Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2
Abstract
:1. Introduction
2. Results
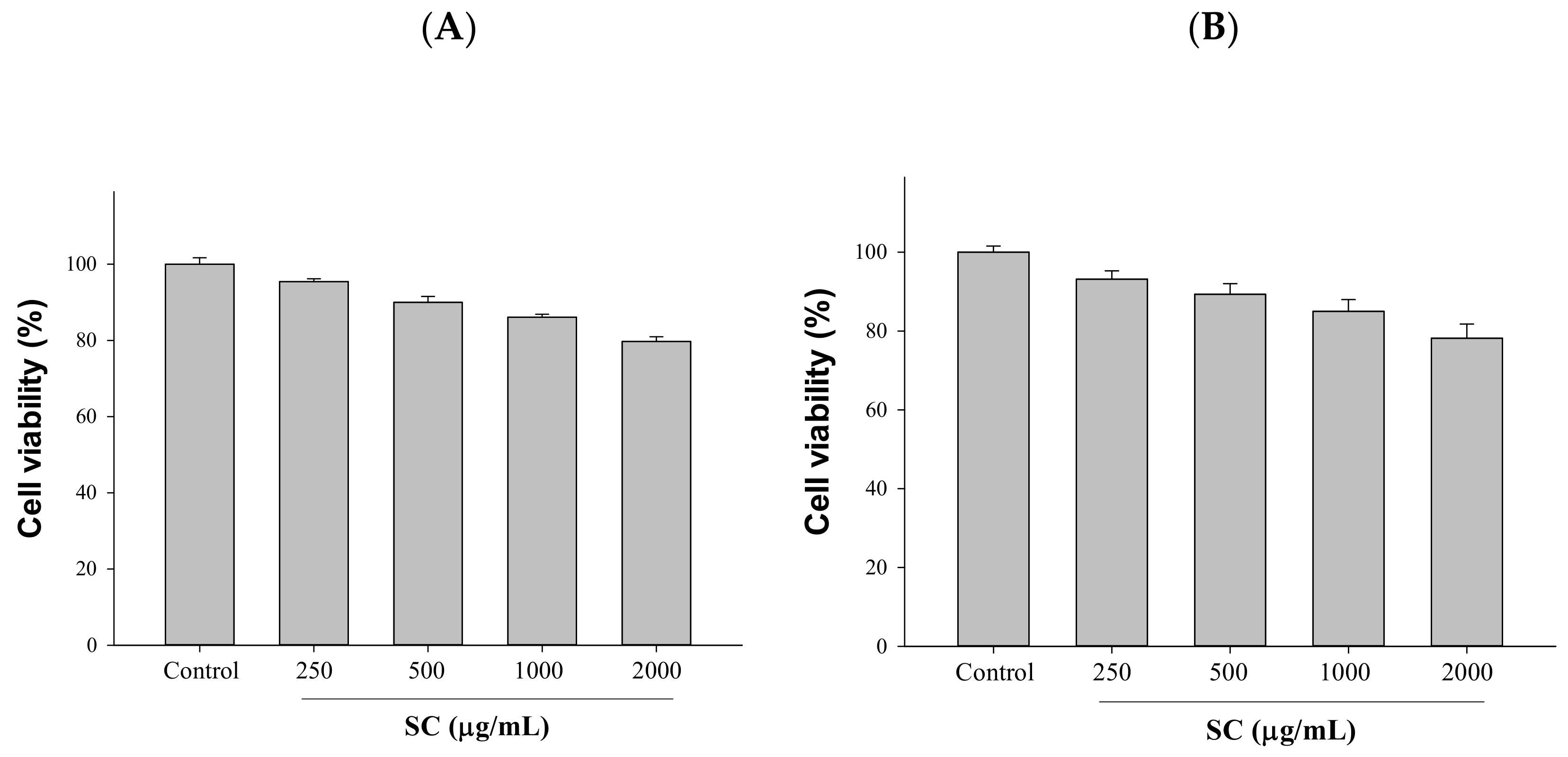
2.1. Cytotoxicity Assessment Was Performed after Exposure to Different Concentrations of SC
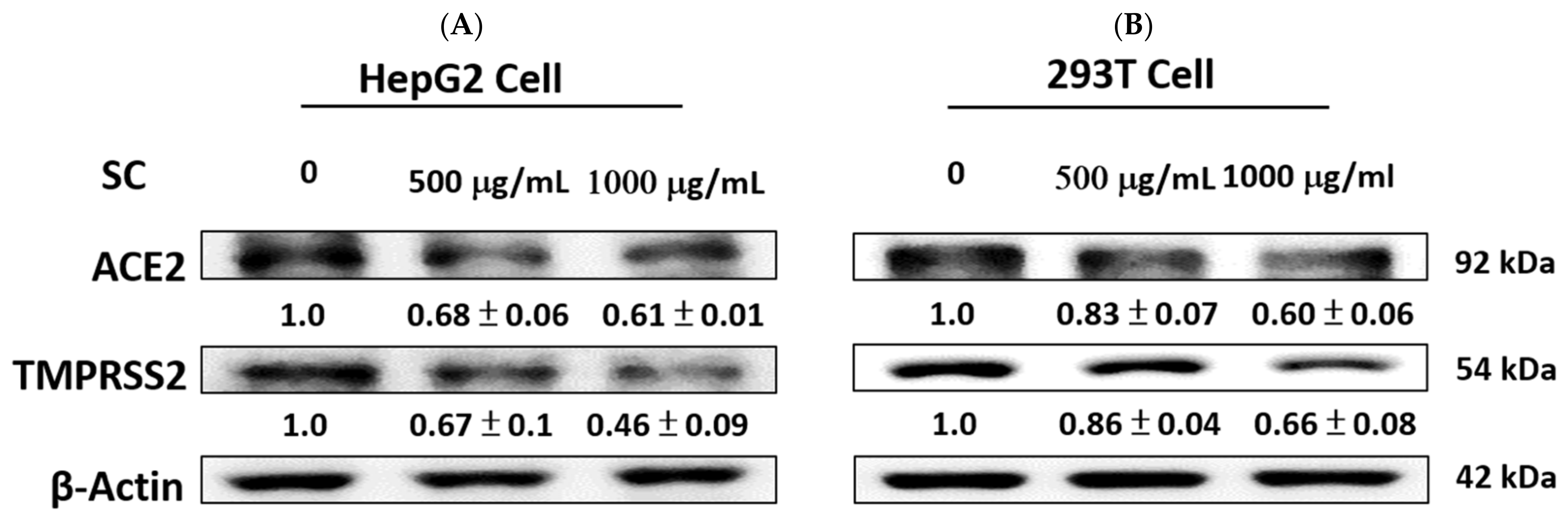
2.2. SC Inhibited ACE2 and TMPRSS2 Expression
2.3. Cytotoxicity Assessment Was Performed after Exposure to Different Concentrations of Adenosine
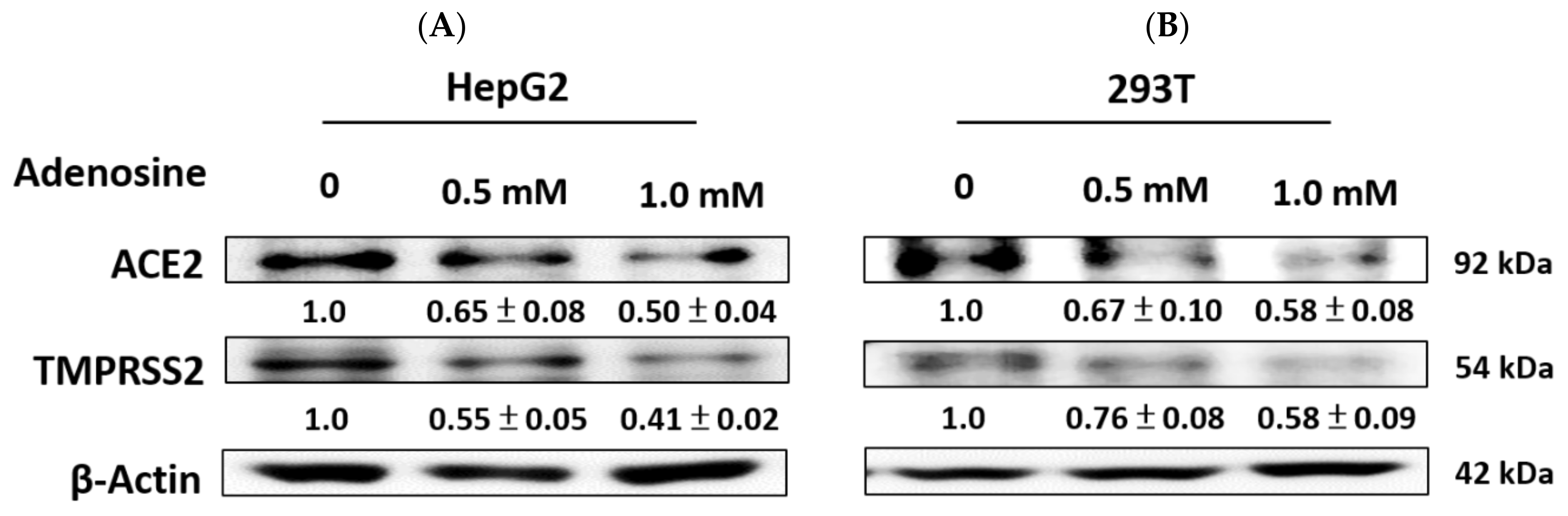
2.4. Adenosine Inhibited ACE2 and TMPRSS2 Expression
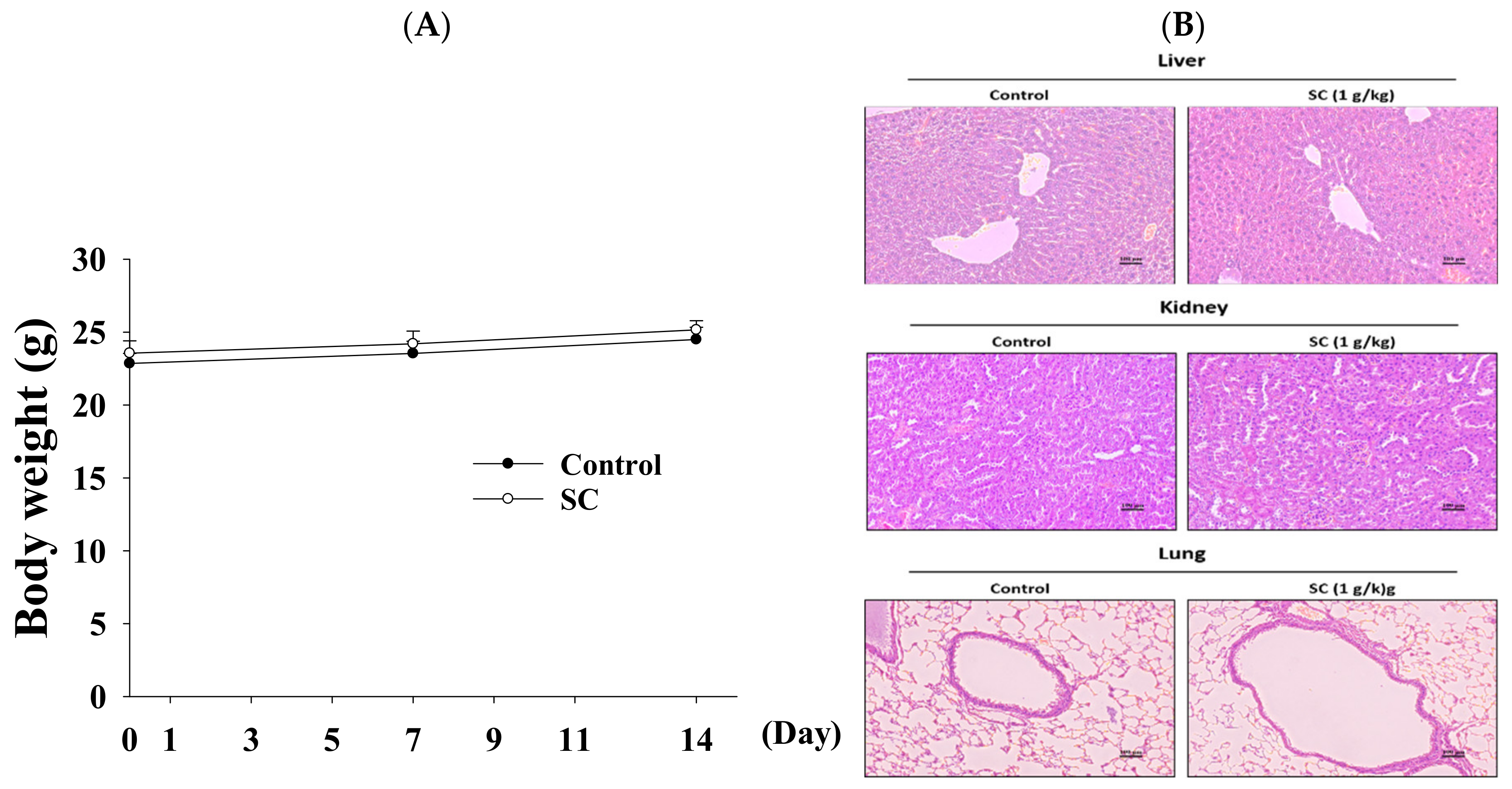
2.5. Acute Oral Toxicity Study of Schizophyllum Commune
2.6. The Function of SC in Animal Model
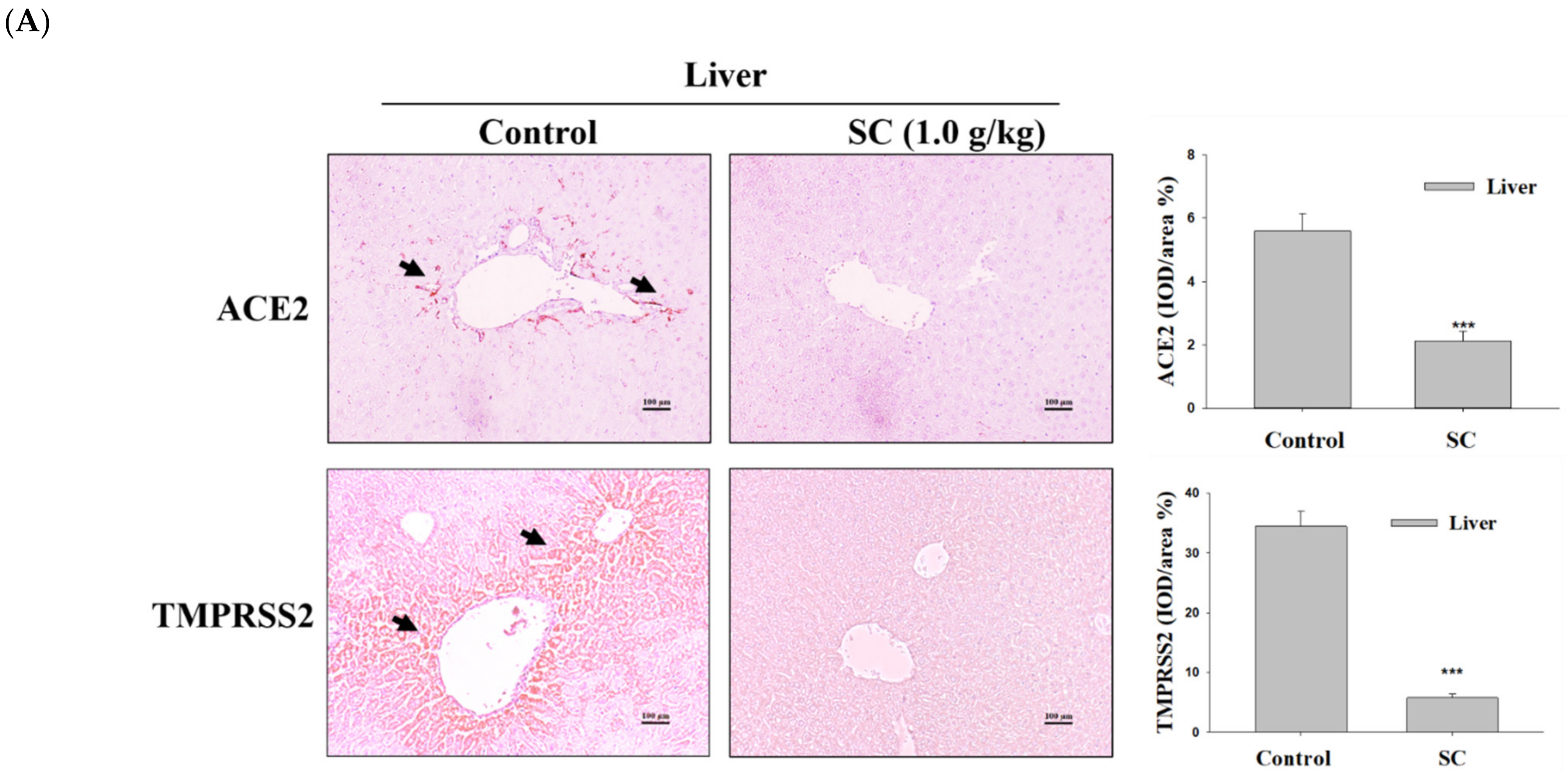
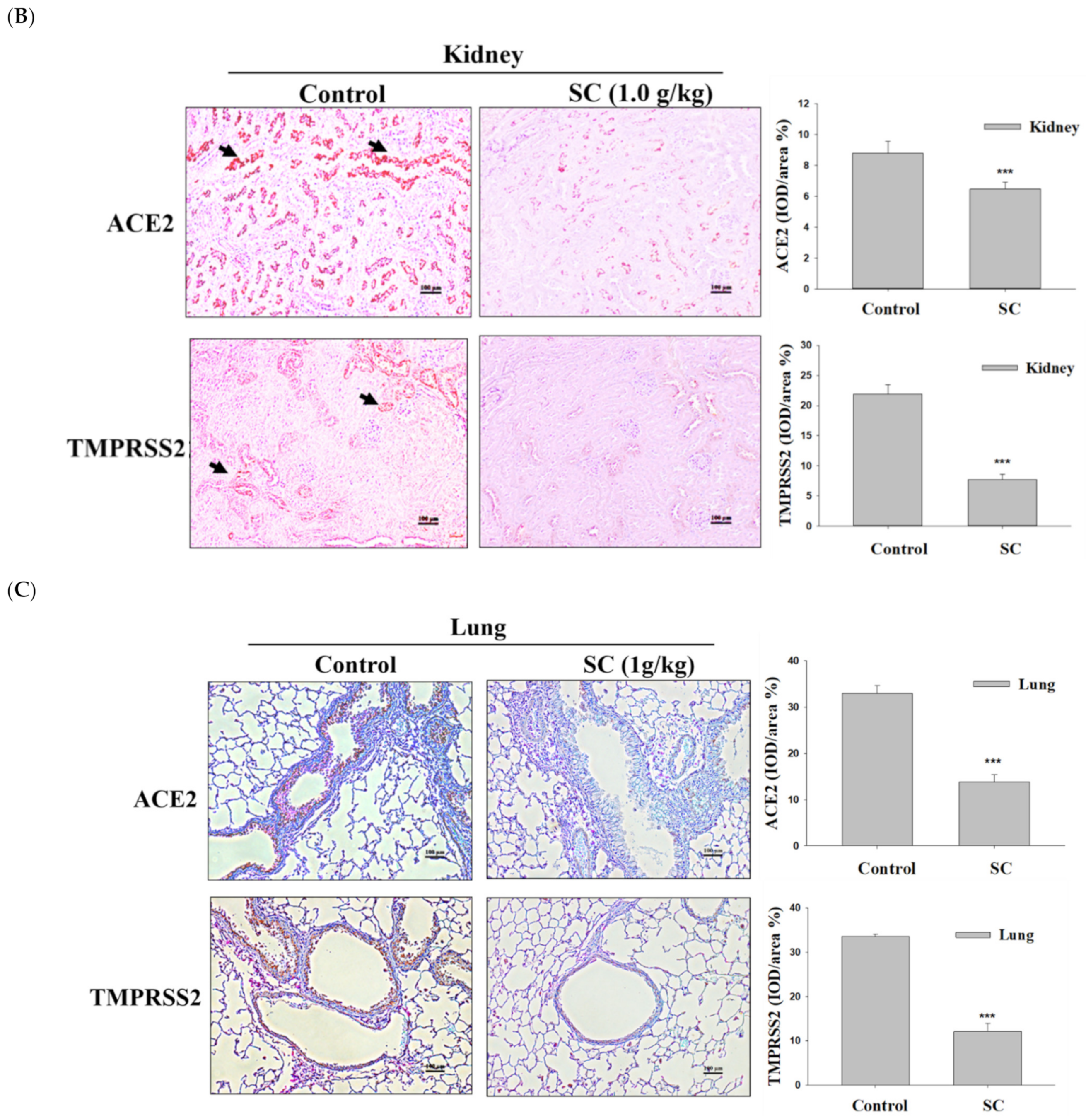
2.7. SC Inhibited ACE2 and TMPRSS2 Protein Expression according to Immunohistochemical Images
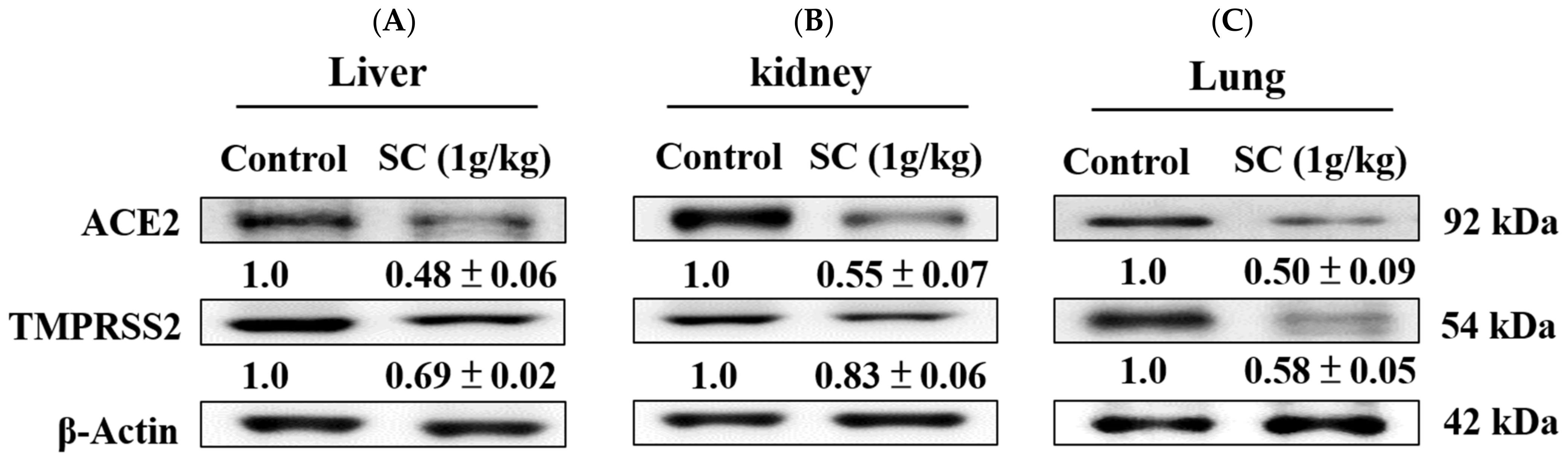
2.8. SC Inhibited ACE2 and TMPRSS2 Protein Expression In Vivo
2.9. HPLC Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Cell Viability
4.4. Western Blot Analysis
4.5. Acute Oral Toxicity
4.6. Mouse Model
4.7. Histopathological Analysis
4.8. Immunohistochemistry (IHC)
4.9. Determination of Adenosine by HPLC
