The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans
Abstract
:1. Introduction
2. The Thermosensory Circuit
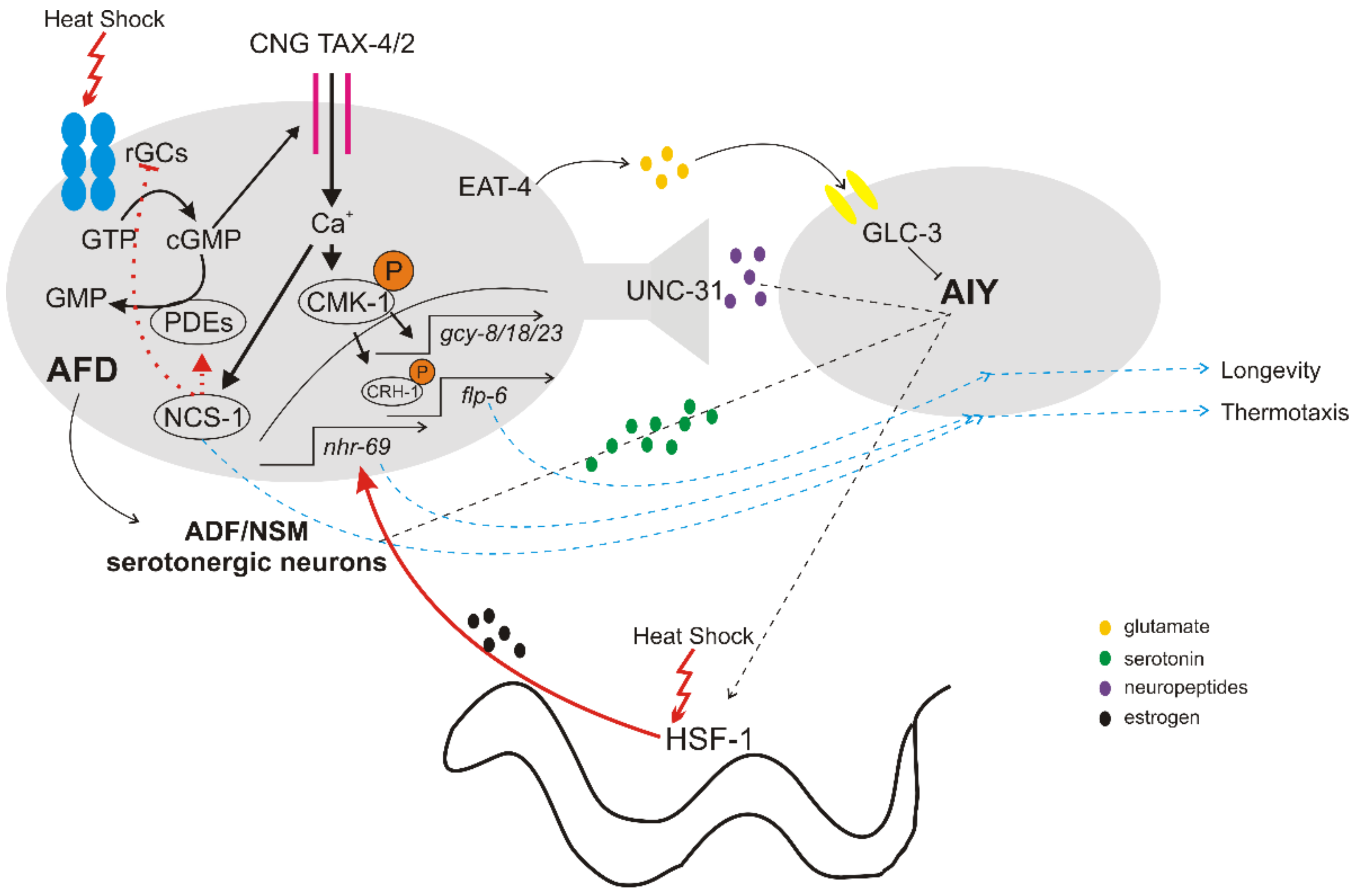
2.1. Detection of Temperature Changes
2.2. Downstream Signaling of Thermosensory Neurons
2.3. Cell Non-Autonomous Regulation of HSR
3. Functional Domains of HSF-1
4. HSF-1 Targets upon Heat Shock
4.1. Heat Shock Proteins, the Main Executive Body of HSR
4.2. Exoskeleton and Cytoskeleton Integrity Genes
5. The Post-Translational Fate of HSF-1
5.1. Phosphorylation
5.2. Sumoylation
5.3. Acetylation
6. Other Factors and Pathways That Aid Coping with Thermal Stress
6.1. The Insulin/IGF-1–Like Signaling (IIS)
6.2. HIF-1
6.3. Autophagy
6.4. Non-Coding RNAs
6.5. m5C Methylation
6.6. Mitochondrial Perturbation
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.E.; Artan, M.; Seo, K.; Lee, S.J. Regulation of lifespan by chemosensory and thermosensory systems: Findings in invertebrates and their implications in mammalian aging. Front. Genet. 2012, 3, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Lee, J.; Kim, Y.; Lee, S.V. Regulatory systems that mediate the effects of temperature on the lifespan of Caenorhabditis elegans. J. Neurogenet. 2020, 34, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008, 320, 811–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakkayil, K.L.; Hoppe, T. Temperature-Dependent Regulation of Proteostasis and Longevity. Front. Aging 2022, 3, 853588. [Google Scholar] [CrossRef]
- Ramot, D.; MacInnis, B.L.; Goodman, M.B. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 2008, 11, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Brunquell, J.; Morris, S.; Lu, Y.; Cheng, F.; Westerheide, S.D. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genom. 2016, 17, 559. [Google Scholar] [CrossRef] [Green Version]
- Higuchi-Sanabria, R.; Frankino, P.A.; Paul, J.W., 3rd; Tronnes, S.U.; Dillin, A. A Futile Battle? Protein Quality Control and the Stress of Aging. Dev. Cell 2018, 44, 139–163. [Google Scholar]
- Occhigrossi, L.; D’Eletto, M.; Barlev, N.; Rossin, F. The Multifaceted Role of HSF1 in Pathophysiology: Focus on Its Interplay with TG2. Int. J. Mol. Sci. 2021, 22, 6366. [Google Scholar] [CrossRef]
- Félix, M.A.; Duveau, F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Harvey, S.C.; Viney, M.E. Thermal variation reveals natural variation between isolates of Caenorhabditis elegans. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Clark, D.A.; Biron, D.; Mahadevan, L.; Samuel, A.D. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J. Exp. Biol. 2006, 209 Pt 23, 4652–4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, I.; Mori, I. Molecular biology of thermosensory transduction in C. elegans. Curr. Opin. Neurobiol. 2015, 34, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glauser, D.A. Temperature sensing and context-dependent thermal behavior in nematodes. Curr. Opin. Neurobiol. 2022, 73, 102525. [Google Scholar] [CrossRef]
- Hedgecock, E.M.; Russell, R.L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1975, 72, 4061–4065. [Google Scholar] [CrossRef] [Green Version]
- Mori, I.; Ohshima, Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 1995, 376, 344–348. [Google Scholar] [CrossRef]
- Goodman, M.B. Thermotaxis navigation behavior. In WormBook: The Online Review of C. elegans Biology; Wormbook: Pasadena, CA, USA, 2014; pp. 1–10. [Google Scholar]
