Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop.
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of PEO
2.2. In Vitro Cell Cytotoxicity
2.3. Real-Time Cell Analysis (RTCA)
2.4. Induction of Cell Apoptosis
2.4.1. PEO-Induced Apoptosis in HepG2 and A549 Cells
2.4.2. Hoechst 33342 Staining
2.5. Cell Cycle Analysis
2.6. PEO Treatment Decreased Mitochondrial Membrane Potential in HepG2 and A549 Cells
2.7. PEO Treatment Reduced ROS Levels in HepG2 and A549 Cells
2.8. PEO Induces Apoptosis through a Mitochondrial Pathway
2.9. Dolabella-3,7-dien-18-ol (DDO) Is One of the Main Active Components in PEO
2.9.1. DDO Induces Apoptosis through a Mitochondrial Pathway
2.9.2. DDO Induces G1 Phase Arrest by Upregulating the Expression of p21Cip1
2.10. Potential Molecular Mechanisms of PEO and DDO against A549 Cells
3. Materials and Methods
3.1. Plant Material
3.2. Simultaneous Distillation–Extraction (SDE) of Essential Oil
3.3. Chemical Composition
3.3.1. GC/MS Analysis of P. acutum Essential Oil (PEO)
3.3.2. Identification of Individual Components
3.4. In Vitro Antitumors Assay
3.4.1. Cytotoxicity Analysis
3.4.2. RTCA
3.4.3. Cell Cycle Analysis
3.4.4. Apoptosis Analysis
3.4.5. Hoechst 33342 Staining
3.5. Mitochondrial Membrane Potential Staining
3.6. Reactive Oxygen Species (ROS) Assay
3.7. Western Blotting Analysis
3.8. Immunofluorescence Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aziz, Z.; Ahmad, A.; Setapar, S.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical constituents of bryophytes. Bio- and chemical diversity, biological activity, and chemosystematics. Prog. Chem. Org. Nat. Prod. 2013, 95, 1–796. [Google Scholar] [CrossRef] [PubMed]
- Eric, S.J.H. Ethnobryology: Traditional uses and folk classification of bryophytes. Bryologist 2008, 111, 169–217. [Google Scholar] [CrossRef]
- Ludwiczuk, A.A.Y. Terpenoids and Aromatic Compounds from Bryophytes and their Central Nervous System Activity. Curr. Org. Chem. 2020, 24, 113–128. [Google Scholar] [CrossRef]
- Wang, M.H.; Yang, B.; Cao, W.; Chen, M. Investigation on Bryophytes and Medicinal Bryophytes in Tourist Route of Fanjingshan Nature Reserve of Guizhou Province. J. Anhui Agric. Sci. 2013, 41, 12846–12848. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Asakawa, Y. Bryophytes as a source of bioactive volatile terpenoids—A review. Food Chem. Toxicol. 2019, 132, 110649. [Google Scholar] [CrossRef]
- Chen, P.P. A Preliminary Chemical Analysis on the Volatiles of Several Mosses. Master’s Thesis, Hunan Normal University, Changsha, China, 2011. [Google Scholar] [CrossRef]
