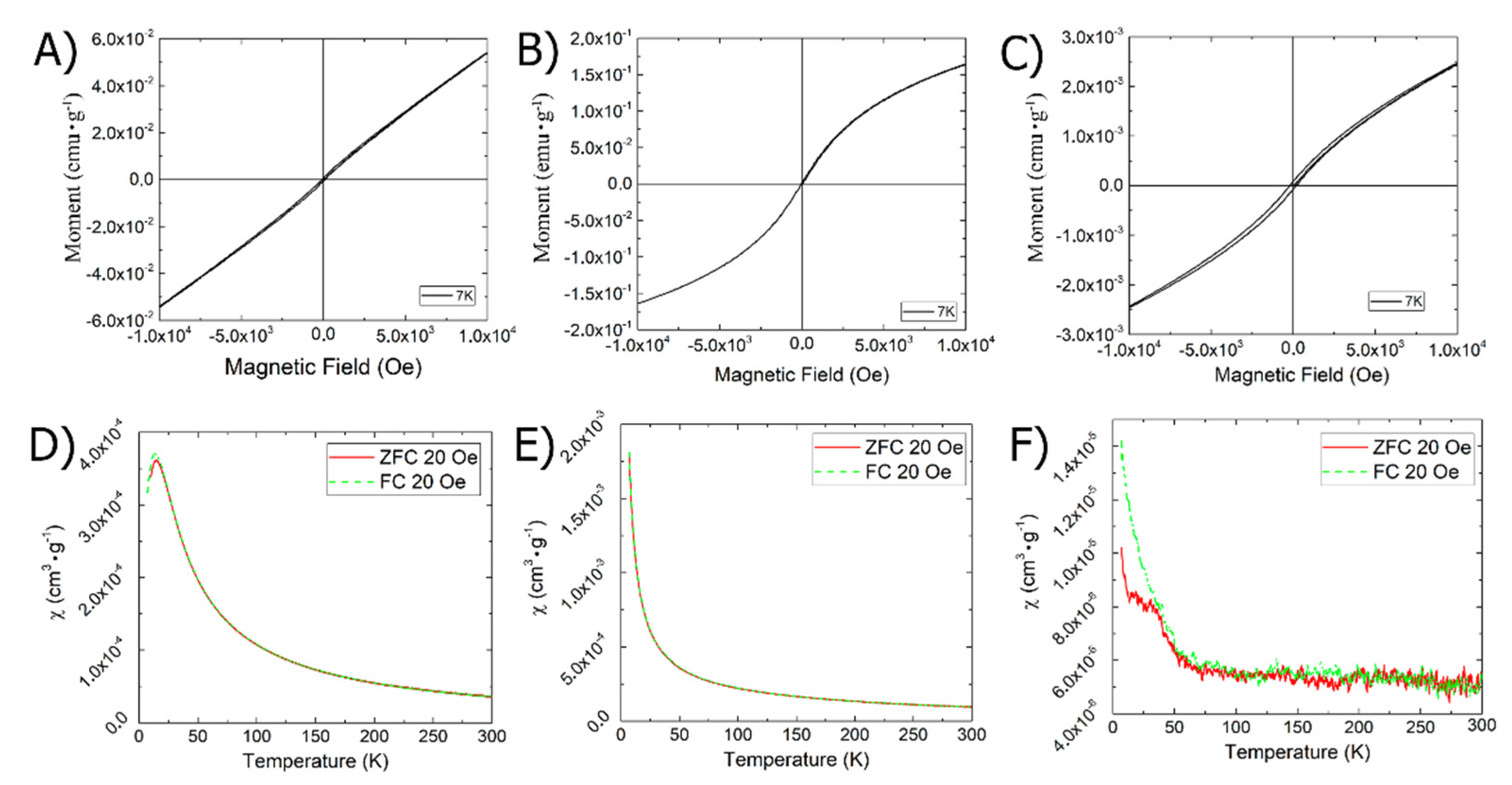
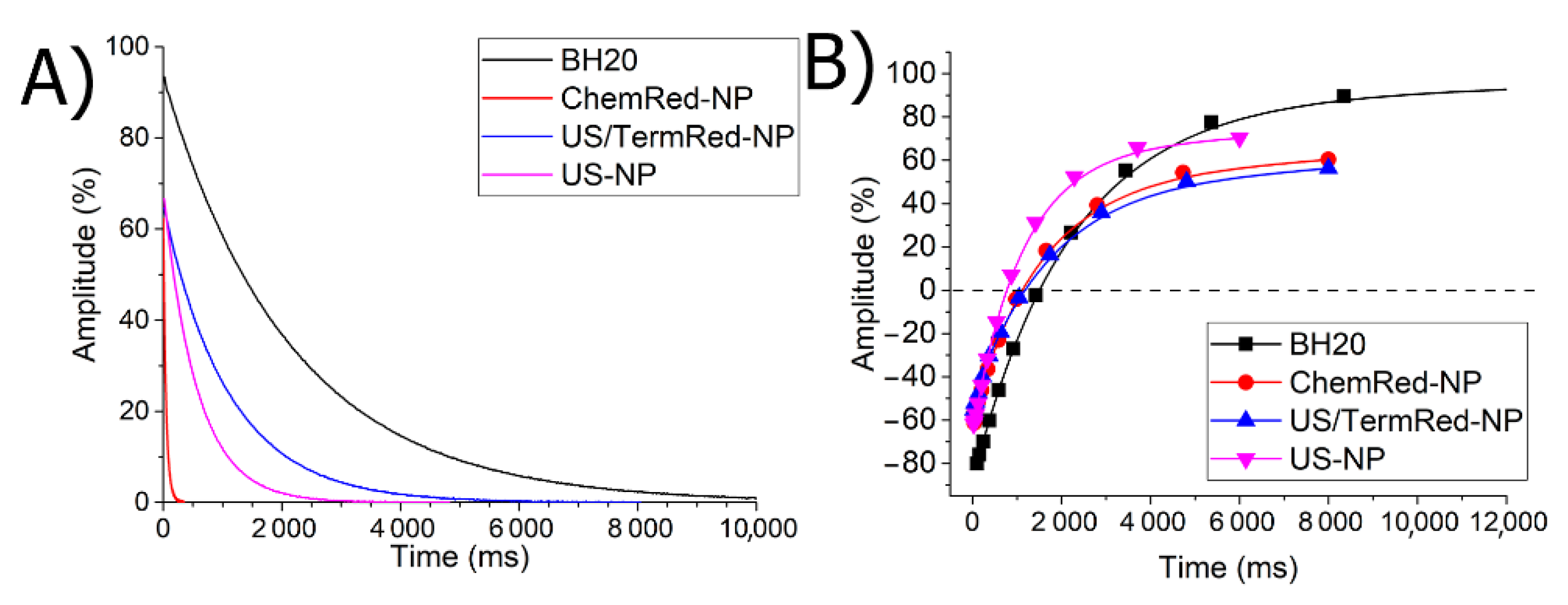
3.4. Magnetic Properties of Iron-Based Composite Nanomaterials in Solid State and Solution
3.5. Aggregation Properties of Iron-Based Composite Nanomaterials
3.6. Hemolytic Activity Assay
4. Materials and Methods
4.1. Materials
4.2. Synthesis Methods
4.2.1. Chemical Reduction (ChemRed-NP)
4.2.2. Polyol Thermolytic Process (TermRed-NP)
4.2.3. Hybrid Ultrasonic/Thermolytic (US/TermRed-NP)
4.3. Ultrasonic (US-NP)
4.4. Characterization Methods
4.5. Hemolysis Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pu, H.; Dai, H.; Zhang, T.; Dong, K.; Wang, Y.; Deng, Y. Metal nanoparticles with clean surface: The importance and progress. Curr. Opin. Electrochem. 2022, 32, 100927. [Google Scholar] [CrossRef]
- Naik, N.; Shivamurthy, B.; Thimmappa, B.H.S.; Gupta, A.; Guo, J.Z.; Seok, I. A Review on Processing Methods and Characterization Techniques of Green Composites. Eng. Sci. 2022, 20, 80–99. [Google Scholar] [CrossRef]
- Stephen, C.; Shivamurthy, B.; Mohan, M.; Mourad, A.-H.I.; Selvam, R.; Thimmappa, B.H.S. Low Velocity Impact Behavior of Fabric Reinforced Polymer Composites– A Review. Eng. Sci. 2022, 18, 75–97. [Google Scholar]
- Shahabaz, S.M.; Sharma, S.; Shetty, N.; Shetty, S.D.; Gowrishankar, M.C. Influence of Temperature on Mechanical Properties and Machining of Fibre Reinforced Polymer Composites: A Review. Eng. Sci. 2021, 16, 26–46. [Google Scholar] [CrossRef]
- Neto, J.C.d.M.; Nascimento, N.R.d.; Bello, R.H.; Verçosa, L.A.d.V.; Neto, J.E.; Costa, J.C.M.d.; Rol, F.; Diaz, R.V. Kaolinite Review: Intercalation and Production of Polymer Nanocomposites. Eng. Sci. 2022, 17, 28–44. [Google Scholar] [CrossRef]
- Seh Zhi, W.; Kibsgaard, J.; Dickens Colin, F.; Chorkendorff, I.; Nørskov Jens, K.; Jaramillo Thomas, F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar]
- Khannanov, A.; Il’yasov, I.; Kiiamov, A.; Vakhitov, I.; Kirgizov, A.; Lamberov, A.; Dimiev, A.M. Catalytic properties of graphene oxide/palladium composites as a function of the fabrication method. New J. Chem. 2019, 43, 19035–19043. [Google Scholar] [CrossRef]
- Parmar, M.; Sanyal, M. Extensive study on plant mediated green synthesis of metal nanoparticles and their application for degradation of cationic and anionic dyes. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100624. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing high performance Pt monolayer core–shell electrocatalysts for fuel cells. Curr. Opin. Electrochem. 2020, 21, 368–375. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Yu, C.-N.; Li, X.-Z.; Wang, L.-F.; Huang, J.; Tong, J.; Lin, Y.; Min, Y.; Liang, Y. Recent Developments of Nanocellulose and Its Applications in Polymeric Composites. ES Food Agrofor. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Wu, Y.; Xie, P.; Pashameah, R.A.; Abo-Dief, H.M.; El-Bahy, S.M.; Wei, Y.; Li, G.; Li, W.; et al. The weakly negative permittivity with low-frequency-dispersion behavior in percolative carbon nanotubes/epoxy nanocomposites at radio-frequency range. Adv. Compos. Hybrid Mater. 2022, 5, 2021–2030. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Chopra, H.; Rahman, M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Patel, M.P.; Patel, J.K. Biomedical Applications of Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 25–36. [Google Scholar]
- Atapour, A.; Khajehzadeh, H.; Shafie, M.; Abbasi, M.; Mosleh-Shirazi, S.; Kasaee, S.R.; Amani, A.M. Gold nanoparticle-based aptasensors: A promising perspective for early-stage detection of cancer biomarkers. Mater. Today Commun. 2022, 30, 103181. [Google Scholar] [CrossRef]
- Xu, X.; Xiang, H.; Wang, Z.; Wu, C.; Lu, C. Doping engineering and functionalization of iron oxide nanoclusters for biomedical applications. J. Alloys Compd. 2022, 923, 166459. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.; Kim, S.-K. Highly efficient heat-dissipation power driven by ferromagnetic resonance in MFe2O4 (M = Fe, Mn, Ni) ferrite nanoparticles. Sci. Rep. 2022, 12, 5232. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, L.; Cao, J.; Liu, T.; Xu, Z.; Yang, B.; Huang, T.; Jiang, X.; Wu, N. Recent advances on the magnetic nanoparticle–based nanocomposites for magnetic induction hyperthermia of tumor: A short review. Adv. Compos. Hybrid Mater. 2021, 4, 925–937. [Google Scholar] [CrossRef]
- Bhiradi, I.; Hiremath, S.S. Energy storage and photosensitivity of in-situ formed silver-copper (Ag-Cu) heterogeneous nanoparticles generated using multi-tool micro electro discharge machining process. J. Alloys Compd. 2022, 897, 162950. [Google Scholar] [CrossRef]
- Alphandéry, E. Light-Interacting iron-based nanomaterials for localized cancer detection and treatment. Acta Biomater. 2021, 124, 50–71. [Google Scholar] [CrossRef]
- Alexiou, C.; Tietze, R.; Schreiber, E.; Jurgons, R.; Richter, H.; Trahms, L.; Rahn, H.; Odenbach, S.; Lyer, S. Cancer therapy with drug loaded magnetic nanoparticles—Magnetic drug targeting. J. Magn. Magn. Mater. 2011, 323, 1404–1407. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Hinge, N.S.; Kathuria, H.; Pandey, M.M. Engineering of structural and functional properties of nanotherapeutics and nanodiagnostics for intranasal brain targeting in Alzheimer’s. Appl. Mater. Today 2022, 26, 101303. [Google Scholar] [CrossRef]