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Mobeen, A.; Chandra, A.; Joshi, S.; Ramachandran, S. A meta-analysis of comorbidities in COVID-19: Which diseases increase the susceptibility of SARS-CoV-2 infection? Comput. Biol. Med. 2021, 130, 104219. [Google Scholar] [CrossRef] [PubMed]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife 2020, 9, e61390. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.; Herrler, G.; Wu, N.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, Y.-S.; Yang, Y.-H.; Shu, L.-H.; Cheng, Y.-C.; Te Liu, H. GB-2 inhibits ACE2 and TMPRSS2 expression: In vivo and in vitro studies. Biomed. Pharmacother. 2020, 132, 110816. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Hua, J.; Zhang, L.; Bian, J.; Liu, B.; Zhao, Z.; Jin, S. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int. J. Environ. Res. Public Health 2021, 18, 284. [Google Scholar] [CrossRef]
- Mišković, J.; Karaman, M.; Rašeta, M.; Krsmanović, N.; Berežni, S.; Jakovljević, D.; Piattoni, F.; Zambonelli, A.; Gargano, M.L.; Venturella, G. Comparison of Two Schizophyllum commune Strains in Production of Acetylcholinesterase Inhibitors and Antioxidants from Submerged Cultivation. J. Fungi 2021, 7, 115. [Google Scholar] [CrossRef]
- Patel, S. Immunomodulatory aspects of medicinal mushrooms. In Medicinal Mushrooms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 169–185. [Google Scholar]