- Saritas, Y.; Sonwa, M.M.; Iznaguen, H.; Konig, W.A.; Muhle, H.; Mues, R. Volatile constituents in mosses (Musci). Phytochemistry 2001, 57, 443–457. [Google Scholar] [CrossRef]
- Xie, W.; Cao, T.; Han, G.C.; Lai, Y.B.; Jin, F.; Sun, X.Y. Studies on Indication of Bryophytes to Air Pollution in Fushun District, Northeast China. Chin. J. Ecol. 1999, 18, 2–6. [Google Scholar]
- Feng, W.W. Studies on Culturing Techniques of Plagiomnium Acutum for Ornamental. Master’s Thesis, Zhongkai University of Agriculture and Engineering, Guangzhou, China, 30 December 2018. [Google Scholar] [CrossRef]
- Mao, L.H.; Wen, C.F.; Ding, H.Q.; Zhou, Q.C.; Tian, D.Q. The Ornamental Application of Bryophytes. Chin. Wild Plant Resour. 2020, 39, 30–32. [Google Scholar] [CrossRef]
- Hanan, Y.A.; Shagufta, P.; Raha, O.; Areej, M.A.; Sultan, Y.; Juergen, W.; Afsar, K.; Rashad, M. Chemical Composition and Antimicrobial Activity of the Essential Oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi Grown in Saudi Arabia. Arab. J. Chem. 2020, 13, 8209–8217. [Google Scholar] [CrossRef]
- Pinho, A.I.; Wallau, G.L.; Nunes, M.E.; Leite, N.F.; Tintino, S.R.; Da, C.L.; Da, C.F.; Da, C.J.; Douglas, M.C.H.; Posser, T. Fumigant activity of the Psidium guajava var. pomifera (Myrtaceae) essential oil in Drosophila melanogaster by means of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 696785. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Y.; Li, G.Z.; Tan, Z.H.; Liu, H. Study on the Component of A New Chemical Type of Leaves Oil from Cinnamomum caphora. J. Fujian Fore. Tech. 2010, 37, 102–104. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L. Gas chromatographic retention indices of biologically and environmentally important organic compounds on capillary columns with low-polar stationary phases. J. Chromatogr. A 2009, 1216, 8998–9007. [Google Scholar] [CrossRef]
- Amico, V.; Currenti, R.; Oriente, G.; Piattelli, M.; Tringali, C. 18-Hydroxy-3,7-dolabelladiene from the brown alga, Dictyota dichotoma. Phytochemistry 1981, 20, 848–849. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Rinkel, J.; Rabe, P.; Beyraghdar, K.A.; Bouwmeester, H.J. 18-Hydroxydolabella-3,7-diene synthase—A diterpene synthase from Chitinophaga pinensis. Beilstein J. Org. Chem. 2017, 13, 1770–1780. [Google Scholar] [CrossRef]
- Duh, C.Y.; Chia, M.C.; Wang, S.K.; Chen, H.J.; El-Gamal, A.A.; Dai, C.F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64, 1028–1031. [Google Scholar] [CrossRef]
