Oxidative Status as an Attribute for Selective Antitumor Activity of Platinum-Containing Nanoparticles against Hepatocellular Carcinoma
Abstract
:1. Introduction
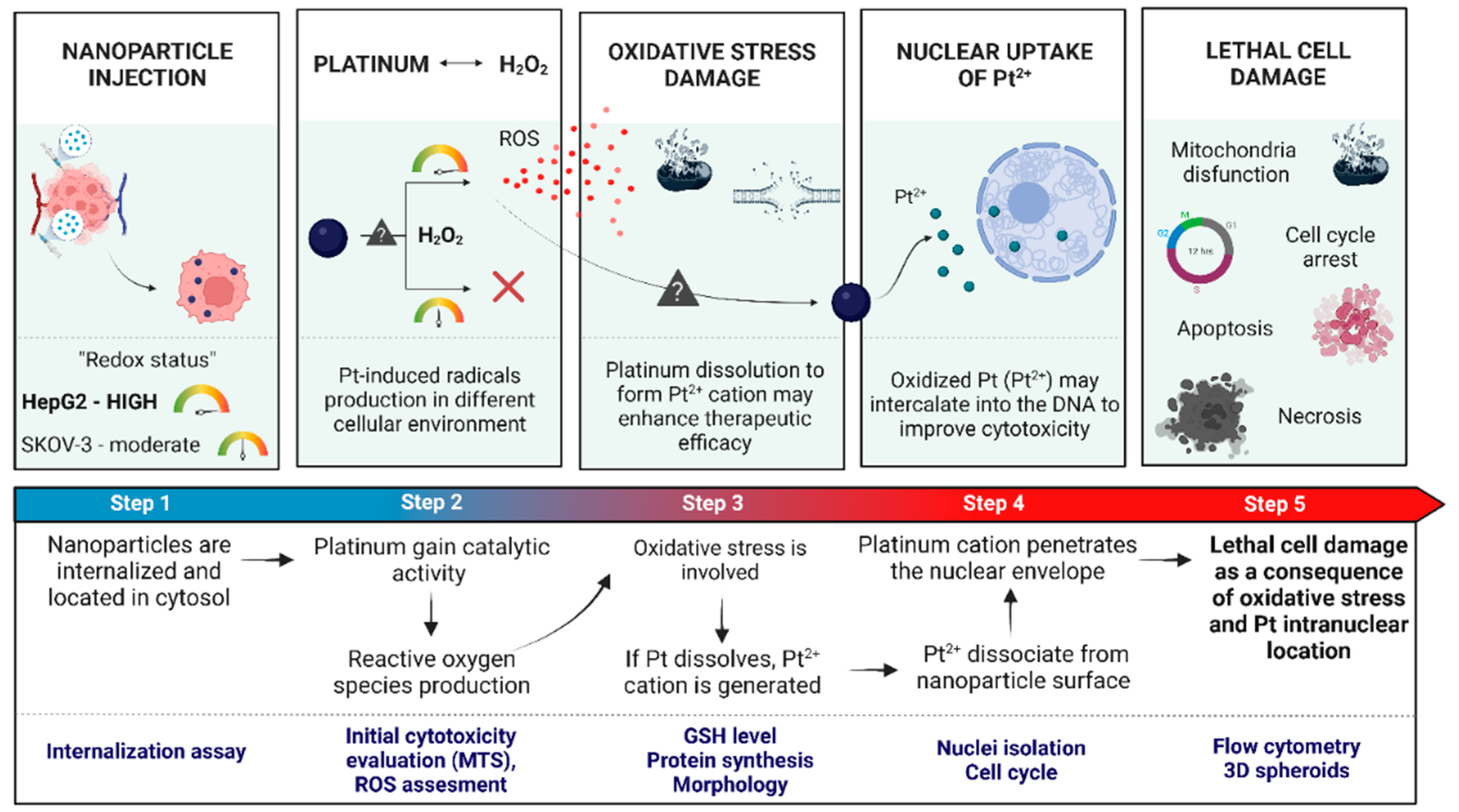
2. Results and Discussion
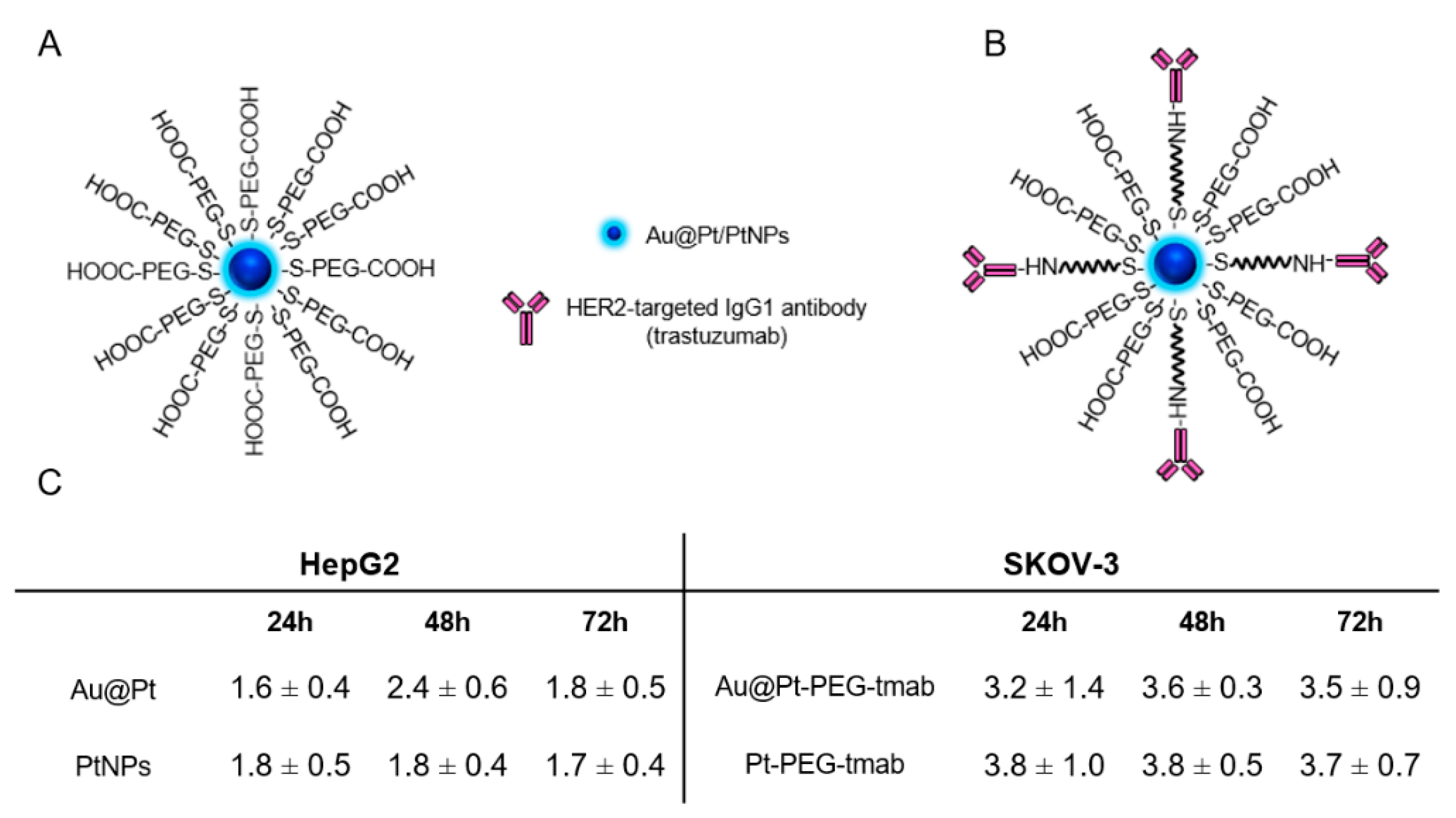
2.1. Characterization of Investigated Platinum Particles
2.2. Uptake of Nanoparticles in HepG2 and SKOV-3 Cells
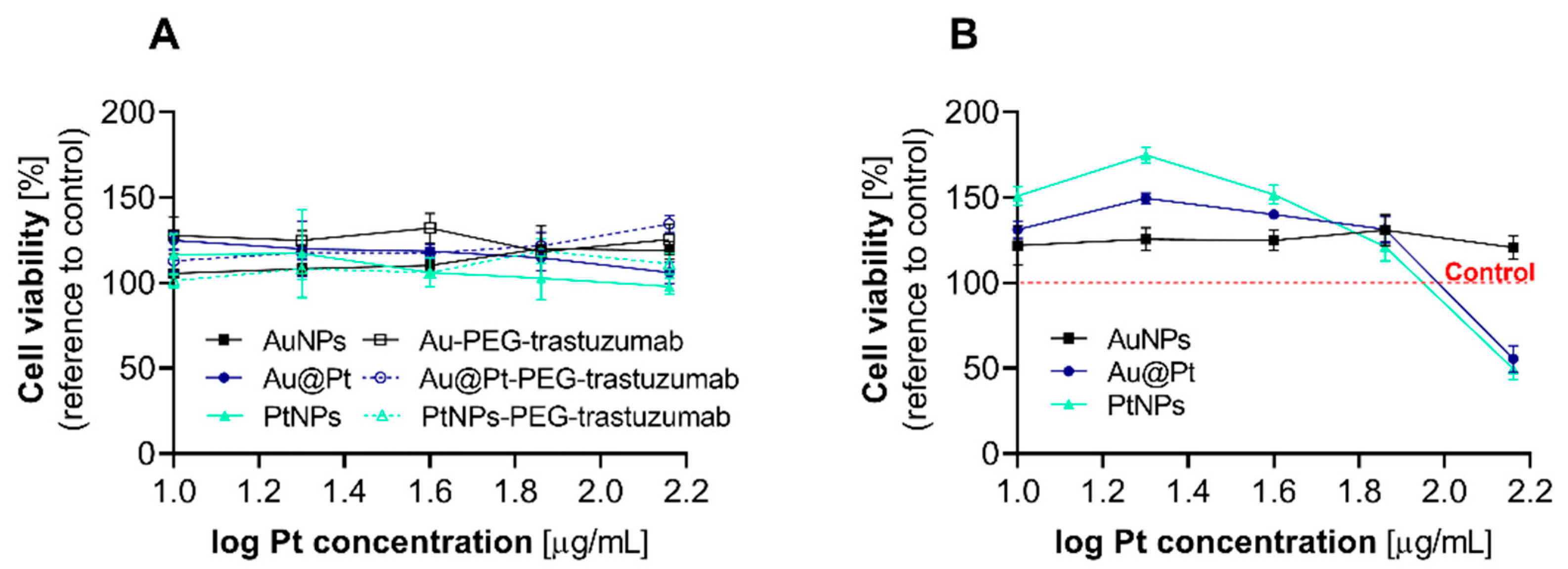
2.3. Cytotoxicity Evaluation after Incubation of Cancer Cells with the Different Pt Particles and Their Bioconjugates
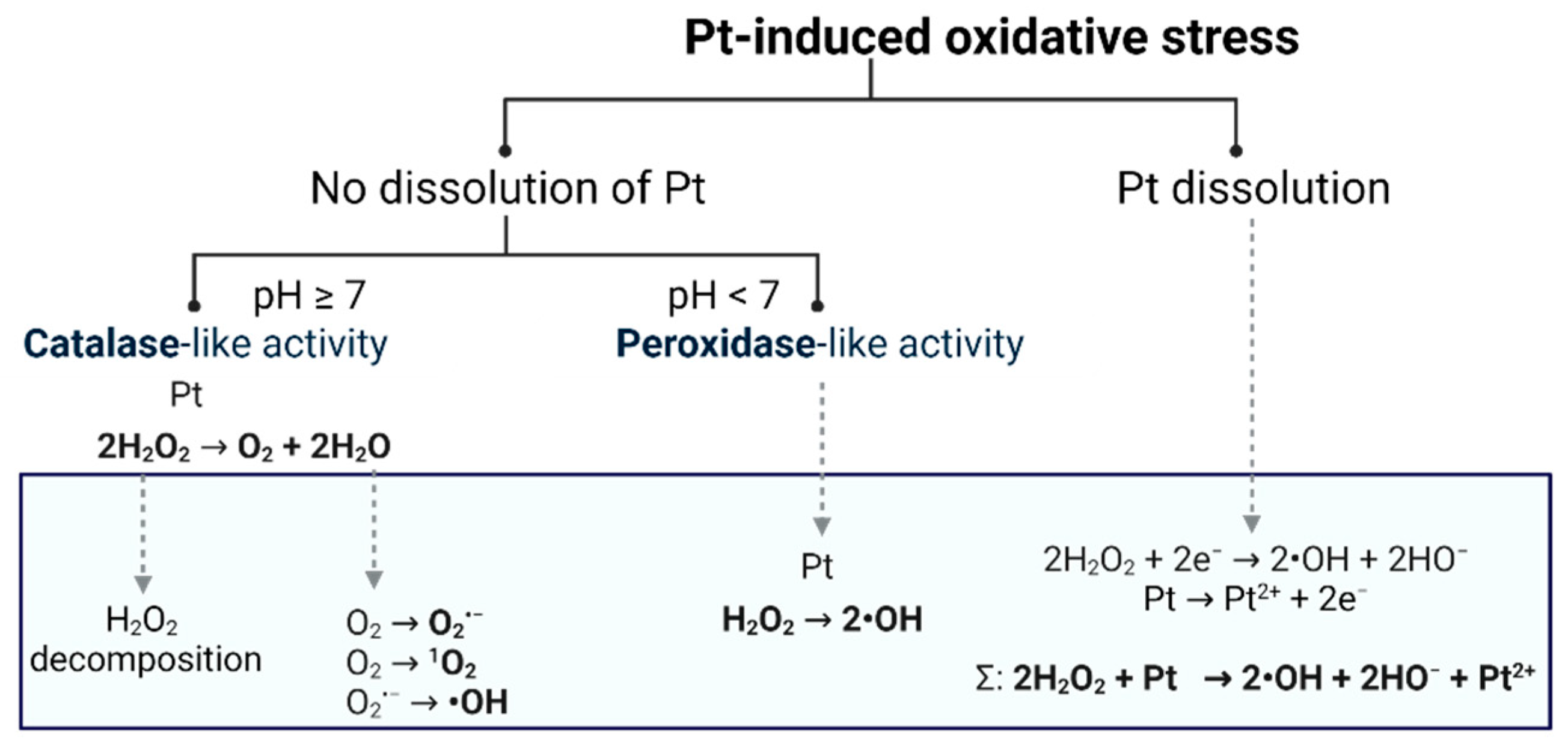
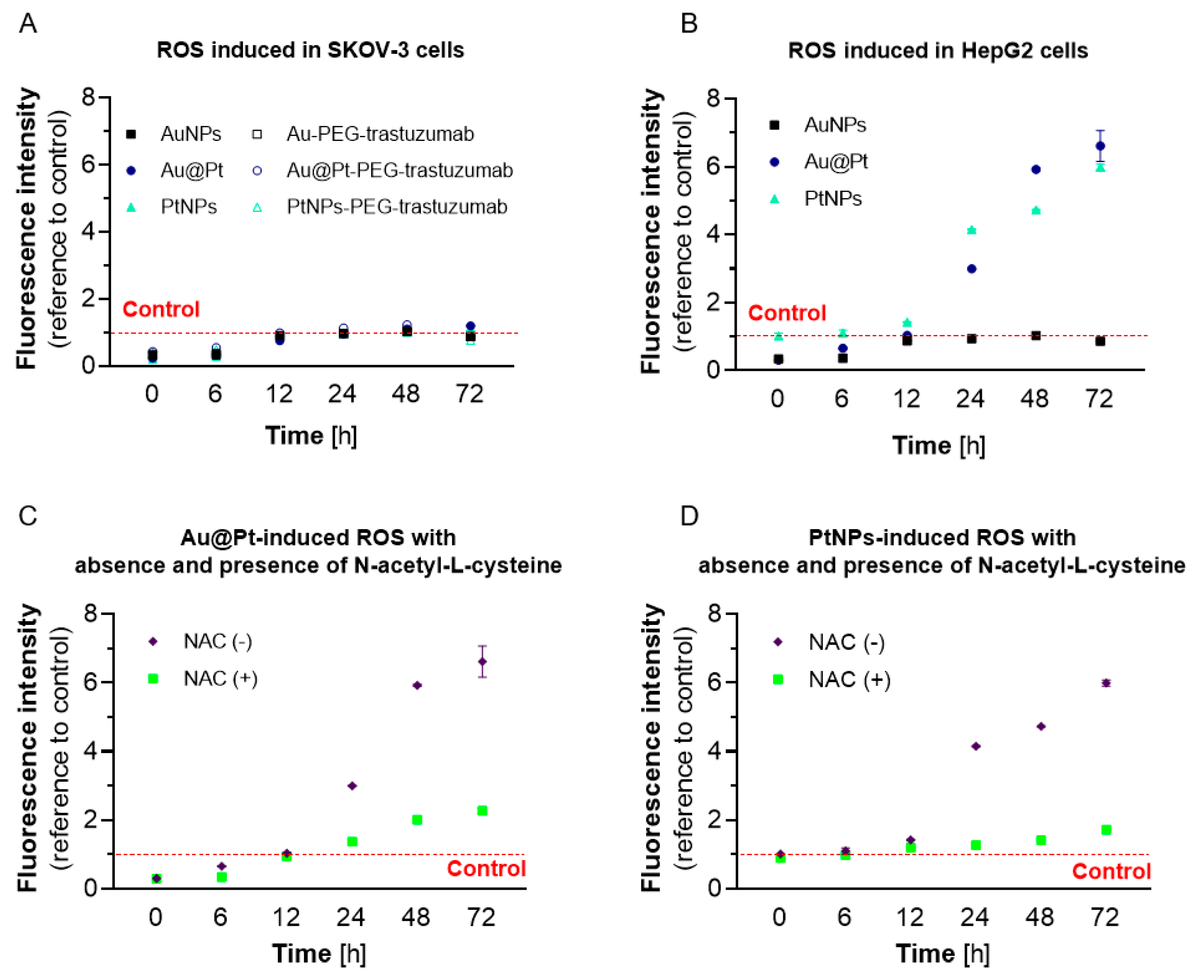
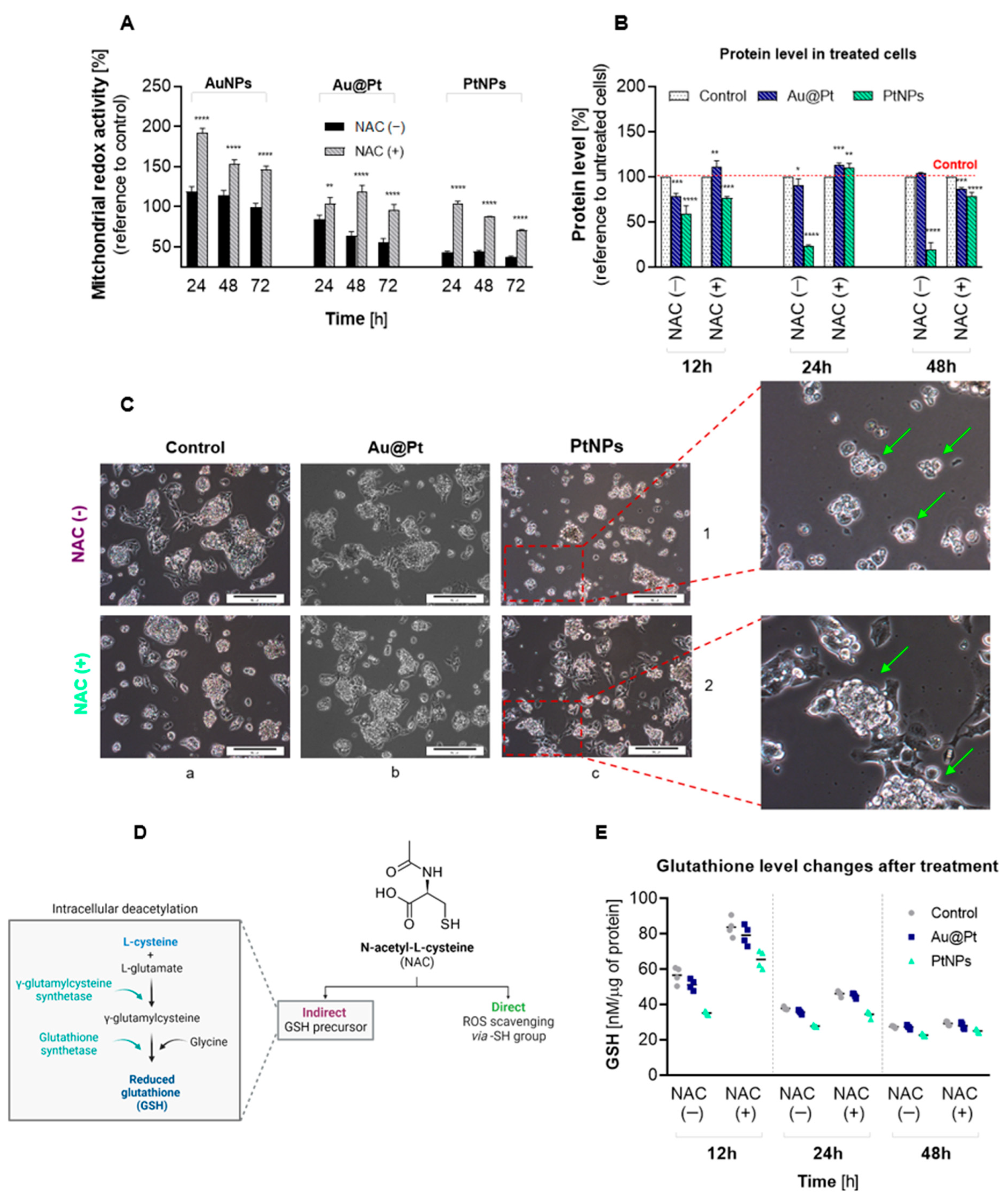
2.4. The Effect of PtNPs, Au@Pt and Their Bioconjugates on Redox Homeostasis in Cancer Cell Lines
2.5. Effects of Oxidative Stress in HepG2 Cells
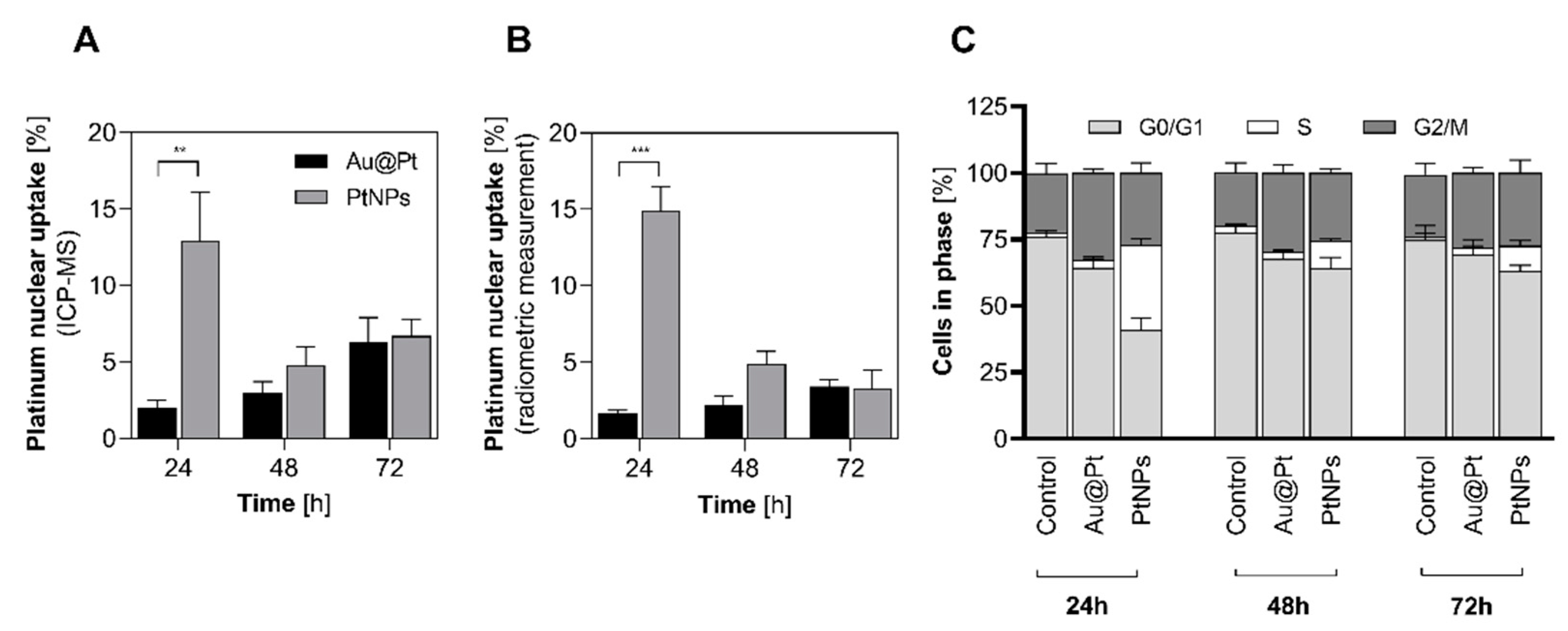
2.6. Nuclear Uptake and Cell Cycle Arrest
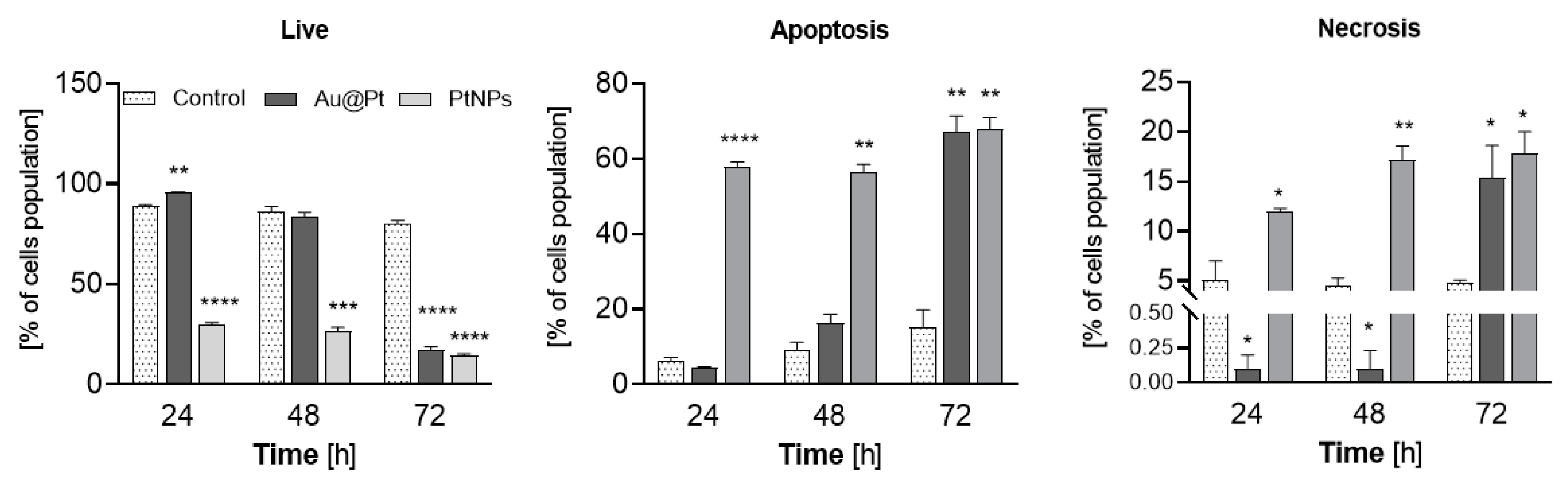
2.7. The Induction of Apoptosis in HepG2 Cells Treated with PtNPs and Au@Pt
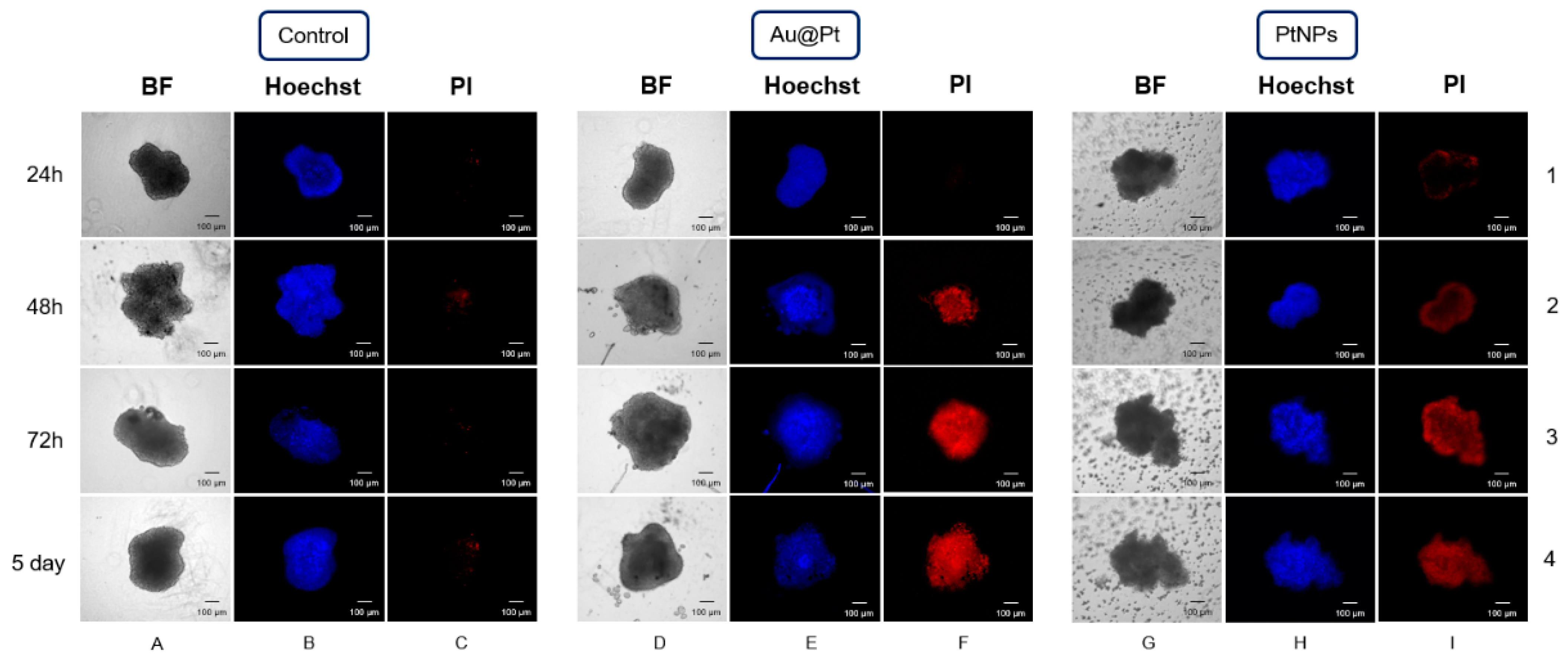
2.8. The Influence of PtNPs and Au@Pt on Three-Dimensional Spheroid Cell Cultures
3. Materials and Methods
3.1. Uptake of Nanoparticles in HepG2 and SKOV-3 Cells