- Cotin, G.; Blanco-Andujar, C.; Perton, F.; Asín, L.; Jesus, M.; Reichardt, W.; Schaffner, D.; Ngyen, D.-V.; Mertz, D.; Kiefer, C. Unveiling the role of surface, size, shape and defects of iron oxide nanoparticles for theranostic applications. Nanoscale 2021, 13, 14552–14571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Jin, S.; Liang, X.-J.; Ma, X. Enhanced anti-tumor activity of a drug through pH-triggered release and dual targeting by calcium phosphate-covered mesoporous silica vehicles. J. Mater. Chem. B 2022, 10, 384–395. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic Nanostructures: Rational Design and Fabrication Strategies toward Diverse Applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Dash, A.; Tomanek, B.; van Veggel, F.C.; Trudel, S. High-field magnetic resonance imaging: Challenges, advantages, and opportunities for novel contrast agents. Chem. Phys. Rev. 2022, 3, 011304. [Google Scholar] [CrossRef]
- Gogoi, M. Magnetic Nanostructures for Cancer Theranostic Applications. Curr. Pathobiol. Rep. 2021, 9, 71–78. [Google Scholar] [CrossRef]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming Hurdles in Nanoparticle Clinical Translation: The Influence of Experimental Design and Surface Modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef]
- Shetake, N.; Balla, M.; Kumar, A.; Pandey, B. Magnetic hyperthermia therapy: An emerging modality of cancer treatment in combination with radiotherapy. J. Radiat. Cancer Res. 2016, 7, 13–17. [Google Scholar]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Derbalah, A.; Abdelsalam, I.; Behiry, S.I.; Abdelkhalek, A.; Abdelfatah, M.; Ismail, S.; Elsharkawy, M.M. Copper oxide nanostructures as a potential method for control of zucchini yellow mosaic virus in squash. Pest Manag. Sci. 2022, 78, 3587–3595. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Ren, J.; Wang, F.; Wei, G.; Yang, Y.; Liu, Y.; Wei, M.; Huan, Y.; Larson, A.C.; Zhang, Z. MRl of Prostate Cancer Antigen Expression for Diagnosis and lmmunotherapy. PLoS ONE 2012, 7, e38350. [Google Scholar] [CrossRef]
- Radzi, M.R.M.; Lim, C.K.; Sulaiman, N.; Jemon, K. Nanoparticles-based Chemo-Phototherapy Synergistic Effects for Breast Cancer Treatment: A Systematic Review. Biointerface Res. Appl. Chem. 2023, 13, 232. [Google Scholar]
- Lv, W.; Liu, Y.; Li, S.; Lv, L.; Lu, H.; Xin, H. Advances of nano drug delivery system for the theranostics of ischemic stroke. J. Nanobiotechnol. 2022, 20, 248. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhou, H.; Du, Z. Anticancer effects of natural phytochemicals in anaplastic thyroid cancer (Review). Oncol. Rep. 2022, 48, 156. [Google Scholar] [CrossRef]
- Al Zarzour, R.H.; Kamarulzaman, E.E.; Saqallah, F.G.; Zakaria, F.; Asif, M.; Abdul Razak, K.N. Medicinal plants’ proposed nanocomposites for the management of endocrine disorders. Heliyon 2022, 8, e10665. [Google Scholar] [CrossRef]
- Zhang, F.; Xin, C.; Dai, Z.; Hu, H.; An, Q.; Wang, F.; Hu, Z.; Sun, Y.; Tian, L.; Zheng, X. Oncocyte Membrane-Camouflaged Multi-Stimuli-Responsive Nanohybrids for Synergistic Amplification of Tumor Oxidative Stresses and Photothermal Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 40633–40644. [Google Scholar] [CrossRef]
