Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of CHT–AH Scaffolds
2.2. Physicochemical and Structural Characterization of CHT-AH Scaffolds
2.2.1. Surface Morphology by SEM
2.2.2. Elemental Composition by EDX
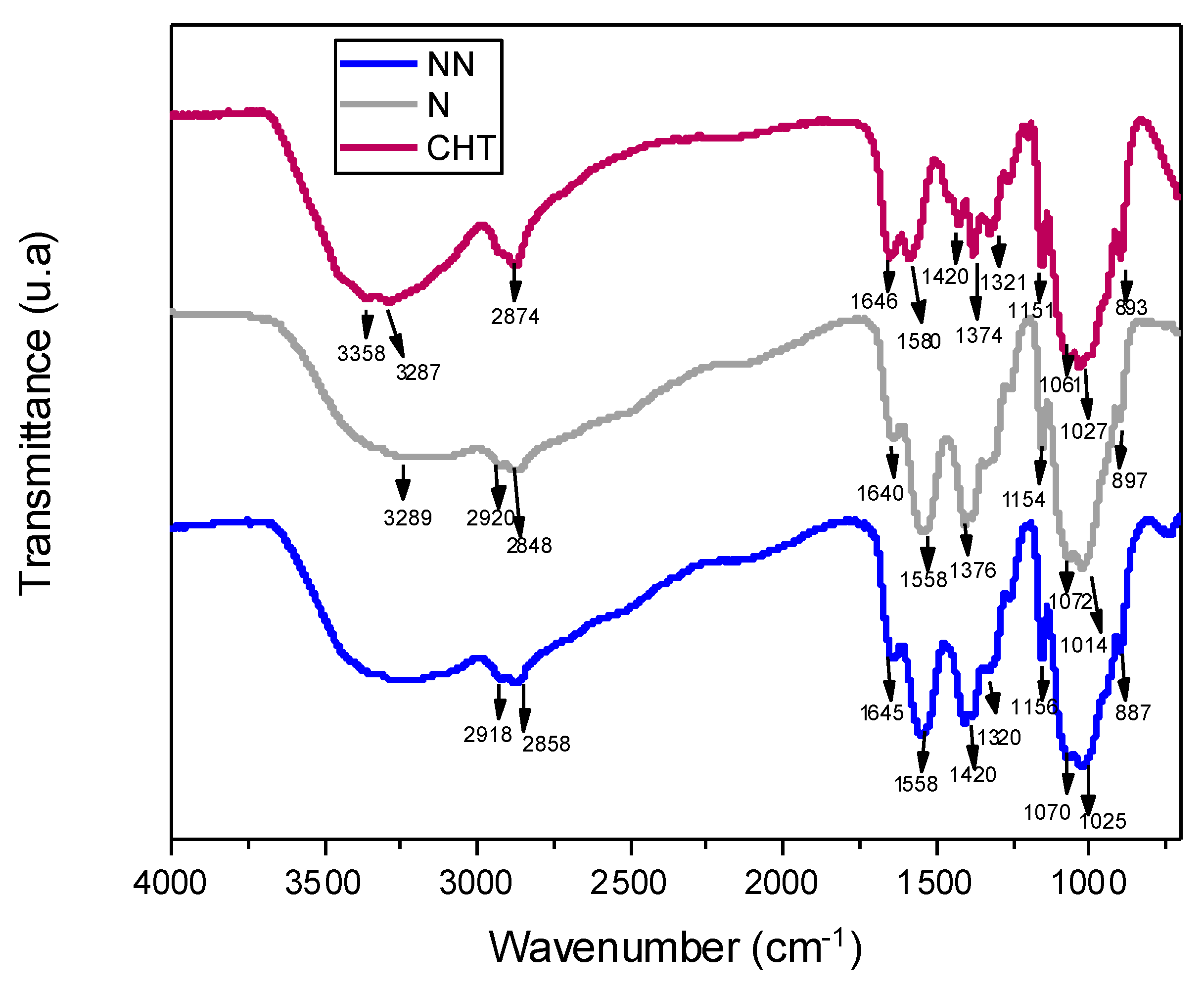
2.2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.4. Raman Spectroscopy
2.2.5. Thermogravimetric Analysis (TGA)
2.3. Compression Mechanical Test
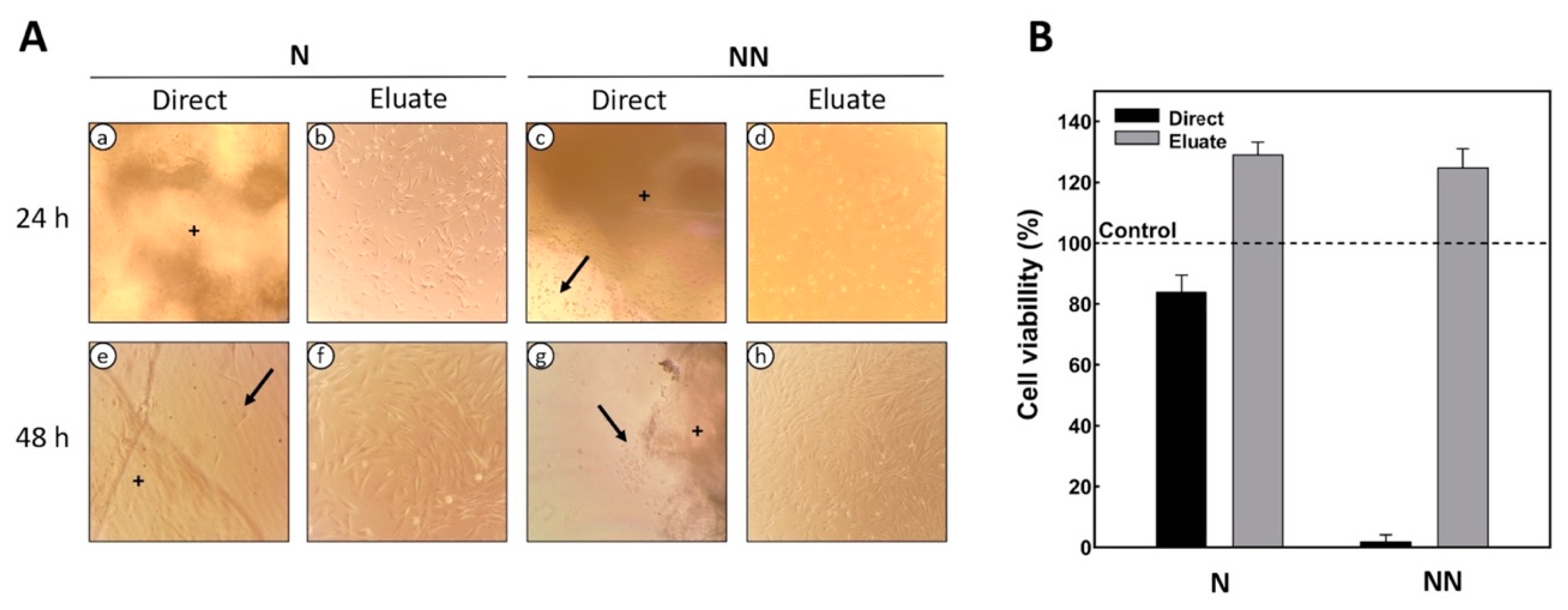
2.4. Evaluation of Cell Viability and Proliferation on the Scaffolds
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Scaffold Preparation
3.2.2. Neutralization Procedure of Chitosan–AH Scaffolds
3.2.3. Characterization of Chitosan–AH Scaffolds
3.2.4. Mechanical Analyses
3.2.5. Cell Viability and Proliferation Studies
Direct Contact
Indirect Contact
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Kheirjou, R.; Rad, J.S.; Khosroshahi, A.F.; Roshangar, L. The useful agent to have an ideal biological scaffold. Cell Tissue Bank. 2021, 22, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent Sericin-Chitosan Scaffold: Physical Properties and Low Cytotoxicity Effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Comparative study on the role of gelatin, chitosan and their combination as tissue engineered scaffolds on healing and regeneration of critical sized bone defects: An in vivo study. J. Mater. Sci. Mater. Med. 2016, 27, 155. [Google Scholar] [CrossRef]
- Chen, F.M. Periodontal tissue engineering and regeneration. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chin. J. Stomatol. 2017, 52, 610–614. [Google Scholar] [CrossRef]
- Berbéri, A.; Fayyad-Kazan, M.; Ayoub, S.; Bou Assaf, R.; Sabbagh, J.; Ghassibe-Sabbagh, M.; Badran, B. Osteogenic potential of dental and oral derived stem cells in bone tissue engineering among animal models: An update. Tissue Cell 2021, 71, 101515. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 169, ISBN 0000000229340. [Google Scholar]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Hirt-Burri, N.; Jeannerat, A.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Peptide-decorated chitosan derivatives enhance fibroblast adhesion and proliferation in wound healing. Carbohydr. Polym. 2016, 142, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dai, F.; Li, H.; Wang, C.; Shi, X.; Cheng, Y.; Deng, H. Chitosan and collagen layer-by-layer assembly modified oriented nanofibers and their biological properties. Carbohydr. Polym. 2021, 254, 117438. [Google Scholar] [CrossRef] [PubMed]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a bone scaffold biomaterial. J. Craniofac. Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef]