- Ghosh, R.; Mohammadi, A.; Kruglyak, L.; Ryu, W.S. Multiparameter behavioral profiling reveals distinct thermal response regimes in Caenorhabditis elegans. BMC Biol. 2012, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Schulze, E.; Baumeister, R. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS ONE 2012, 7, e32360. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.B.; Sengupta, P. The extraordinary AFD thermosensor of C. elegans. Pflug. Arch. 2018, 470, 839–849. [Google Scholar] [CrossRef]
- Goodman, M.B.; Sengupta, P. How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli. Genetics 2019, 212, 25–51. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.; Thomson, N.; White, J.G.; Brenner, S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975, 160, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Beverly, M.; Anbil, S.; Sengupta, P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. 2011, 31, 11718–11727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biron, D.; Wasserman, S.; Thomas, J.H.; Samuel, A.D.; Sengupta, P. An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 11002–11007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhara, A.; Okumura, M.; Kimata, T.; Tanizawa, Y.; Takano, R.; Kimura, K.D.; Inada, H.; Matsumoto, K.; Mori, I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 2008, 320, 803–807. [Google Scholar] [CrossRef]
- Yu, S.; Avery, L.; Baude, E.; Garbers, D.L. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 3384–3387. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, C.O.; Etchberger, J.F.; Posy, S.L.; Frøkjaer-Jensen, C.; Lockery, S.; Honig, B.; Hobert, O. Searching for neuronal left/right asymmetry: Genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 2006, 173, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Inada, H.; Ito, H.; Satterlee, J.; Sengupta, P.; Matsumoto, K.; Mori, I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 2006, 172, 2239–2252. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, S.M.; Beverly, M.; Bell, H.W.; Sengupta, P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 2011, 21, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Mori, I.; Rhee, J.S.; Akaike, N.; Ohshima, Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 1996, 17, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Coburn, C.M.; Bargmann, C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 1996, 17, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A.; Biron, D.; Sengupta, P.; Samuel, A.D. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006, 26, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, A.; Yu, Y.V.; Hapiak, V.M.; Bell, H.W.; O’Leary, T.; Sengupta, P. Receptor-type Guanylyl Cyclases Confer Thermosensory Responses in C. elegans. Neuron 2016, 90, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; O’Halloran, D.; Goodman, M.B. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J. Gen. Physiol. 2013, 142, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.V.; Bell, H.W.; Glauser, D.; Van Hooser, S.D.; Goodman, M.B.; Sengupta, P. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron 2014, 84, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Nakano, S.; Amano, M.; Tsuboi, D.; Nishioka, T.; Ikeda, S.; Yokoyama, G.; Kaibuchi, K.; Mori, I. Single-Cell Memory Regulates a Neural Circuit for Sensory Behavior. Cell Rep. 2016, 14, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Sugi, T.; Nonomura, M.; Mori, I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 2011, 12, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Glauser, D.A.; Chen, W.C.; Agin, R.; Macinnis, B.L.; Hellman, A.B.; Garrity, P.A.; Tan, M.W.; Goodman, M.B. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics 2011, 188, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saro, G.; Lia, A.S.; Thapliyal, S.; Marques, F.; Busch, K.E.; Glauser, D.A. Specific Ion Channels Control Sensory Gain, Sensitivity, and Kinetics in a Tonic Thermonociceptor. Cell Rep. 2020, 30, 397–408.e4. [Google Scholar] [CrossRef] [Green Version]