- Shaari, N.S.; Ibrahim, S.M.; Md Jamil, F.; Zubir, Z.A.; Abdullah, A.; Muhammad, N.A.; Wan Jaafar, W.M.N.; Malik, M. Synthesis and Characterization of Magnetic Mesoporous γ-FE2O3/SIO2; Advanced Materials Research; Trans Tech Publications Ltd.: Bach, Switzerland, 2014; pp. 128–133. [Google Scholar]
- Mann, J.; Doluda, V.Y.; Leonard, C.; Losovyj, Y.B.; Morgan, D.G.; Bukalov, S.S.; Shifrina, Z.; Stein, B.D.; Cherkasov, N.; Rebrov, E.V.; et al. Metal oxide–zeolite composites in transformation of methanol to hydrocarbons: Do iron oxide and nickel oxide matter? RSC Adv. 2016, 6, 75166–75177. [Google Scholar] [CrossRef]
- Jaquish, R.; Reilly, A.K.; Lawson, B.P.; Golikova, E.; Sulman, A.M.; Stein, B.D.; Lakina, N.V.; Tkachenko, O.P.; Sulman, E.M.; Matveeva, V.G. Immobilized glucose oxidase on magnetic silica and alumina: Beyond magnetic separation. Int. J. Biol. Macromol. 2018, 120, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Huang, Y.; Liu, Y.; Jiang, D.; Wu, Z.; Li, B.; Xu, Q.; Murugadoss, V.; Jiang, Q.; Huang, M.; et al. An overview of regenerable wood-based composites: Preparation and applications for flame retardancy, enhanced mechanical properties, biomimicry, and transparency energy saving. Adv. Compos. Hybrid Mater. 2022, 5, 1612–1657. [Google Scholar] [CrossRef]
- Khannanov, A.; Kiiamov, A.; Valimukhametova, A.; Tayurskii, D.A.; Börrnert, F.; Kaiser, U.; Eigler, S.; Vagizov, F.G.; Dimiev, A.M. γ-Iron Phase Stabilized at Room Temperature by Thermally Processed Graphene Oxide. J. Am. Chem. Soc. 2018, 140, 9051–9055. [Google Scholar] [CrossRef] [PubMed]
- Khannanov, A.A.; Valimukhametova, A.R.; Kiiamov, A.G.; Vakhitov, I.R.; Dimiev, A.M. The Mechanistic Details for the Growth of Palladium Nanoparticles on Graphene Oxide Support. ChemistrySelect 2017, 2, 10546–10554. [Google Scholar] [CrossRef]
- Valimukhametova, A.; Khannanov, A.; Kiiamov, A.; Vakhitov, I.; Gilmutdinov, I.; Vagizov, F.G.; Dimiev, A.M. Growth of invar nanoparticles on a graphene oxide support. CrystEngComm 2019, 21, 4092–4097. [Google Scholar] [CrossRef]
- Khannanov, A.; Kiiamov, A.; Valimukhametova, A.; Vagizov, F.G.; Dimiev, A.M. Direct growth of oriented nanocrystals of gamma-iron on graphene oxide substrates. Detailed analysis of the factors affecting unexpected formation of the gamma-iron phase. New J. Chem. 2019, 43, 12923–12931. [Google Scholar] [CrossRef]
- Xie, P.; Shi, Z.; Feng, M.; Sun, K.; Liu, Y.; Yan, K.; Liu, C.; Moussa, T.A.A.; Huang, M.; Meng, S.; et al. Recent advances in radio-frequency negative dielectric metamaterials by designing heterogeneous composites. Adv. Compos. Hybrid Mater. 2022, 5, 679–695. [Google Scholar] [CrossRef]
- Tso, C.-P.; Zhung, C.-M.; Shih, Y.-H.; Tseng, Y.-M.; Wu, S.-C.; Doong, R.-A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef]