- Leathers, T.-D.; Nunnally, M.-S.; Price, N.-P. Co-production of schizophyllan and arabinoxylan from corn fiber. Biotechnol Lett. 2006, 28, 623–626. [Google Scholar] [CrossRef]
- Wong, J.-H.; Ng, T.-B.; Chan, H.-H.-L.; Liu, Q.; Man, G.-C.-W.; Zhang, C.-Z.; Guan, S.; Ng, C.-C.-W.; Fang, E.-F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Raskovalova, T.; Lokshin, A.; Huang, X.; Su, Y.; Mandic, M.; Zarour, H.-M.; Jackson, E.-K.; Gorelik, E. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 2007, 67, 5949–5956. [Google Scholar] [CrossRef] [Green Version]
- Naidu, A.S.; Shahidi, F.; Wang, C.-K.; Sato, K.; Wirakartakusumah, A.; Aworhf, O.C.; Halliwell, B.; Clemensh, R.A. SARS-CoV-2-induced Host Metabolic Reprogram (HMR): Nutritional Interventions for Global Management of COVID-19 and Post-Acute Sequelae of COVID-19 (PASC). J. Food Bioact. 2022, 18, 1–42. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, C.G.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Stewart, C.A.; Gay, C.M.; Ramkumar, K.; Cargill, K.R.; Cardnell, R.J.; Nilsson, M.B.; Heeke, S.; Park, E.M.; Kundu, S.T.; Diao, L. SARS-CoV-2 infection induces EMT-like molecular changes, including ZEB1-mediated repression of the viral receptor ACE2, in lung cancer models. BioRxiv 2020. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid.-Based Complement. Altern. Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.; Jiang, X.; Zhang, Z.; Zheng, Y.; Wang, Z.; Zhu, Y.; Gao, L.; Huang, H.; Wang, X. Systemic analysis of tissue cells potentially vulnerable to SARS-CoV-2 infection by the protein-proofed single-cell RNA profiling of ACE2, TMPRSS2 and Furin proteases. BioRxiv 2020. [Google Scholar] [CrossRef]
- Steiger, S.; Rossaint, J.; Zarbock, A.; Anders, H.-J. Secondary immunodeficiency related to kidney disease (SIDKD)—Definition, unmet need, and mechanisms. J. Am. Soc. Nephrol. 2022, 33, 259–278. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Velez, G.; Parsons, D.E.; Li, K.; Ortiz, M.E.; Sharma, S.; McCray, P.B.; Bassuk, A.G.; Mahajan, V.B. Structure-based phylogeny identifies avoralstat as a TMPRSS2 inhibitor that prevents SARS-CoV-2 infection in mice. J. Clin. Investig. 2021, 131, e147973. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, A.; Bharate, S.B. Natural products in mitigation of SARS CoV Infections. Curr. Med. Chem. 2021, 28, 4454–4483. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, J.; Chen, Z.; Zhang, M.; Peng, H.; Liu, J.; Ding, L.; Liu, M.; Zhao, C.; Zhao, P. Nonmuscle myosin heavy chain IIA facilitates SARS-CoV-2 infection in human pulmonary cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2111011118. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 2021, 12, 848. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Lumbers, E.R.; Head, R.; Smith, G.R.; Delforce, S.J.; Jarrott, B.; Martin, J.H.; Pringle, K.G. The interacting physiology of COVID-19 and the renin-angiotensin-aldosterone system: Key agents for treatment. Pharmacol. Res. Perspect. 2022, 10, e00917. [Google Scholar] [CrossRef]
- Shukla, A.K.; Banerjee, M. Angiotensin-converting-enzyme 2 and renin-angiotensin system inhibitors in COVID-19: An update. High Blood Press Cardiovasc Prev. 2021, 28, 129–139. [Google Scholar] [CrossRef]
- Cure, M.C.; Cure, E. Prolonged NHE activation may be both cause and outcome of cytokine release syndrome in COVID-19. Curr. Pharm. Des. 2022, 28, 1815–1822. [Google Scholar] [CrossRef]
- Wang, M.; Xiong, H.; Chen, H.; Li, Q.; Ruan, X.Z. Renal injury by SARS-CoV-2 infection: A systematic review. Kidney Diseases 2021, 7, 100–110. [Google Scholar] [CrossRef]
- Ghoda, A.; Ghoda, M. Liver injury in COVID-19 infection: A systematic review. Cureus 2020, 12, e9487. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.-R. COVID-19 Pandemic along with Pandemic of Lifestyle-Associated Diseases Victimizes Patients in an Inflammation Context! Dubai Med. J. 2020, 3, 55–57. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Dianat, N.; Steichen, C.; Vallier, L.; Weber, A. Dubart-Kupperschmitt, A., Human pluripotent stem cells for modelling human liver diseases and cell therapy. Curr. Gene Ther. 2013, 13, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Vora, S.-M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Españo, E.; Kim, J.; Lee, K.; Kim, J.-K. Phytochemicals for the treatment of COVID-19. J. Microbiol. 2021, 59, 959–977. [Google Scholar] [CrossRef]
- Thongsiri, C.; Nagai-Yoshioka, Y.; Yamasaki, R.; Adachi, Y.; Usui, M.; Nakashima, K.; Nishihara, T.; Ariyoshi, W. Schizophyllum commune β-glucan: Effect on interleukin-10 expression induced by lipopolysaccharide from periodontopathic bacteria. Carbohydr. Polym. 2021, 253, 117285. [Google Scholar] [CrossRef]
- Ellan, K.; Thayan, R.; Phan, C.; Sabaratnam, V. Anti-inflammatory effect of mushrooms in dengue-infected human monocytes. Trop. Biomed. 2019, 36, 1087–1098. [Google Scholar]
- Hobbs, C. The chemistry, nutritional value, immunopharmacology, and safety of the traditional food of medicinal split-gill fugus Schizophyllum commune Fr.: Fr.(Schizophyllaceae). A literature review. Int. J. Med. Mushrooms 2005, 7, 127–140. [Google Scholar] [CrossRef]
- Kakumu, S.; Ishikawa, T.; Wakita, T.; Yoshioka, K.; Ito, Y.; Shinagawa, T. Effect of sizofiran, a polysaccharide, on interferon gamma, antibody production and lymphocyte proliferation specific for hepatitis B virus antigen in patients with chronic hepatitis B. Int. J. Immunopharmacol. 1991, 13, 969–975. [Google Scholar] [CrossRef]