- Schild, L.C.; Glauser, D.A. Dual Color Neural Activation and Behavior Control with Chrimson and CoChR in Caenorhabditis elegans. Genetics 2015, 200, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, D.; Thapliyal, S.; Glauser, D.A. Ca(2+)/CaM binding to CaMKI promotes IMA-3 importin binding and nuclear translocation in sensory neurons to control behavioral adaptation. Elife 2021, 10, e71443. [Google Scholar] [CrossRef]
- Narayan, A.; Laurent, G.; Sternberg, P.W. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Kuhara, A.; Nakamura, F.; Okochi, Y.; Mori, I. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011, 30, 1376–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horoszok, L.; Raymond, V.; Sattelle, D.B.; Wolstenholme, A.J. GLC-3: A novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br. J. Pharmacol. 2001, 132, 1247–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, F.; Saro, G.; Lia, A.S.; Poole, R.J.; Falquet, L.; Glauser, D.A. Identification of avoidance genes through neural pathway-specific forward optogenetics. PLoS Genet. 2019, 15, e1008509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne Rodgers, J.; Ryu, W.S. Targeted thermal stimulation and high-content phenotyping reveal that the C. elegans escape response integrates current behavioral state and past experience. PLoS ONE 2020, 15, e0229399. [Google Scholar] [CrossRef]
- Marques, F.; Falquet, L.; Vandewyer, E.; Beets, I.; Glauser, D.A. Signaling via the FLP-14/FRPR-19 neuropeptide pathway sustains nociceptive response to repeated noxious stimuli in C. elegans. PLoS Genet. 2021, 17, e1009880. [Google Scholar] [CrossRef]
- Lee, S.J.; Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 2009, 19, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Chen, H.J.; Tseng, W.C.; Hsu, J.M.; Huang, T.T.; Chen, C.H.; Pan, C.L. A C. elegans Thermosensory Circuit Regulates Longevity through crh-1/CREB-Dependent flp-6 Neuropeptide Signaling. Dev. Cell 2016, 39, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Sugi, T.; Nishida, Y.; Mori, I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat. Neurosci. 2011, 14, 984–992. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Stringham, E.G.; Candido, E.P. Targeted single-cell induction of gene products in Caenorhabditis elegans: A new tool for developmental studies. J. Exp. Zool. 1993, 266, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Plagens, R.N.; Mossiah, I.; Kim Guisbert, K.S.; Guisbert, E. Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans. BMC Biol. 2021, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Morimoto, R.I. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 14204–14209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatum, M.C.; Ooi, F.K.; Chikka, M.R.; Chauve, L.; Martinez-Velazquez, L.A.; Steinbusch, H.W.M.; Morimoto, R.I.; Prahlad, V. Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr. Biol. 2015, 25, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10 (Suppl. S10–17). [Google Scholar] [CrossRef]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Teixeira-Castro, A.; Ailion, M.; Jalles, A.; Brignull, H.R.; Vilaça, J.L.; Dias, N.; Rodrigues, P.; Oliveira, J.F.; Neves-Carvalho, A.; Morimoto, R.I.; et al. Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: Rescue by the DAF-16 and HSF-1 pathways. Hum. Mol. Genet. 2011, 20, 2996–3009. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten-Hawle, P.; Porter, R.S.; Morimoto, R.I. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 2013, 153, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- Douglas, P.M.; Baird, N.A.; Simic, M.S.; Uhlein, S.; McCormick, M.A.; Wolff, S.C.; Kennedy, B.K.; Dillin, A. Heterotypic Signals from Neural HSF-1 Separate Thermotolerance from Longevity. Cell Rep. 2015, 12, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Chauve, L.; Hodge, F.; Murdoch, S.; Masoudzadeh, F.; Mann, H.J.; Lopez-Clavijo, A.F.; Okkenhaug, H.; West, G.; Sousa, B.C.; Segonds-Pichon, A.; et al. Neuronal HSF-1 coordinates the propagation of fat desaturation across tissues to enable adaptation to high temperatures in C. elegans. PLoS Biol. 2021, 19, e3001431. [Google Scholar] [CrossRef] [PubMed]
- Maman, M.; Carvalhal Marques, F.; Volovik, Y.; Dubnikov, T.; Bejerano-Sagie, M.; Cohen, E. A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans. J. Neurosci. 2013, 33, 6102–6111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumsta, C.; Ching, T.T.; Nishimura, M.; Davis, A.E.; Gelino, S.; Catan, H.H.; Yu, X.; Chu, C.C.; Ong, B.; Panowski, S.H.; et al. Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging Cell 2014, 13, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Gracida, X.; Dion, M.F.; Harris, G.; Zhang, Y.; Calarco, J.A. An Elongin-Cullin-SOCS Box Complex Regulates Stress-Induced Serotonergic Neuromodulation. Cell Rep. 2017, 21, 3089–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Min, S.; Prahlad, V. Gene bookmarking by the heat shock transcription factor programs the insulin-like signaling pathway. Mol. Cell 2021, 81, 4843–4860. [Google Scholar] [CrossRef]
- Das, S.; Ooi, F.K.; Cruz Corchado, J.; Fuller, L.C.; Weiner, J.A.; Prahlad, V. Serotonin signaling by maternal neurons upon stress ensures progeny survival. Elife 2020, 9, e55246. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.L.; Erdenebat, P.; Morphis, A.C.; Kumar, L.; Wang, L.; Chamera, T.; Georgescu, C.; Wren, J.D.; Li, J. Insulin/IGF-1 signaling and heat stress differentially regulate HSF1 activities in germline development. Cell Rep 2021, 36, 109623. [Google Scholar] [CrossRef]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127 Pt 2, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Hajdu-Cronin, Y.M.; Chen, W.J.; Sternberg, P.W. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics 2004, 168, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Anckar, J.; Sistonen, L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv. Exp. Med. Biol. 2007, 594, 78–88. [Google Scholar]
- Green, M.; Schuetz, T.J.; Sullivan, E.K.; Kingston, R.E. A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol. Cell Biol. 1995, 15, 3354–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chauve, L.; Phelps, G.; Brielmann, R.M.; Morimoto, R.I. E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev. 2016, 30, 2062–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, N.A.; Douglas, P.M.; Simic, M.S.; Grant, A.R.; Moresco, J.J.; Wolff, S.C.; Yates, J.R., 3rd; Manning, G.; Dillin, A. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 2014, 346, 360–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.F.; Morimoto, R.I. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 2004, 15, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, N.L.; Plagens, R.N.; Kim Guisbert, K.S.; Guisbert, E. Standardized Methods for Measuring Induction of the Heat Shock Response in Caenorhabditis elegans. J. Vis. Exp. 2020, 161, e61030. [Google Scholar] [CrossRef]