- Wang, S.K.; Huang, M.J.; Duh, C.Y. Cytotoxic constituents from the formosan soft coral Clavularia inflata var. luzoniana. J. Nat. Prod. 2006, 69, 1411–1416. [Google Scholar] [CrossRef]
- Yan, G.; Du, Q.; Wei, X.; Miozzi, J.; Kang, C.; Wang, J.; Han, X.; Pan, J.; Xie, H.; Chen, J. Application of Real-Time Cell Electronic Analysis System in Modern Pharmaceutical Evaluation and Analysis. Molecules 2018, 23, 3280. [Google Scholar] [CrossRef]
- Hacker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Kim, D.H.; Yoo, J.S.; Jo, E.S.; Rugamba, A.; Jang, K.J.; Yang, Y.M. Tannic Acid Inhibits Non-small Cell Lung Cancer (NSCLC) Stemness by Inducing G0/G1 Cell Cycle Arrest and Intrinsic Apoptosis. Anticancer Res. 2020, 40, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Zampieri, L.X.; Capelôa, T.; Van de Velde, J.A.; Sonveaux, P. Mitochondria in cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Pandey, S. Exploiting Mitochondrial Vulnerabilities to Trigger Apoptosis Selectively in Cancer Cells. Cancers 2019, 11, 916. [Google Scholar] [CrossRef]
- Sehamuddin, G.; Anees, R.; Siraj, P.; Faisal, T. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radical Bio. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Granville, D.J.; Cassidy, B.A.; Ruehlmann, D.O.; Choy, J.C.; Brenner, C.; Kroemer, G.; van Breemen, C.; Margaron, P.; Hunt, D.W.; McManus, B.M. Mitochondrial release of apoptosis-inducing factor and cytochrome c during smooth muscle cell apoptosis. Am. J. Pathol. 2001, 159, 305–311. [Google Scholar] [CrossRef]
- Sawada, M.; Nakashima, S.; Banno, Y.; Yamakawa, H.; Hayashi, K.; Takenaka, K.; Nishimura, Y.; Sakai, N.; Nozawa, Y. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000, 7, 761–772. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego, P.T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, B.; Usluer, S. p21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Hall, S.J.; Sehgal, I.; Wang, J.; Timme, T.L.; Yang, G.; Connell-Crowley, L.; Elledge, S.J.; Zhang, W.W.; Harper, J.W. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995, 55, 5151–5155. [Google Scholar] [PubMed]
- Tsao, Y.P.; Huang, S.J.; Chang, J.L.; Hsieh, J.T.; Pong, R.C.; Chen, S.L. Adenovirus-mediated p21((WAF1/SDII/CIP1)) gene transfer induces apoptosis of human cervical cancer cell lines. J. Virol. 1999, 73, 4983–4990. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Wang, W.Z.; Li, K.Z.; Guan, W.X.; Yan, W. Effect of p27(KIP1) on cell cycle and apoptosis in gastric cancer cells. World J. Gastroenterol. 2005, 11, 7072–7077. [Google Scholar] [CrossRef]