3.2. Cytotoxicity Studies
3.3. Reactive Oxygen Species (ROS)
3.4. Glutathione Level Changes
3.5. Protein Level Changes
3.6. Nuclear Uptake and Cell Cycle Arrest
3.7. Flow Cytometry–Apoptosis
3.8. Spheroids
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B.; Kim, H. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. Int. J. Mol. Sci. 2021, 22, 5111. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.; Chen, H.; Xiong, F.; Fang, Y.; Huang, H.; Wu, J. Platinum-based chemotherapy: Via nanocarriers and co-delivery of multiple drugs. Biomater. Sci. 2021, 9, 6023–6036. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Li, M.; Yin, J.-J.; Nie, Z. pH dependent catalytic activities of platinum nanoparticles with respect to the decomposition of hydrogen peroxide and scavenging of superoxide and singlet oxygen. Nanoscale 2014, 6, 11904–11910. [Google Scholar] [CrossRef]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-Coated Platinum Nanoparticles with Selective Toxicity against Liver Cancer Cells. Angew. Chem. Int. Ed. 2018, 58, 4901–4905. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs—Phenanthriplatin. Anticancer Res. 2019, 34, 471–476. [Google Scholar]
- Ma, J.; Goryaynov, A.; Sarma, A.; Yang, W. Self-regulated viscous channel in the nuclear pore complex. Proc. Natl. Acad. Sci. USA 2012, 109, 7326–7331. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.S.; Tse, W.K.F.; Yu, L.C.; Li, K.M.; Mak, N.K.; Gupta, R.C.; Tse, K.F.W. Energy and Redox States in the C6 Glioma Cells Following Acute Exposure to Zn, Se+4, and Se+6 and the Correlation with Apoptosis. Toxicol. Mech. Methods 2006, 16, 13–19. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards principled design of cancer nanomedicine to accelerate clinical translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M.; Ahmad, M.F. Nanoparticle-Protein Interaction: The Significance and Role of Protein Corona. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Cheng, C.; Liu, X.; Jiang, H.; Wang, X. A highly sensitive electrochemical biosensor for evaluation of oxidative stress based on the nano-interface of graphene nanocomposites blended with Au, Fe3O4 and Pt nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 18441–18449. [Google Scholar] [CrossRef] [PubMed]