- Anand, K.; Varghese, S. Role of surfactants on the stability of nano-zinc oxide dispersions. Part. Sci. Technol. 2017, 35, 67–70. [Google Scholar]
- Singh, S.; Singh, G.; Sehrawat, S.; Rawat, P.; Molugulu, N.; Singh, V.; Ahmed, F.J.; Kesharwani, P. Chapter 25—Conclusion and future considerations of dendrimers. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 449–458. [Google Scholar]
- Patle, R.Y.; Meshram, J.S. The advanced synthetic modifications and applications of multifunctional PAMAM dendritic composites. React. Chem. Eng. 2022, 7, 9–40. [Google Scholar] [CrossRef]
- Quintana-Sánchez, S.; Barrios-Gumiel, A.; Sánchez-Nieves, J.; Copa-Patiño, J.L.; de la Mata, F.J.; Gómez, R. Bacteria capture with magnetic nanoparticles modified with cationic carbosilane dendritic systems. Mater. Sci. Eng. C 2021, 133, 112622. [Google Scholar] [CrossRef]
- Salami-Kalajahi, M.; Golshan, M. Chapter 14—Dendrimer hybrids with other nanoparticles as therapeutics. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 253–272. [Google Scholar]
- Graham-Gurysh, E.G.; Carpenter, B.W.; Beck, W.A.; Varma, D.M.; Vincent, B.G.; Bachelder, E.M.; Ainslie, K.M. Chapter Fourteen—Delivery strategies for cancer vaccines and immunoadjuvants. In Systemic Drug Delivery Strategies; Amiji, M.M., Milane, L.S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 359–408. [Google Scholar]
- Cai, J.; Murugadoss, V.; Jiang, J.; Gao, X.; Lin, Z.; Huang, M.; Guo, J.; Alsareii, S.A.; Algadi, H.; Kathiresan, M. Waterborne polyurethane and its nanocomposites: A mini-review for anti-corrosion coating, flame retardancy, and biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 641–650. [Google Scholar] [CrossRef]
- Akkurt, N.; Altan, C.L.; Sarac, M.F. Continuous Flow–Assisted Polyol Synthesis of Citric Acid Functionalized Iron Oxide Nanoparticles. J. Supercond. Nov. Magn. 2022, 35, 615–623. [Google Scholar] [CrossRef]
- Kutyreva, M.P.; Ulakhovich, N.A.; Gataulina, A.R.; Khannanov, A.A.; Malinovskikh, O.A.; Yurtaeva, S.V.; Medyantseva, E.P. Synthesis and properties of hyperbranched polyester polyacrylic acids and their metal complexes. Russ. Chem. Bull. 2014, 63, 239–246. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Ibrahim, M.M.; Wu, H.; Wu, Y.; Li, Y.; Mersal, G.A.M.; El Azab, I.H.; El-Bahy, S.M.; Huang, M.; et al. Flexible polystyrene/graphene composites with epsilon-near-zero properties. Adv. Compos. Hybrid Mater. 2022, 5, 1054–1066. [Google Scholar] [CrossRef]
- Maleki, B.; Jafari-Soghieh, F.; Alinezhad, H.; Ghani, M.; Jamshidi, A. Development of PAMAM dendrimer-modified magnetic polyoxometalate: A novel platform to reinforce mechanical and thermal properties of diglycidyl ether of bisphenol A/isophorone diamine hardener epoxy. High Perform. Polym. 2022, 34, 768–777. [Google Scholar] [CrossRef]