- Turci, F.; Giulia, M.; Corazzari, I.; Nistic, R.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for periodontal tissue engineering: Chitosan-based scaffolds. A systematic review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef]
- Reyna-Urrutia, V.A.; Mata-Haro, V.; Cauich-Rodriguez, J.V.; Herrera-Kao, W.A.; Cervantes-Uc, J.M. Effect of two crosslinking methods on the physicochemical and biological properties of the collagen-chitosan scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011, 809743. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, L.; Chen, C.; Liu, Y.; Wu, C.; Dai, C. A construction of novel iron-foam-based calcium phosphate/chitosan coating biodegradable scaffold material. Mater. Sci. Eng. C 2013, 33, 1022–1031. [Google Scholar] [CrossRef]
- Mania, S.; Partyka, K.; Pilch, J.; Augustin, E.; Cieślik, M.; Ryl, J.; Jinn, J.R.; Wang, Y.J.; Michałowska, A.; Tylingo, R. Obtaining and characterization of the PLA/chitosan foams with antimicrobial properties achieved by the emulsification combined with the dissolution of chitosan by CO2 saturation. Molecules 2019, 24, 4532. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Silvestro, I.; Sergi, R.; Scotto d’Abusco, A.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan scaffolds with enhanced mechanical strength and elastic response by combination of freeze gelation, photo-crosslinking and freeze-drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef] [PubMed]
- Arasukumar, B.; Prabakaran, G.; Gunalan, B.; Moovendhan, M. Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol. 2019, 135, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.D.A.; Sun, Z.; Ali, M.A. Bio-scaffolds produced from irradiated squid pen and crab chitosan with hydroxyapatite/β-tricalcium phosphate for bone-tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1446–1456. [Google Scholar] [CrossRef]
- Yan, J.; Wu, T.; Ding, Z.; Li, X. Preparation and characterization of carbon nanotubes/chitosan composite foam with enhanced elastic property. Carbohydr. Polym. 2016, 136, 1288–1296. [Google Scholar] [CrossRef]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N. Journal of Science: Advanced Materials and Devices Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Tamburaci, S.; Kimna, C.; Tihminlioglu, F. Bioactive diatomite and POSS silica cage reinforced chitosan/Na-carboxymethyl cellulose polyelectrolyte scaffolds for hard tissue regeneration. Mater. Sci. Eng. C 2019, 100, 196–208. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Karimi, Z.; Ghorbani, M.; Hashemibeni, B.; Bahramian, H. Evaluation of the proliferation and viability rates of nucleus pulposus cells of human intervertebral disk in fabricated chitosan-gelatin scaffolds by freeze drying and freeze gelation methods. Adv. Biomed. Res. 2015, 4, 251. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Ahmad, M.; Huma, T.; Khalid, I.; Ahmad, I. Evaluation of Low Molecular Weight Cross Linked Chitosan Nanoparticles, to Enhance the Bioavailability of 5-Flourouracil. Dose Response 2021, 19, 15593258211025353. [Google Scholar] [CrossRef] [PubMed]