- Mirfat, A.-H.-S.; Noorlidah, A.; Vikineswary, S. Scavenging activity of Schizophyllum commune extracts and its correlation to total phenolic content. J. Trop. Agric. Food Sci. 2010, 38, 231–238. [Google Scholar]
- Geiger, J.-D.; Khan, N.; Murugan, M.; Boison, D. Possible role of adenosine in COVID-19 pathogenesis and therapeutic opportunities. Front. Pharmacol. 2020, 11, 594487. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Function 2012, 3, 1118–1130. [Google Scholar] [CrossRef]
- Mochizuki, S.; Miyamoto, N.; Sakurai, K. Oligonucleotide delivery to antigen presenting cells by using schizophyllan. Drug Metab. Pharmacokinet. 2022, 42, 100434. [Google Scholar] [CrossRef]
- Falcone, C.; Caracciolo, M.; Correale, P.; Macheda, S.; Vadalà, E.G.; La Scala, S.; Tescione, M.; Danieli, R.; Ferrarelli, A.; Tarsitano, M.G. Can adenosine fight COVID-19 acute respiratory distress syndrome? J. Clin. Med. 2020, 9, 3045. [Google Scholar] [CrossRef]
- Lucas, R.; Verin, A.D.; Black, S.M.; Catravas, J.D. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem. Pharmacol. 2009, 77, 1763–1772. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Berg, N.