- Naruse, I.; Hoshino, H.; Dobashi, K.; Minato, K.; Saito, R.; Mori, M. Over-expression of p27kip1 induces growth arrest and apoptosis mediated by changes of pRb expression in lung cancer cell lines. Int. J. Cancer 2000, 88, 377–383. [Google Scholar] [CrossRef]
- Gogada, R.; Amadori, M.; Zhang, H.; Jones, A.; Verone, A.; Pitarresi, J.; Jandhyam, S.; Prabhu, V.; Black, J.D.; Chandra, D. Curcumin induces Apaf-1-dependent, p21-mediated caspase activation and apoptosis. Cell Cycle 2011, 10, 4128–4137. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.R.; Wang, Y.M.; Zhang, J.; Wang, Q.; Cheng, A.W.; Guo, X.; Wang, X.K.; Sun, J.Y. Supercritical CO2 fluid extraction of croton crassifolius Geisel root: Chemical composition and anti-proliferative, autophagic, apoptosis-inducing, and related molecular effects on A549 tumour cells. Phytomedicine 2019, 61, 152846. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lee, W.S. P27/Kip1 is responsible for magnolol-induced U373 apoptosis in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.K.; Mitrea, D.M.; Ou, L.; Kriwacki, R.W. Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem. Soc. Trans. 2012, 40, 981–988. [Google Scholar] [CrossRef]
- Jiang, F.; Li, M.; Wang, H.; Ding, B.; Zhang, C.; Ding, Z.; Yu, X.; Lv, G. Coelonin, an Anti-Inflammation Active Component of Bletilla striata and Its Potential Mechanism. Int. J. Mol. Sci. 2019, 20, 4422. [Google Scholar] [CrossRef]
- Mao, J.; Tian, Y.; Wang, C.; Jiang, K.; Li, R.; Yao, Y.; Zhang, R.; Sun, D.; Liang, R.; Gao, Z. CBX2 Regulates Proliferation and Apoptosis via the Phosphorylation of YAP in Hepatocellular Carcinoma. J. Cancer 2019, 10, 2706–2719. [Google Scholar] [CrossRef]
- Shang, H.; Li, L.; Ma, L.; Tian, Y.; Jia, H.; Zhang, T.; Yu, M.; Zou, Z. Design and Synthesis of Molecular Hybrids of Sophora Alkaloids and Cinnamic Acids as Potential Antitumor Agents. Molecules 2020, 25, 1168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Huang, R.Z.; Chen, H.F.; Liao, Z.X.; Sun, J.Y.; Xia, X.K.; Wang, F.X. Three new C-27-carboxylated-lupane-triterpenoid derivatives from Potentilla discolor Bunge and their in vitro antitumor activities. PLoS ONE 2017, 12, e175502. [Google Scholar] [CrossRef]
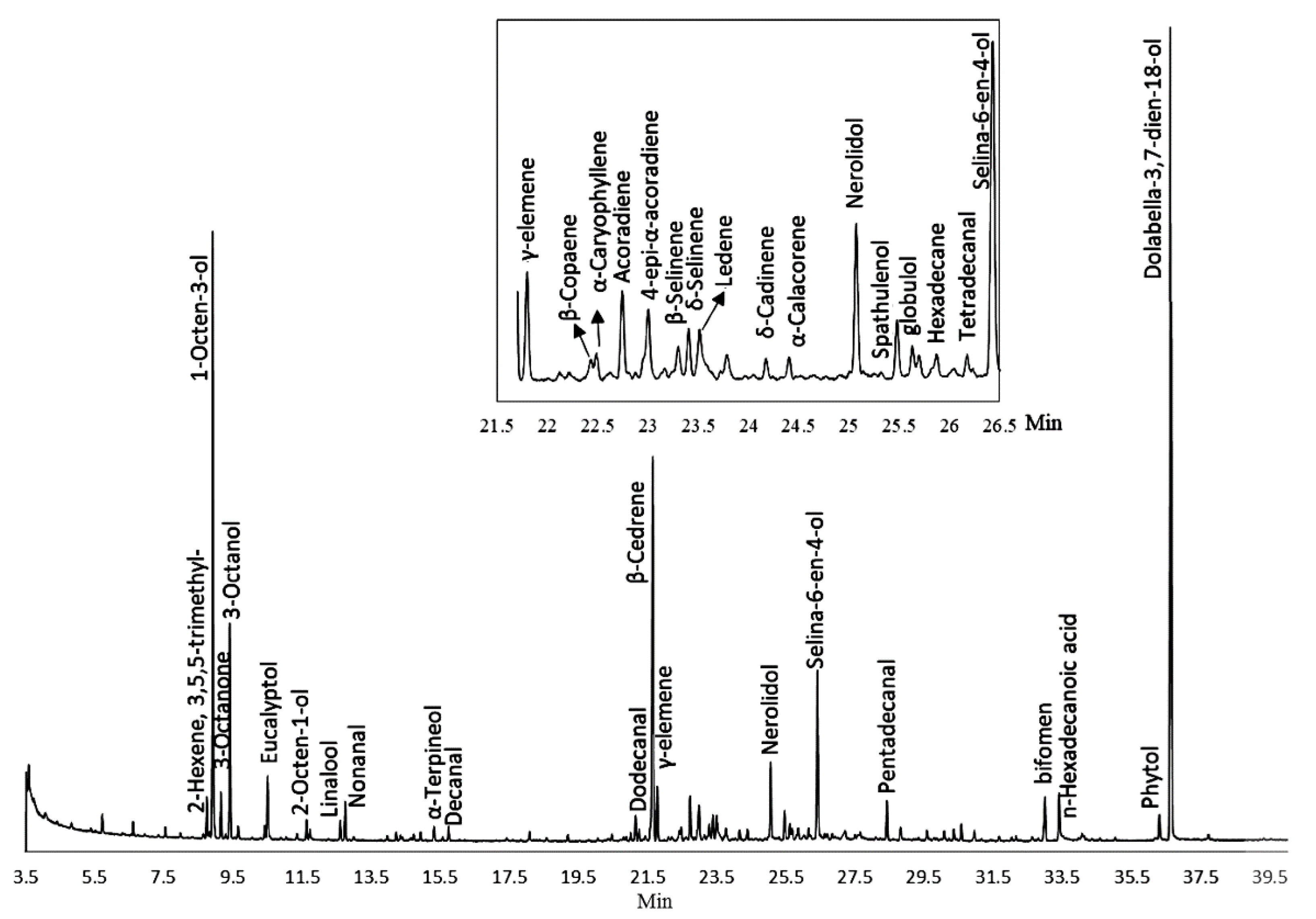












| Class | Component | Molecular Formula | RI 1 | RI (NIST) 2 | RI Range 3 | Area % |
|---|---|---|---|---|---|---|
| β-Myrcene | C10H16 | 991 | 991 ± 2 | 980–995 | 0.06 | |
| β-Ocimene, (E)- | C10H16 | 1049 | 1049 ± 2 | 1038–1056 | 0.05 | |
| Linalool | C10H18O | 1099 | 1099 ± 2 | 1088–1109 | 0.37 | |
| Limonene | C10H16 | 1028 | 1027–1032 | 0.25 | ||