- Chikhaliwala, P.; Schlegel, W.; Lang, H.; Chandra, S. Inkjet printed patterns of polyamidoamine dendrimer functionalized magnetic nanostructures for future biosensing device application. J. Mater. Sci. 2021, 56, 5802–5816. [Google Scholar] [CrossRef]
- Shende, P.; Sahu, P. Enzyme bioconjugated PAMAM dendrimers for estimation of glucose in saliva. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 469–475. [Google Scholar] [CrossRef]
- Yu, L.; Luo, B.; Li, Z.; He, J.; Lan, F.; Wu, Y. PAMAM–PMAA brush-functionalized magnetic composite nanospheres: A smart nanoprobe with tunable selectivity for effective enrichment of mono-, multi-, or global phosphopeptides. J. Mater. Chem. B 2020, 8, 1266–1276. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Xie, X.; Feng, Y.; Li, T.; Li, S.; Qin, X.; Yang, H.; Wu, C.; Zheng, C.; et al. Dendrimer-Functionalized Superparamagnetic Nanobeacons for Real-Time Detection and Depletion of HSP90α mRNA and MR Imaging. Theranostics 2019, 9, 5784–5796. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B Biointerfaces 2019, 173, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Bahadur, D. Doxorubicin-loaded dendritic-Fe3O4 supramolecular nanoparticles for magnetic drug targeting and tumor regression in spheroid murine melanoma model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Tian, Z.; Wang, Q.; Peng, S.; Luo, J.-B.; Zhou, Q.-H.; Lin, J. Surface design and preparation of multi-functional magnetic nanoparticles for cancer cell targeting, therapy, and imaging. RSC Adv. 2018, 8, 35437–35447. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xu, K.; Wang, J.; Cui, X.; Zhang, J.; Weng, Y. Phosphorous-functionalized PAMAM dendrimers supported on mesoporous silica for Zr(iv) and Hf(iv) separation. RSC Adv. 2021, 11, 34754–34765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Q.; Yan, C.; Li, J.; Zhou, W.; Gao, H.; Zhang, S.; Lu, R. Hyperbranched aromatic polyamide modified magnetic nanoparticles for the extraction of benzoylurea insecticides. J. Sep. Sci. 2021, 44, 1931–1938. [Google Scholar] [CrossRef]
- Tabatabaiee Bafrooee, A.A.; Ahmad Panahi, H.; Moniri, E.; Miralinaghi, M.; Hasani, A.H. Removal of Hg2+ by carboxyl-terminated hyperbranched poly(amidoamine) dendrimers grafted superparamagnetic nanoparticles as an efficient adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 9547–9567. [Google Scholar] [CrossRef]
- Rezaei, J.T.S.; Malekzadeh, M.A.; Ramazani, A.; Niknejad, H. pH-Sensitive Magnetite Nanoparticles Modified with Hyperbranched Polymers and Folic Acid for Targeted Imaging and Therapy. Curr. Drug Deliv. 2019, 16, 839–848. [Google Scholar] [CrossRef]
- Shabanian, M.; Khoobi, M.; Hemati, F.; Khonakdar, H.A.; Ebrahimi, S.E.S.; Wagenknecht, U.; Shafiee, A. New PLA/PEI-functionalized Fe3O4 nanocomposite: Preparation and characterization. J. Ind. Eng. Chem. 2015, 24, 211–218. [Google Scholar] [CrossRef]
