The Role of L-Arginine-NO System in Female Reproduction: A Narrative Review
Abstract
1. Introduction
2. Clinical and Physiological Significance of L-Arginine Methabolism
2.1. L-Arginine Transport into the Cells
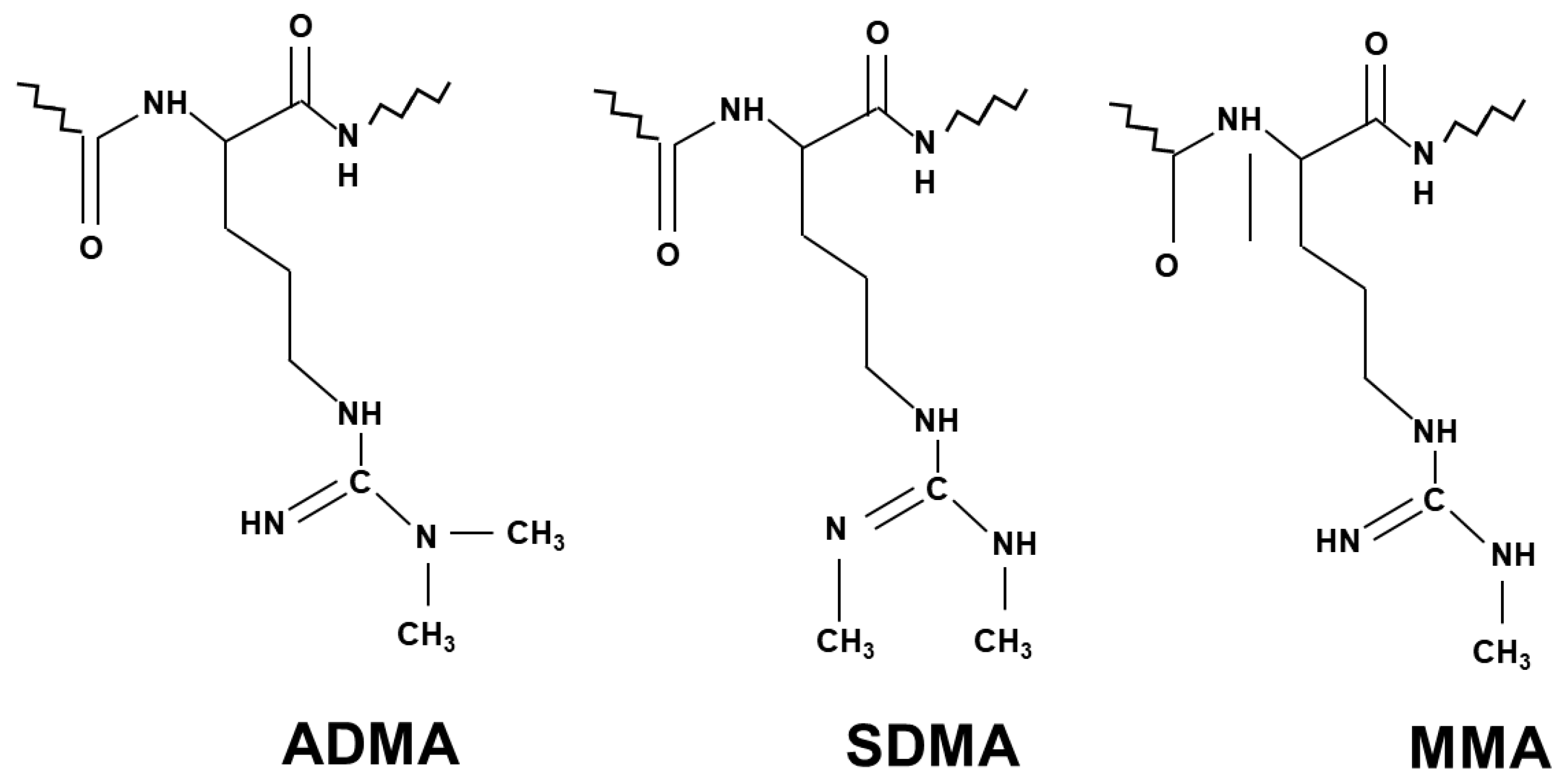
2.2. The Process of Arginine Methylation
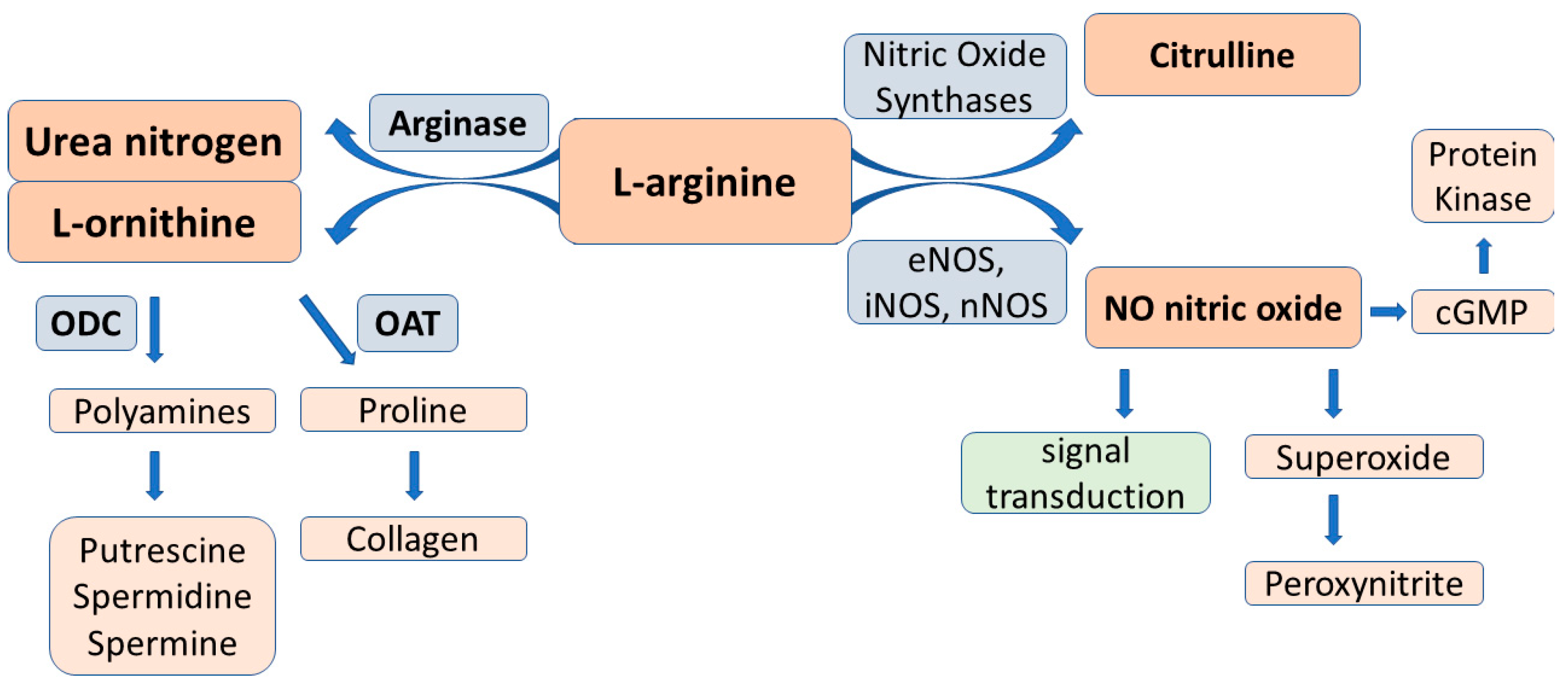
2.3. L-Arginine-Arginase Pathway
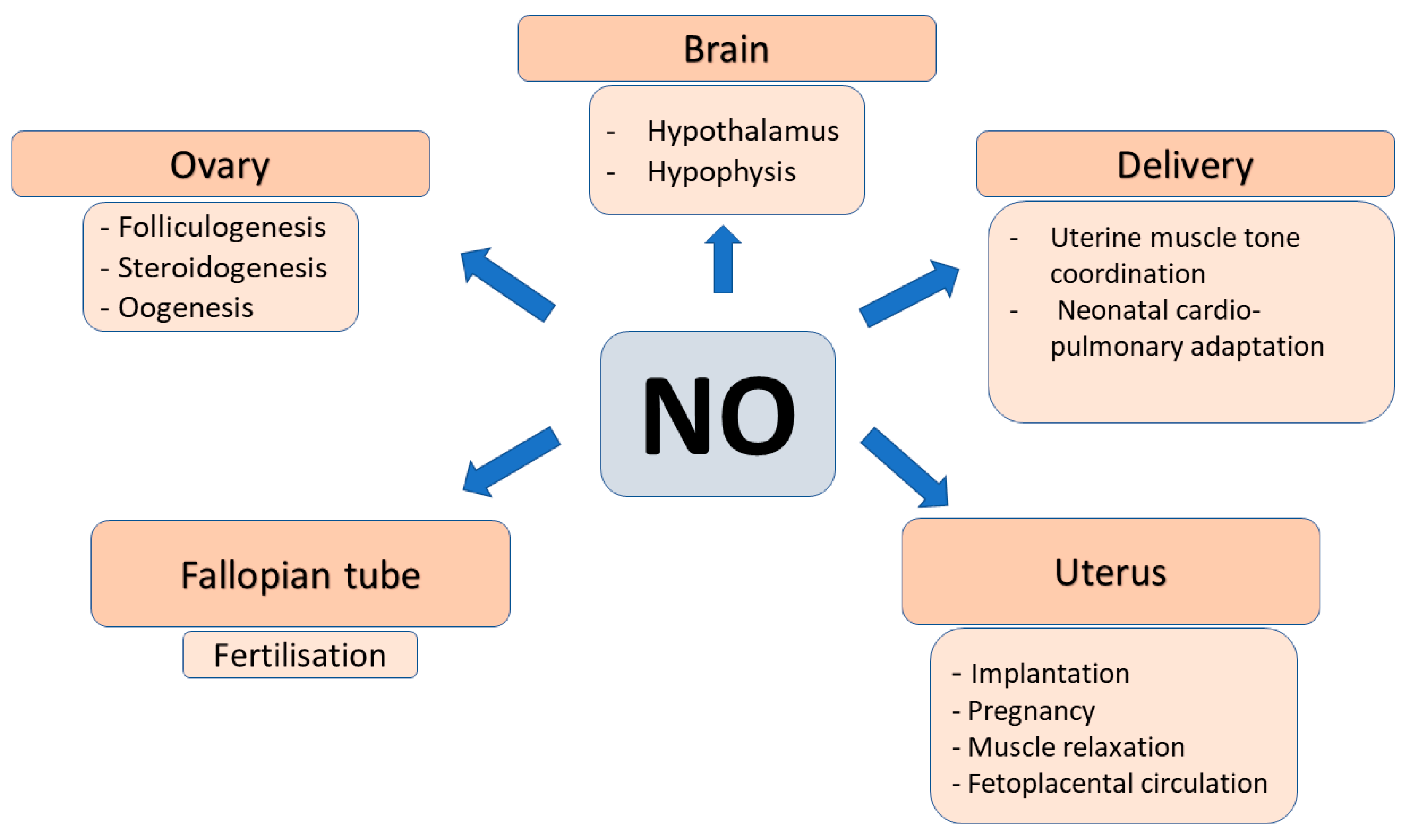
2.4. Role of NO in Female Reproduction
2.5. Oxidative Stress and L-Arginine NO System
2.6. L-Arginine Supplementation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AZI | antizyme |
| CAT | cationic amino acid transporter |
| cGMP | cyclic guanosine monophosphate |
| DDAH | dimethylarginine dimethylaminohydrolase |
| DNA | dezoxyribonucleic acid |
| FF | follicular fluid |
| FOXO3a | forkhead box O3 |
| 5-HT | 5- hydroxyl- tryptamine (serotonin) |
| hCG | human chorionic gonadotropin |
| IDO | indoleamine-2,3-dioxygenase |
| IVF | in vitro fertilization |
| L-NA | Nitro-l-arginine |
| L-NAME | Nitro-l-arginine methyl ester |
| MMA | mono-methylarginine |
| mRNA | messenger ribonucleic acid |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| ODC | ornithine decarboxylase |
| PRMT | protein arginine methyltransferase |
| ROS | reactive oxygen species |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SDMA | symmetric dimethylarginine |
| sGC | soluble guanylate cyclase |
| SLC | solute carrier |
| cGMP | cyclic guanosyl monophosphate |
| DDAH | dimethylarginine dimethylaminohydrolase |
| DNA | dezoxyribonucleic acid |
| FF | follicular fluid |
References
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Cziraki, A.; Lenkey, Z.; Sulyok, E.; Szokodi, I.; Koller, A. L-arginine-nitric oxide-asymmetric dimethylarginine pathway and the coronary circulation: Translation of basic science results to clinical practice. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Closs, E.I.; Boissel, A.; Habermeier, A.; Rotmann, A. Structure and function of cationic amino acid transporters (CATs). J. Membr. Biol. 2006, 213, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J. Amino acid transport and metabolism regulate early embryo development: Species differences, clinical significance and evolutionary implications. Cells 2010, 10, 3154. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.; Wellis, D.; Malter, H.; Munné, S.; Cohen, J.; Steuerwald, N.M. Expression profiles of individual human oocytes using microarray technology. Reprod. Biomed. Online 2004, 3, 325–337. [Google Scholar] [CrossRef]
- Gao, H.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Bazer, F.W. Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol. Reprod. 2009, 80, 602–609. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Koppán, M.; Várnagy, Á.; Prémusz, V.; Gödöny, K.; Rascher, W.; Rauh, M. Tryptophan catabolism to serotonin and kynurenine in women undergoing in-vitro fertilization. Psihol. Res. 2020, 69, 1113–1124. [Google Scholar] [CrossRef]
- Vesela, J.; Rehak, P.; Mihalik, J.; Czikkova, S.; Pokorny, J. Expression of serotonin receptors in mouse oocytes and preimplantation embryos. Psyhol. Res. 2003, 52, 223–228. [Google Scholar]
- Il’ Kova, G.; Rehak, P.; Vesela, J.; Cikos, S.; Fabian, D.; Czikkova, S.; Koppel, J. Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote 2004, 12, 205–213. [Google Scholar] [CrossRef]
- Amireault, P.; Dube, F. Intracellular cAMP and calcium signalling by serotonin in mouse cumulus-oocyte complexes. Mol. Pharmacol. 2005, 68, 1678–1687. [Google Scholar] [CrossRef]
- Groebner, A.E.; Schulke, K.; Schefold, J.C.; Fusch, G.; Singowatz, F.; Reichenbach, H.D.; Wolf, E.; Meyer, H.H.; Ulbrich, S.E. Immunological mechanisms to establish embryo tolerance in early bovine pregnancy. Reprod. Fertil. Dev. 2011, 23, 619–632. [Google Scholar] [CrossRef]
- Schrocksnadel, H.; Baier-Bitterlich, G.; Dapunt, O.; Wachter, H.; Fuchs, D. Decreased plasma tryptophan in pregnancy. Obstet. Gynecol. 1996, 88, 47–50. [Google Scholar] [CrossRef]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Passhine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef]
- Santillan, M.K.; Pelham, C.J.; Ketsawatsomkron, P.; Santillan, D.A.; Davis, D.R.; Devor, E.J.; Gibson-Corley, K.N.; Scroggins, S.M.; Grobe, J.L.; Yang, B.; et al. Pregnant mice lacking indoleamine 2,3-digoxygenase exhibit preeclampsia phenotypes. Physiol. Rep. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calvcer, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of NO synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar]