| γ-Terpinene | C10H16 | 1059 | 1060 ± 3 | 1049–1069 | 0.11 | |
| Terpinen-4-ol | C10H18O | 1178 | 1182 ± 0 | 1165–1189 | 0.18 | |
| α-Terpineol | C10H18O | 1191 | 1189 ± 2 | 1178–1203 | 0.28 | |
| α-Pinene | C10H16 | 933 | 921–944 | 0.16 | ||
| Camphene | C10H16 | 948 | 952 ± 2 | 936–959 | 0.09 | |
| β-Pinene | C10H16 | 976 | 979 ± 2 | 964–988 | 0.11 | |
| δ-4-Carene | C10H16 | 1017 | 1009 ± 7 | 0.04 | ||
| Eucalyptol | C10H18O | 1031 | 1032 ± 2 | 1.38 | ||
| 2-Bornanone | C10H16O | 1146 | 1143 ± 9 | 0.11 | ||
| β-Nerolidol | C15H26O | 1565 | 1564 ± 2 | 1560–1565 | 1.46 | |
| β-Elemene | C15H24 | 1395 | 1391 ± 2 | 1374–1402 | 0.1 | |
| γ-Elemene | C15H24 | 1430 | 1433 ± 3 | 1410–1486 | 0.96 | |
| α-Caryophyllene | C15H24 | 1458 | 1454 ± 3 | 0.24 | ||
| Germacrene D | C15H24 | 1486 | 1481 ± 3 | 1464–1493 | 0.38 | |
| Acoradiene | C15H24 | 1469 | 1471 ± 8 | 1447–1478 | 0.88 | |
| 4-epi-α-acoradiene | C15H24 | 1479 | 1475 | 0.94 | ||
| β-Selinene | C15H24 | 1491 | 1486 ± 3 | 1473–1496 | 0.31 | |
| δ-Selinene | C15H24 | 1495 | 1493 ± 4 | 0.46 | ||
| δ-Cadinene | C15H24 | 1527 | 1524 ± 2 | 1508–1539 | 0.2 | |
| α-Calacorene | C15H20 | 1548 | 1542 ± 3 | 1522–1549 | 0.01 | |
| Selina-6-en-4-ol | C15H26O | 1624 | 3.82 | |||
| α-Cadinol | C15H26O | 1660 | 1653 ± 2 | 1635–1664 | 0.34 | |
| Drimenol | C15H26O | 1769 | 1761 ± 11 | 0.23 | ||
| α-Copaene | C15H24 | 1379 | 1376 ± 2 | 1363–1391 | 0.14 | |
| α-Cedrene | C15H24 | 1417 | 1411 ± 3 | 1397–1435 | 0.1 | |
| β-Cedrene | C15H24 | 1426 | 1421 ± 3 | 1415–1434 | 11.39 | |
| Aromandendrene | C15H24 | 1444 | 1440 ± 1 | 0.1 | ||
| β-Copaene | C15H24 | 1456 | 1426–1449 | 0.27 | ||
| Ledene | C15H24 | 1500 | 1493 ± 4 | 0.64 | ||
| Spathulenol | C15H24O | 1583 | 1577 ± 5 | 1562–1590 | 0.57 | |
| Globulol | C15H26O | 1589 | 1583 ± 3 | 1568–1592 | 0.35 | |
| Dolabella-3,7-dien-18-ol | C20H34O | 2133 | 2162 | 25.5 | ||
| bifomen | C20H32 | 1938 | 1931 | 1 | ||
| Hexanal | C6H12O | 802 | 800 ± 2 | 782–810 | 0.34 | |
| Heptanal | C7H14O | 903 | 901 ± 2 | 894–913 | 0.23 | |
| 3-Octanone | C8H16O | 987 | 986 ± 3 | 971–994 | 1.07 | |
| Octanal | C8H16O | 1004 | 1003 ± 2 | 993–1012 | 0.22 | |
| Benzeneacetaldehyde | C8H8O | 1044 | 1045 ± 4 | 1032–1063 | 0.04 | |
| Nonanal | C9H18O | 1104 | 1104 ± 2 | 1093–1118 | 0.64 | |
| 2-Nonenal, (E)- | C9H16O | 1160 | 1162 ± 3 | 1142–1151 | 0.09 | |
| Decanal | C10H20O | 1205 | 1206 ± 2 | 1195–1217 | 0.25 | |
| Undecanal | C11H22O | 1308 | 1307 ± 2 | 1295–1319 | 0.09 | |
| Dodecanal | C12H24O | 1409 | 1409 ± 4 | 1397–1420 | 0.22 | |
| Tetradecanal | C14H28O | 1613 | 1613 ± 2 | 1609–1615 | 0.21 | |