- Chiang, W.C.; Ching, T.T.; Lee, H.C.; Mousigian, C.; Hsu, A.L. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 2012, 148, 322–334. [Google Scholar] [CrossRef] [Green Version]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef]
- Trinklein, N.D.; Murray, J.I.; Hartman, S.J.; Botstein, D.; Myers, R.M. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 2004, 15, 1254–1261. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Tsu, B.V.; Daugherty, M.D.; Pasquinelli, A.E. Diversification of the Caenorhabditis heat shock response by Helitron transposable elements. Elife 2019, 8, e51139. [Google Scholar] [CrossRef] [PubMed]
- Guisbert, E.; Czyz, D.M.; Richter, K.; McMullen, P.D.; Morimoto, R.I. Identification of a tissue-selective heat shock response regulatory network. PLoS Genet. 2013, 9, e1003466. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.A.; Lamitina, T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 2013, 12, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, C.; Morimoto, R.I. Stress and the cell nucleus: Dynamics of gene expression and structural reorganization. Gene Expr. 1999, 7, 261–270. [Google Scholar] [PubMed]
- Melnick, M.; Gonzales, P.; Cabral, J.; Allen, M.A.; Dowell, R.D.; Link, C.D. Heat shock in C. elegans induces downstream of gene transcription and accumulation of double-stranded RNA. PLoS ONE 2019, 14, e0206715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [Green Version]
- Shalgi, R.; Hurt, J.A.; Krykbaeva, I.; Taipale, M.; Lindquist, S.; Burge, C.B. Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 2013, 49, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Dutta, N.; Garcia, G.; Higuchi-Sanabria, R. Hijacking Cellular Stress Responses to Promote Lifespan. Front. Aging 2022, 3, 860404. [Google Scholar] [CrossRef]
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Snutch, T.P.; Baillie, D.L. Alterations in the pattern of gene expression following heat shock in the nematode Caenorhabditis elegans. Can. J. Biochem. Cell Biol. 1983, 61, 480–487. [Google Scholar] [CrossRef]
- McColl, G.; Rogers, A.N.; Alavez, S.; Hubbard, A.E.; Melov, S.; Link, C.D.; Bush, A.I.; Kapahi, P.; Lithgow, G.J. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 2010, 12, 260–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi-Sanabria, R.; Paul, J.W., 3rd; Durieux, J.; Benitez, C.; Frankino, P.A.; Tronnes, S.U.; Garcia, G.; Daniele, J.R.; Monshietehadi, S.; Dillin, A. Spatial regulation of the actin cytoskeleton by HSF-1 during aging. Mol. Biol. Cell 2018, 29, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Sóti, C.; Csermely, P. Molecular chaperones and the aging process. Biogerontology 2000, 1, 225–233. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Sun, F.; Zhang, J.; Feng, J.; Liu, H.; Rajendran, K.V.; Sun, L.; Zhang, Y.; Jiang, Y.; et al. RNA-Seq reveals expression signatures of genes involved in oxygen transport, protein synthesis, folding, and degradation in response to heat stress in catfish. Physiol. Genom. 2013, 45, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Dams, S.D.; de Liefde-van Beest, M.; Nuijs, A.M.; Oomens, C.W.; Baaijens, F.P. Heat shocks enhance procollagen type I and III expression in fibroblasts in ex vivo human skin. Skin Res. Technol. 2011, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nagata, K. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 2011, 499, 167–182. [Google Scholar] [PubMed]