- Chuc-Gamboa, M.G.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Escobar-García, D.M.; Pozos-Guillén, A.; San Román del Barrio, J. The Effect of PEGDE Concentration and Temperature on Physicochemical and Biological Properties of Chitosan. Polymers 2019, 11, 1830. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Eid, M.M.; Ahmed, M.K. Synthesis, characterization, and evaluation of antimicrobial activity of novel Chitosan/Tigecycline composite. Int. J. Biol. Macromol. 2020, 147, 194–199. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Przekora, A.; Benko, A.; Blazewicz, M.; Ginalska, G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomed. Mater. 2016, 11, 45001. [Google Scholar] [CrossRef]
- Galli, C.; Parisi, L.; Elviri, L.; Bianchera, A.; Smerieri, A.; Lagonegro, P.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Chitosan scaffold modified with D-(+) raffinose and enriched with thiol-modified gelatin for improved osteoblast adhesion. Biomed. Mater. 2016, 11, 15004. [Google Scholar] [CrossRef]
- Desbrie, J.; Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argu, W. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar]
- Zhang, K.; Peschel, D.; Helm, J.; Groth, T.; Fischer, S. FT Raman investigation of novel chitosan sulfates exhibiting osteogenic capacity. Carbohydr. Polym. 2011, 83, 60–65. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. A 2014, 102, 2704–2712. [Google Scholar] [CrossRef]
- Zaja̧c, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.H.S.; Garcia, I.M.; de Souza da Motta, A.; Leitune, V.C.B.; Collares, F.M. Triclosan-loaded chitosan as antibacterial agent for adhesive resin. J. Dent. 2019, 83, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, K. Study on preparation and performance of PEG-based polyurethane foams modified by the chitosan with different molecular weight. Int. J. Biol. Macromol. 2019, 140, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Robau-Porrua, A.; Pérez-Rodríguez, Y.; Soris-Rodríguez, L.M.; Pérez-Acosta, O.; González, J.E. The effect of diameter, length and elastic modulus of a dental implant on stress and strain levels in peri-implant bone: A 3D finite element analysis. Biomed. Mater. Eng. 2020, 30, 541–558. [Google Scholar] [CrossRef]
- D1621–1673; Astm Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM Standard: West Conshohocken, PA, USA, 1991.