-K.; Mills, T.; Zhang, K.; Eltzschig, H.-K.; Yuan, X. Adenosine at the interphase of hypoxia and inflammation in Lung Injury. Front. Immunol. 2021, 11, 604944. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.-K.; Huang, W.-C.; Sun, Y.-W.; Deng, J.-S.; Chien, L.-H.; Chou, Y.-N.; Jiang, W.-P.; Lin, J.-G.; Huang, G.-J. Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. Int. J. Mol. Sci. 2022, 23, 14766. https://doi.org/10.3390/ijms232314766
Sun T-K, Huang W-C, Sun Y-W, Deng J-S, Chien L-H, Chou Y-N, Jiang W-P, Lin J-G, Huang G-J. Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. International Journal of Molecular Sciences. 2022; 23(23):14766. https://doi.org/10.3390/ijms232314766
Chicago/Turabian StyleSun, Te-Kai, Wen-Chin Huang, Yu-Wen Sun, Jeng-Shyan Deng, Liang-Hsuan Chien, Ya-Ni Chou, Wen-Ping Jiang, Jaung-Geng Lin, and Guan-Jhong Huang. 2022. "Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2" International Journal of Molecular Sciences 23, no. 23: 14766. https://doi.org/10.3390/ijms232314766
APA StyleSun, T.-K., Huang, W.-C., Sun, Y.-W., Deng, J.-S., Chien, L.-H., Chou, Y.-N., Jiang, W.-P., Lin, J.-G., & Huang, G.-J. (2022). Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. International Journal of Molecular Sciences, 23(23), 14766. https://doi.org/10.3390/ijms232314766