- Jahandar, M.; Zarrabi, A.; Shokrgozar, M.A.; Mousavi, H. Synthesis, characterization and application of polyglycerol coated Fe3O4nanoparticles as a nano-theranostics agent. Mater. Res. Express 2015, 2, 125002. [Google Scholar] [CrossRef]
- Smith, C.E.; Ernenwein, D.; Shkumatov, A.; Clay, N.E.; Lee, J.; Melhem, M.; Misra, S.; Zimmerman, S.C.; Kong, H. Hydrophilic packaging of iron oxide nanoclusters for highly sensitive imaging. Biomaterials 2015, 69, 184–190. [Google Scholar] [CrossRef]
- Lu, T.; Wu, Y.; Zhao, C.; Su, F.; Liu, J.; Ma, Z.; Han, Q. One-step fabrication and characterization of Fe3O4/HBPE-DDSA/INH nanoparticles with controlled drug release for treatment of tuberculosis. Mater. Sci. Eng. C 2018, 93, 838–845. [Google Scholar] [CrossRef]
- Kannan, R.M.; Perumal, O.P.; Kannan, S. Dendrimers and Hyperbranched Polymers for Drug Delivery. In Biomedical Applications of Nanotechnology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007; pp. 105–129. [Google Scholar]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Khannanov, A.A.; Rossova, A.A.; Ignatyeva, K.A.; Ulakhovich, N.A.; Gerasimov, A.V.; Boldyrev, A.E.; Evtugyn, V.G.; Rogov, A.M.; Cherosov, M.A.; Gilmutdinov, I.F.; et al. Superparamagnetic cobalt nanoparticles in hyperbranched polyester polyol matrix with anti-protease activity. J. Magn. Magn. Mater. 2022, 547, 168808. [Google Scholar] [CrossRef]
- Medvedeva, O.I.; Kambulova, S.S.; Bondar, O.V.; Gataulina, A.R.; Ulakhovich, N.A.; Gerasimov, A.V.; Evtugyn, V.G.; Gilmutdinov, I.F.; Kutyreva, M.P. Magnetic Cobalt and Cobalt Oxide Nanoparticles in Hyperbranched Polyester Polyol Matrix. J. Nanotechnol. 2017, 2017, 7607658. [Google Scholar] [CrossRef]
- Maksimov, A.F.; Zhukova, A.A.; Ernandes, A.M.P.; Kutyreva, M.P.; Gataulina, A.R.; Kutyrev, G.A. Synthesis of Ethyl 2,2-Bis{[(benzoylcarbamothioyl)oxy]methyl}propanoate and Its Complexes with Copper(II) and Cobalt(II) Ions. Russ. J. Gen. Chem. 2020, 90, 1285–1291. [Google Scholar] [CrossRef]
- Mashhadi Malekzadeh, A.; Ramazani, A.; Tabatabaei Rezaei, S.J.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, A.; Ansari, M.M.; Vyawahare, A.; Jayamurugan, G.; Khan, R. Hyperbranched Polymer-Functionalized Magnetic Nanoparticle-Mediated Hyperthermia and Niclosamide Bimodal Therapy of Colorectal Cancer Cells. ACS Biomater. Sci. Eng. 2020, 6, 1102–1111. [Google Scholar] [CrossRef]
- Arce, E.; Nieto, P.M.; Díaz, V.; Castro, R.G.; Bernad, A.; Rojo, J. Glycodendritic Structures Based on Boltorn Hyperbranched Polymers and Their Interactions with Lens culinaris Lectin. Bioconjugate Chem. 2003, 14, 817–823. [Google Scholar] [CrossRef]
- Seiler, M. Hyperbranched polymers: Phase behavior and new applications in the field of chemical engineering. Fluid Phase Equilibria 2006, 241, 155–174. [Google Scholar] [CrossRef]