- Bendale, Y.; Bendale, V.; Paul, S. Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr. Med. Res. 2017, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green Platinum Nanoparticles Interaction With HEK293 Cells: Cellular Toxicity, Apoptosis, and Genetic Damage. Dose-Response 2018, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironava, T.; Simon, M.; Rafailovich, M.H.; Rigas, B. Platinum folate nanoparticles toxicity: Cancer vs. normal cells. Toxicol. In Vitro 2013, 27, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelka, J.; Gehrke, H.; Esselen, M.; Türk, M.; Crone, M.; Bräse, S.; Muller, T.; Blank, H.; Send, W.; Zibat, V.; et al. Cellular Uptake of Platinum Nanoparticles in Human Colon Carcinoma Cells and Their Impact on Cellular Redox Systems and DNA Integrity. Chem. Res. Toxicol. 2009, 22, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Jeyaraj, M.; La, H.; Yoo, H.; Choi, Y.; Do, J.T.; Park, C.; Kim, J.-H.; Hong, K. Anisotropic Platinum Nanoparticle-Induced Cytotoxicity, Apoptosis, Inflammatory Response, and Transcriptomic and Molecular Pathways in Human Acute Monocytic Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 440. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.; Alrokayan, S. Pt-Coated Au Nanoparticle Toxicity Is Preferentially Triggered Via Mitochondrial Nitric Oxide/Reactive Oxygen Species in Human Liver Cancer (HepG2) Cells. ACS Omega 2021, 6, 15431–15441. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Han, X.; Zhuang, H.; Nie, G.; Xu, H. Nanomedicine Assembled by Coordinated Selenium–Platinum Complexes Can Selectively Induce Cytotoxicity in Cancer Cells by Targeting the Glutathione Antioxidant Defense System. ACS Biomater. Sci. Eng. 2018, 4, 1954–1962. [Google Scholar] [CrossRef]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Effect of Surface Coating of Gold Nanoparticles on Cytotoxicity and Cell Cycle Progression. Nanomaterials 2018, 8, 1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yun, K.-H.; Lee, H.; Goh, S.-H.; Suh, Y.-G.; Choi, Y. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 2019, 197, 12–19. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Bai, X.-L.; Liang, T.-B. Intratumoral heterogeneity of hepatocellular carcinoma: From single-cell to population-based studies. World J. Gastroenterol. 2020, 26, 3720–3736. [Google Scholar] [CrossRef]
- Valiyaveettil, S.; Asharani, P.; Hande, M.P.; Xinyi, N. DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Wang, P.; Cui, J.; Wen, J.; Guo, Y.; Zhang, L.; Chen, X. Cisplatin induces HepG2 cell cycle arrest through targeting specific long noncoding RNAs and the p53 signaling pathway. Oncol. Lett. 2016, 12, 4605–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Martin, L. Do Cells Cycle? Proc. Natl. Acad. Sci. USA 1973, 70, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wawrowicz, K.; Majkowska-Pilip, A.; Gaweł, D.; Chajduk, E.; Pieńkowski, T.; Bilewicz, A. Au@Pt Core-Shell Nanoparticle Bioconjugates for the Therapy of HER2+ Breast Cancer and Hepatocellular Carcinoma. Model Studies on the Applicability of 193mPt and 195mPt Radionuclides in Auger Electron Therapy. Molecules 2021, 26, 2051. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar] [CrossRef]
- Singh, S.K.; Abbas, S.; Saxena, A.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Critical role of three-dimensional tumorsphere size on experimental outcome. BioTechniques 2020, 69, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Torgersen, M.L.; Kumari, R.V.; Yadava, S.K.; Pust, S.; Iversen, T.G.; Skotland, T.; Giri, J.; Sandvig, K. Biological response and cytotoxicity induced by lipid nanocapsules. J. Nanobiotechnol. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining Glutathione and Glutathione Disulfide Using the Fluorescence Probe o-Phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef]