- Gomez-Pastor, R.; Burchfiel, E.T.; Neef, D.W.; Jaeger, A.M.; Cabiscol, E.; McKinstry, S.U.; Doss, A.; Aballay, A.; Lo, D.C.; Akimov, S.S.; et al. Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat. Commun. 2017, 8, 14405. [Google Scholar] [CrossRef] [Green Version]
- Batista-Nascimento, L.; Neef, D.W.; Liu, P.C.; Rodrigues-Pousada, C.; Thiele, D.J. Deciphering human heat shock transcription factor 1 regulation via post-translational modification in yeast. PLoS ONE 2011, 6, e15976. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, S.; Loew, C.; Körner, R.; Pinkert, S.; Theis, M.; Hayer-Hartl, M.; Buchholz, F.; Hartl, F.U. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 2014, 156, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Brunquell, J.; Raynes, R.; Bowers, P.; Morris, S.; Snyder, A.; Lugano, D.; Deonarine, A.; Westerheide, S.D. CCAR-1 is a negative regulator of the heat-shock response in Caenorhabditis elegans. Aging Cell 2018, 17, e12813. [Google Scholar] [CrossRef] [Green Version]
- Tonkiss, J.; Calderwood, S.K. Regulation of heat shock gene transcription in neuronal cells. Int. J. Hyperth. 2005, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Guettouche, T.; Boellmann, F.; Lane, W.S.; Voellmy, R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Laskovs, M.; Williams, R.I.; Mahadevan, A.; Labbadia, J. A Mitochondrial Stress-Specific Form of HSF1 Protects against Age-Related Proteostasis Collapse. Dev. Cell 2020, 54, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, R.; Denison, C.; Bening-Abu-Shach, U.; Chisholm, A.D.; Gygi, S.P.; Broday, L. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev. Cell 2009, 17, 724–735. [Google Scholar] [CrossRef] [Green Version]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Rivard, R.S.; Morris, J.M.; Youngman, M.J. The PP2A/4/6 subfamily of phosphoprotein phosphatases regulates DAF-16 and confers resistance to environmental stress in postreproductive adult C. elegans. PLoS ONE 2020, 15, e0229812. [Google Scholar] [CrossRef]
- Biglou, S.G.; Bendena, W.G.; Chin-Sang, I. An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Peptides 2021, 145, 170640. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef] [Green Version]
- Tepper, R.G.; Ashraf, J.; Kaletsky, R.; Kleemann, G.; Murphy, C.T.; Bussemaker, H.J. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 2013, 154, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Nettleton, D.; Jiang, M.; Kim, S.K.; Powell-Coffman, J.A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 20580–20588. [Google Scholar] [CrossRef] [Green Version]
- Treinin, M.; Shliar, J.; Jiang, H.; Powell-Coffman, J.A.; Bromberg, Z.; Horowitz, M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol. Genom. 2003, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Carranza, A.D.V.; Saragusti, A.; Chiabrando, G.A.; Carrari, F.; Asis, R. Effects of chlorogenic acid on thermal stress tolerance in C. elegans via HIF-1, HSF-1 and autophagy. Phytomedicine 2020, 66, 153132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Leboutet, R.; Largeau, C.; Zentout, S.; Lefebvre, C.; Delahodde, A.; Culetto, E.; Legouis, R. Autophagy facilitates mitochondrial rebuilding after acute heat stress via a DRP-1-dependent process. J. Cell Biol. 2021, 220, e201909139. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, M.; Nakai, A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010, 277, 4112–4125. [Google Scholar] [PubMed]