- Lucock, M. Follic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef]
- Pogribna, M.; Melnyk, S.; Pogribny, I.; Chango, A.; Yi, P.; James, S.J. Homocystine metabolism in children with Down syndrome. Am. J. Hum. Genet. 2001, 69, 88–95. [Google Scholar] [CrossRef]
- Hobbes, C.A.; Cleves, M.A.; Melnyk, S.; Zhao, W.; James, S.J. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am. J. Clin. Nutr. 2005, 81, 147–153. [Google Scholar] [CrossRef]
- Shea, T.B.; Rogeres, E. Lifetime requirement of the methionine cycle for neuronal development and maintenance. Cuur. Opin. Psychiatry 2014, 27, 138–142. Available online: www.co-psychiatry.com (accessed on 1 January 2021).
- Baumann, C.B.; Olson, M.; Wang, K.; Fazleabas, A.; De La Funte, R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction 2015, 150, 297–331. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.W.H.; Cho, L.; Brennan, D.M.; Haten, S.L. Targeted metabolomics evaluation of arginine methylation and cardiovascular risk. Potential Mechanisms beyond nitric oxide synthase inhibition. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Bódis, J.; Várnagy, Á.; Sulyok, E.; Kovács, G.L.; Martens-Lobenhoffer, J.; Bode-Böger, S.M. Negative association of l-arginine methylation products with oocyte numbers. Hum. Reprod. Adv. Acc. 2010, 25, 3095–3100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pahlich, S.; Zakaryan, R.P.; Gehring, H. Protein arginine methylation: Cellular functions and methods of analysis. Biochim. Biophys. Acta 2006, 1764, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.S. The protein aarginine methyltransferase family: An update about function, new perspectives and physiological role in humans. Cell Mol. Life Sci. 2009, 66, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.R.; Scherer, C.A.; Chen, J.I.N.; Roshon, M.J.; Ruley, H.E. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol. Cell Biol. 2000, 20, 4859–4869. [Google Scholar] [CrossRef]