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Pérez, R.A.; Manero, J.M.; Mur, J.G. Influence of the elastic modulus on the osseointegration of dental implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef]
- Changotade, S.; Radu Bostan, G.; Consalus, A.; Poirier, F.; Peltzer, J.; Lataillade, J.-J.; Lutomski, D.; Rohman, G. Preliminary In Vitro Assessment of Stem Cell Compatibility with Cross-Linked Poly(ε-caprolactone urethane) Scaffolds Designed through High Internal Phase Emulsions. Stem Cells Int. 2015, 2015, 283796. [Google Scholar] [CrossRef]
- Flinck, M.; Kramer, S.H.; Pedersen, S.F. Roles of pH in control of cell proliferation. Acta Physiol. 2018, 223, e13068. [Google Scholar] [CrossRef]
- Sweidan, K.; Jaber, A.M.; Al-Jbour, N.; Obaidat, R.; Al-Remawi, M.; Badwan, A. Further investigation on the degree of deacetylation of chitosan determined by Potentiometric titration. J. Excip. Food Chem. 2011, 2, 16–25. [Google Scholar]



| Scaffolds | %C | %O | %N | %Na |
|---|---|---|---|---|
| Neutralized | 48 ± 5 | 38 ± 2 | 11 ± 4 | 3 ± 1 |
| Non-neutralized | 51 ± 5 | 41 ± 1 | 7 ± 4 | 1 ± 1 |
| Scaffolds | Td (°C) | T (°C) at 50% Weight Loss |
|---|---|---|
| Neutralized | 285 | 278 |
| Non-neutralized | 316 | 263 |
| Scaffolds | Elastic Modulus (Ec) kPa | Compressive Strength (σ 10) kPa |
|---|---|---|
| Neutralized | 8.8 ± 1.2 | 99.2 ± 15.7 |
| Non-neutralized | 19.1 ± 0.9 CV 4.7% | 127.0 ± 10.9 CV 8.6 |
| p-Values of t-test | 0.011 * | 0.176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azueta-Aguayo, P.H.; Chuc-Gamboa, M.G.; Aguilar-Pérez, F.J.; Aguilar-Ayala, F.J.; Rodas-Junco, B.A.; Vargas-Coronado, R.F.; Cauich-Rodríguez, J.V. Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds. Int. J. Mol. Sci. 2022, 23, 14822. https://doi.org/10.3390/ijms232314822
Azueta-Aguayo PH, Chuc-Gamboa MG, Aguilar-Pérez FJ, Aguilar-Ayala FJ, Rodas-Junco BA, Vargas-Coronado RF, Cauich-Rodríguez JV. Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds. International Journal of Molecular Sciences. 2022; 23(23):14822. https://doi.org/10.3390/ijms232314822
Chicago/Turabian StyleAzueta-Aguayo, Paola Hassibe, Martha Gabriela Chuc-Gamboa, Fernando Javier Aguilar-Pérez, Fernando Javier Aguilar-Ayala, Beatriz A. Rodas-Junco, Rossana Faride Vargas-Coronado, and Juan Valerio Cauich-Rodríguez. 2022. "Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds" International Journal of Molecular Sciences 23, no. 23: 14822. https://doi.org/10.3390/ijms232314822
APA StyleAzueta-Aguayo, P. H., Chuc-Gamboa, M. G., Aguilar-Pérez, F. J., Aguilar-Ayala, F. J., Rodas-Junco, B. A., Vargas-Coronado, R. F., & Cauich-Rodríguez, J. V. (2022). Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds. International Journal of Molecular Sciences, 23(23), 14822. https://doi.org/10.3390/ijms232314822