- Gawęda, W.; Pruszyński, M.; Cędrowska, E.; Rodak, M.; Majkowska-Pilip, A.; Gaweł, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials 2020, 10, 2067. [Google Scholar] [CrossRef]
- Onsum, M.D.; Geretti, E.; Paragas, V.; Kudla, A.J.; Moulis, S.P.; Luus, L.; Wickham, T.J.; McDonagh, C.F.; MacBeath, G.; Hendriks, B.S. Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am. J. Pathol. 2013, 183, 1446–1460. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and Its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.; Christianson, T.; Rougeot, C.; Rochefort, H.; Clinton, G.M. SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J. Steroid Biochem. Mol. Biol. 1995, 55, 279–289. [Google Scholar] [CrossRef]
- Islam, M.T.; Saenz-Arana, R.; Wang, H.; Bernal, R.; Noveron, J.C. Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4-nitrophenol and methyl orange. New J. Chem. 2018, 42, 6472–6478. [Google Scholar] [CrossRef]
| Diameter * | Pt Atoms per NP ** | SA:V | Pt Atoms on the Surface ** | % of the Pt Surface Atoms | |
|---|---|---|---|---|---|
| Au@Pt | 30 nm (core) 9.70 nm (shell) | 788,882 | 0.16 | 70,432 | 8.9% |
| PtNPs | 2 nm | 273 | 3.0 | 161 | 61% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrowicz, K.; Majkowska-Pilip, A.; Szwed, M.; Żelechowska-Matysiak, K.; Chajduk, E.; Bilewicz, A. Oxidative Status as an Attribute for Selective Antitumor Activity of Platinum-Containing Nanoparticles against Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 14773. https://doi.org/10.3390/ijms232314773
Wawrowicz K, Majkowska-Pilip A, Szwed M, Żelechowska-Matysiak K, Chajduk E, Bilewicz A. Oxidative Status as an Attribute for Selective Antitumor Activity of Platinum-Containing Nanoparticles against Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2022; 23(23):14773. https://doi.org/10.3390/ijms232314773
Chicago/Turabian StyleWawrowicz, Kamil, Agnieszka Majkowska-Pilip, Marzena Szwed, Kinga Żelechowska-Matysiak, Ewelina Chajduk, and Aleksander Bilewicz. 2022. "Oxidative Status as an Attribute for Selective Antitumor Activity of Platinum-Containing Nanoparticles against Hepatocellular Carcinoma" International Journal of Molecular Sciences 23, no. 23: 14773. https://doi.org/10.3390/ijms232314773