- Kim, S.; Günesdogan, U.; Zylicz, J.; Hackett, J.A.; Cougot, D.; Bao, S.; Lee, C.; Dietmann, S.; Allen, G.E.; Sengupta, R.; et al. PRMT5 Protects genomic integrity during global DNA demethylation in primordial germ cells and preimplantation embryos. Mol. Cell 2014, 56, 564–579. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, D.; Liu, X.; Yu, G.; Cai, X.; Xu, C.; Rong, F. Zebrafish prmt5 arginine methyltransferase is essential for germ cell development. Development 2019, 146, 179572. [Google Scholar] [CrossRef]
- Yadav, N.; Lee, J.; Kim, J.; Shen, J.; Hu, M.C.T.; Aldaz, C.M.; Mark, T.; Betford, T. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 6464–6468. [Google Scholar] [CrossRef]
- Chamani, I.J.; Keele, D. Epigenetics and female reproductive aging. Front. Endocrinol. 2019, 10, 473. [Google Scholar] [CrossRef]
- Yu, B.; Russanova, V.R.; Gravina, S.; Hartley, S.; Mullikin, J.C.; Ignezweski, A.; Graham, J.; Segars, J.H.; De Cherney, A.H.; Howard, B.H. DNA methyloma and transcriptome sequencing in human ovarian granulosa cells links age-related changes in gene expression to gene body methylation and 3’-end CG density. Oncotarget 2015, 6, 3627–3643. [Google Scholar] [CrossRef]
- Jenkinson, C.P.; Grody, W.W.; Cederbaum, S.P. Comparative properties of arginases. Comp. Biochem. Physiol. 1996, 114, 107–132. [Google Scholar] [CrossRef]
- Bachetti, T.; Comini, L.; Francolini, G.; Bastianon, D.; Valetti, B.; Cadei, M.; Grigolato, P.; Suzuki, H.; Finazzi, D.; Albertini, A.; et al. Arginase pathway in human endothelial cells in pathophysiological conditions. J. Mol. Cell Cardiol. 2003, 7, 515–523. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Hawker, J.R.; Haynes, T.E.; Kepka-Lenhart, D.; Mistry, S.K.; Morris, S.M., Jr.; Wu, G. Regulatory role of arginase I and II in nitric oxide, polyamine and proline synthesis in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E75–E82. [Google Scholar] [CrossRef]
- Lefevre, P.L.C.; Palin, M.-F.; Murphy, B.D. Polyamines in the reproductive landscap. Endocrin. Rev. 2011, 32, 694–712. [Google Scholar] [CrossRef]