- Kumsta, C.; Hansen, M. Hormetic heat shock and HSF-1 overexpression improve C. elegans survival and proteostasis by inducing autophagy. Autophagy 2017, 13, 1076–1077. [Google Scholar] [CrossRef] [Green Version]
- Civelek, M.; Mehrkens, J.F.; Carstens, N.M.; Fitzenberger, E.; Wenzel, U. Inhibition of mitophagy decreases survival of Caenorhabditis elegans by increasing protein aggregation. Mol. Cell Biochem. 2019, 452, 123–131. [Google Scholar] [CrossRef]
- Nehammer, C.; Podolska, A.; Mackowiak, S.D.; Kagias, K.; Pocock, R. Specific microRNAs regulate heat stress responses in Caenorhabditis elegans. Sci. Rep. 2015, 5, 8866. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Brunquell, J.; Snyder, A.; Cheng, F.; Westerheide, S.D. HSF-1 is a regulator of miRNA expression in Caenorhabditis elegans. PLoS ONE 2017, 12, e0183445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, W.P.; Pagliuso, D.C.; Garrigues, J.M.; Chen, J.S.; Aalto, A.P.; Pasquinelli, A.E. Remodeling of the Caenorhabditis elegans non-coding RNA transcriptome by heat shock. Nucleic Acids Res. 2019, 47, 9829–9841. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, D.C.; Bodas, D.M.; Pasquinelli, A.E. Recovery from heat shock requires the microRNA pathway in Caenorhabditis elegans. PLoS Genet. 2021, 17, e1009734. [Google Scholar] [CrossRef] [PubMed]
- Tuorto, F.; Herbst, F.; Alerasool, N.; Bender, S.; Popp, O.; Federico, G.; Reitter, S.; Liebers, R.; Stoecklin, G.; Gröne, H.J.; et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015, 34, 2350–2362. [Google Scholar] [CrossRef] [Green Version]
- Navarro, I.C.; Tuorto, F.; Jordan, D.; Legrand, C.; Price, J.; Braukmann, F.; Hendrick, A.G.; Akay, A.; Kotter, A.; Helm, M.; et al. Translational adaptation to heat stress is mediated by RNA 5-methylcytosine in Caenorhabditis elegans. EMBO J. 2021, 40, e105496. [Google Scholar] [CrossRef]
- Labbadia, J.; Brielmann, R.M.; Neto, M.F.; Lin, Y.F.; Haynes, C.M.; Morimoto, R.I. Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging. Cell Rep. 2017, 21, 1481–1494. [Google Scholar] [CrossRef] [Green Version]
- Taouktsi, E.; Kyriakou, E.; Smyrniotis, S.; Borbolis, F.; Bondi, L.; Avgeris, S.; Trigazis, E.; Rigas, S.; Voutsinas, G.E.; Syntichaki, P. Organismal and Cellular Stress Responses upon Disruption of Mitochondrial Lonp1 Protease. Cells 2022, 11, 1363. [Google Scholar] [CrossRef]
- Gibellini, L.; De Gaetano, A.; Mandrioli, M.; Van Tongeren, E.; Bortolotti, C.A.; Cossarizza, A.; Pinti, M. The biology of Lonp1: More than a mitochondrial protease. Int. Rev. Cell Mol. Biol. 2020, 354, 1–61. [Google Scholar]
- Voos, W.; Pollecker, K. The Mitochondrial Lon Protease: Novel Functions off the Beaten Track? Biomolecules 2020, 10, 253. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, E.; Taouktsi, E.; Syntichaki, P. The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 14907. https://doi.org/10.3390/ijms232314907
Kyriakou E, Taouktsi E, Syntichaki P. The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. International Journal of Molecular Sciences. 2022; 23(23):14907. https://doi.org/10.3390/ijms232314907
Chicago/Turabian StyleKyriakou, Eleni, Eirini Taouktsi, and Popi Syntichaki. 2022. "The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans" International Journal of Molecular Sciences 23, no. 23: 14907. https://doi.org/10.3390/ijms232314907
APA StyleKyriakou, E., Taouktsi, E., & Syntichaki, P. (2022). The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. International Journal of Molecular Sciences, 23(23), 14907. https://doi.org/10.3390/ijms232314907