- Žagar, E.; Grdadolnik, J. An infrared spectroscopic study of H-bond network in hyperbranched polyester polyol. J. Mol. Struct. 2003, 658, 143–152. [Google Scholar] [CrossRef]
- Solodov, A.N.; Shayimova, J.R.; Burilova, E.A.; Shurtakova, D.V.; Zhuravleva, Y.I.; Cherosov, M.A.; Tian, Y.; Kiiamov, A.G.; Amirov, R.R. Understanding the Nucleation and Growth of Iron Oxide Nanoparticle Formation by a “Heating-Up” Process: An NMR Relaxation Study. J. Phys. Chem. C 2021, 125, 20980–20992. [Google Scholar] [CrossRef]
- Klajnert, B.; Walach, W.; Bryszewska, M.; Dworak, A.; Shcharbin, D. Cytotoxicity, haematotoxicity and genotoxicity of high molecular mass arborescent polyoxyethylene polymers with polyglycidol-block-containing shells. Cell Biol. Int. 2006, 30, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Sonia, S.; Jayasudha, R.; Jayram, N.D.; Kumar, P.S.; Mangalaraj, D.; Prabagaran, S.R. Synthesis of hierarchical CuO nanostructures: Biocompatible antibacterial agents for Gram-positive and Gram-negative bacteria. Curr. Appl. Phys. 2016, 16, 914–921. [Google Scholar] [CrossRef]





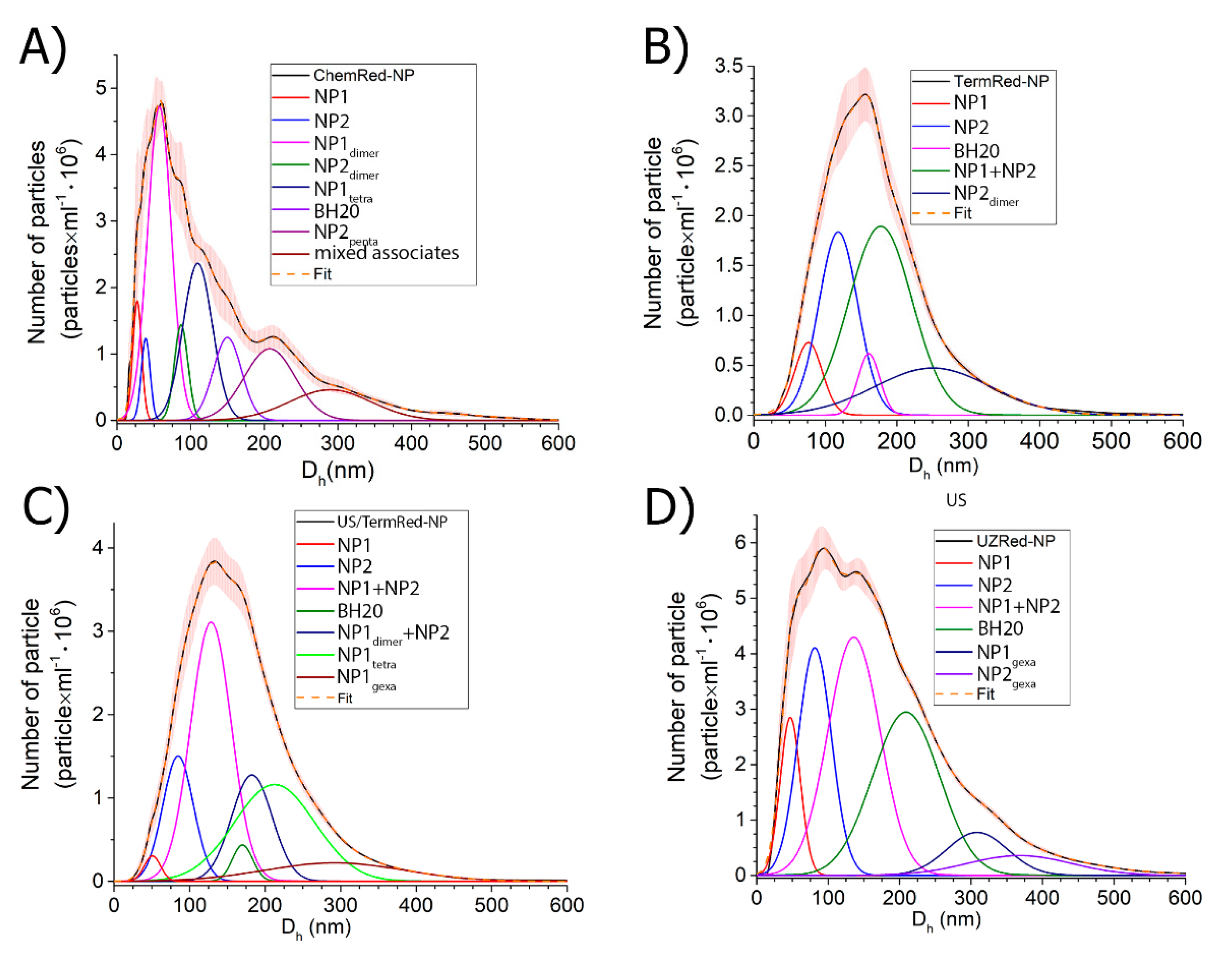

| Sample | Dh(mean) by NTA; (nm) | Dh(mode) by NTA; (nm) | D by SEM; (nm) | Total Particle Concentration in Solution; (Particles × mL−1) |
|---|---|---|---|---|
| ChemRed-NP | 133 ± 7 | 27, 40 | 25 | 6.24 × 108 |
| TermRed-NP | 172 ± 2 | 77, 118 | 50, 85 | 4.92 × 108 |
| US-NP | 169 ± 6 | 50, 80 | 30, 55 | 1.25 × 109 |
| US/TermRed-NP | 168 ± 4 | 35, 52 | 25 | 6.03 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khannanov, A.; Burmatova, A.; Ignatyeva, K.; Vagizov, F.; Kiiamov, A.; Tayurskii, D.; Cherosov, M.; Gerasimov, A.; Vladimir, E.; Kutyreva, M. Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix. Int. J. Mol. Sci. 2022, 23, 14764. https://doi.org/10.3390/ijms232314764
Khannanov A, Burmatova A, Ignatyeva K, Vagizov F, Kiiamov A, Tayurskii D, Cherosov M, Gerasimov A, Vladimir E, Kutyreva M. Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix. International Journal of Molecular Sciences. 2022; 23(23):14764. https://doi.org/10.3390/ijms232314764
Chicago/Turabian StyleKhannanov, Artur, Anastasia Burmatova, Klara Ignatyeva, Farit Vagizov, Airat Kiiamov, Dmitrii Tayurskii, Mikhail Cherosov, Alexander Gerasimov, Evtugyn Vladimir, and Marianna Kutyreva. 2022. "Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix" International Journal of Molecular Sciences 23, no. 23: 14764. https://doi.org/10.3390/ijms232314764
APA StyleKhannanov, A., Burmatova, A., Ignatyeva, K., Vagizov, F., Kiiamov, A., Tayurskii, D., Cherosov, M., Gerasimov, A., Vladimir, E., & Kutyreva, M. (2022). Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix. International Journal of Molecular Sciences, 23(23), 14764. https://doi.org/10.3390/ijms232314764