| Pentadecanal | C15H30O | 1715 | 1715 ± 3 | 0.69 | ||
| Hexadecanal | C16H32O | 1817 | 1817 ± 6 | 0.34 | ||
| Nonanoic acid | C9H18O2 | 1266 | 1273 ± 7 | 1260–1293 | 0.09 | |
| n-Hexadecanoic acid | C16H32O2 | 1959 | 1968 ± 7 | 1.05 | ||
| 3-Hexen-1-ol, (E)- | C6H12O | 855 | 852 ± 3 | 837–863 | 0.07 | |
| 1-Hexanol | C6H14O | 868 | 868 ± 4 | 859–885 | 0.22 | |
| 1-Heptanol | C7H16O | 970 | 970 ± 2 | 945–980 | 0.09 | |
| 1-Octen-3-ol | C8H16O | 979 | 980 ± 2 | 967–991 | 14.17 | |
| 3-Octanol | C8H18O | 996 | 994 ± 3 | 981–1005 | 5.68 | |
| 2-Octen-1-ol, (Z)- | C8H16O | 1068 | 1067 ± 4 | 1059–1071 | 0.38 | |
| 1-Octyn-3-ol | C8H14O | 1071 | 0.15 | |||
| Phenylethyl Alcohol | C8H10O | 1112 | 1116 ± 5 | 1100–1129 | 0.07 | |
| 3-Nonen-1-ol, (Z)- | C9H18O | 1154 | 1156 ± 3 | 0.17 | ||
| 1-Nonanol | C9H20O | 1171 | 1173 ± 2 | 1167–1184 | 0.13 | |
| 10-Undecen-1-ol | C11H22O | 1678 | 1664 | 0.15 | ||
| Phytol | C20H40O | 2114 | 2114 ± 5 | 2110–2122 | 0.53 | |
| Ethylbenzene | C8H10 | 860 | 855 ± 10 | 0.03 | ||
| p-Xylene | C8H10 | 868 | 865 ± 7 | 0.11 | ||
| 2-Hexene, 3,5,5-trimethyl- | C9H18 | 973 | 985 ± 4 | 0.65 | ||
| Dodecane | C12H26 | 1200 | 1200 | 0.07 | ||
| Tetradecane | C14H30 | 1400 | 1400 | 1400 | 0.14 | |
| n-Hexadecane | C16H34 | 1600 | 1600 | 0.26 | ||
| 1-Docosene | C22H44 | 2194 | 2193 ± 2 | 0.36 | ||
| Safrole | C10H10O2 | 1290 | 1287 ± 2 | 1280–1294 | 0.16 | |
| Methyleugenol | C11H14O2 | 1406 | 1402 ± 3 | 0.41 | ||
| Butanoic acid, ethyl ester | C6H12O2 | 804 | 802 ± 2 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, L.; Li, S.; Hua, C.; Gao, H.; Ning, D.; Li, C.; Zhang, C.; Jiang, F. Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop. Int. J. Mol. Sci. 2022, 23, 14790. https://doi.org/10.3390/ijms232314790
Li M, Wang L, Li S, Hua C, Gao H, Ning D, Li C, Zhang C, Jiang F. Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop. International Journal of Molecular Sciences. 2022; 23(23):14790. https://doi.org/10.3390/ijms232314790
Chicago/Turabian StyleLi, Meiya, Linyan Wang, Shiqing Li, Chenglong Hua, Hang Gao, Dandan Ning, Changyu Li, Chunchun Zhang, and Fusheng Jiang. 2022. "Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop." International Journal of Molecular Sciences 23, no. 23: 14790. https://doi.org/10.3390/ijms232314790
APA StyleLi, M., Wang, L., Li, S., Hua, C., Gao, H., Ning, D., Li, C., Zhang, C., & Jiang, F. (2022). Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop. International Journal of Molecular Sciences, 23(23), 14790. https://doi.org/10.3390/ijms232314790