- Kim, J.H.; Bugaj, L.J.; Oh, Y.J.; Bivalacqua, T.J.; Ryoo, S.; Soucy, K.G.; Santhanam, L.; Webb, A.; Camara, A.; Sikka, G.; et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol. 2009, 107, 1249–1257. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A multifaceted enzyme important in health and disease. Physiol. Rev. 2018, 98, 641–675. [Google Scholar] [CrossRef]
- Hein, T.W.; Zhang, C.; Wang, W.; Chang, C.I.; Thengchaisri, N.; Kuo, L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilatation in coronary arterioles: Counteracting role of arginase. FASEB J. 2003, 17, 2328–2330. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Kilic, K.; Akman, O.; Ömür, A.D.; Uçar, Ö. Arginase activity of ovarian structures in cows of brown swiss and its cross-breeds. Veter. Arch. 2015, 85, 261–271. [Google Scholar]
- Razmi, N.G.; Jelodar, A.G.; Nazifi, S.; Dehgani, A. Arginase status ln cattle reproductive system. Vet. Archiv. 2005, 75, 31–38. [Google Scholar]
- Budani, M.C.; Tiboni, G.M. Novel insights on the role of nitric oxide in ovary: A review of literature. Int. J. Environ. Res. Public Health 2021, 18, 980. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Role of nitric oxide in the regulation of reproduction: A review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Somvanshi, R.K.; Paik, S.; Kumar, U. Colocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hypothalamus. J. Mol. Neurosci. 2015, 55, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Mc Cosh, R.B.; Lopez, J.A.; Szeligo, B.M.; Bedenbaugh, M.N.; Hileman, S.M.; Coolen, L.M.; Lehman, M.N.; Goodman, R.L. Evidence that nitric oxide is critical for LH surge generation in female sheep. Endocrinology 2020, 161, bqaa010. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Kubota, T.; Aso, T. Effect of nitric oxide on steroidogenesis in porcine granulosa cells during different stages of follicular development. Eur. J. Endocrinol. 2021, 144, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kabagu, S.; Kodama, H.; Fukuda, J.; Karube, A.; Murata, M.; Tanaka, T. Inhibitory effects of nitric oxide on the expression and activity of aromatase in human granulosa cells. Mol. Hum. Reprod. 1999, 5, 396–401. [Google Scholar]
- Constantin, S.; Reynolds, D.; Oh, A.; Pizano, K.; Wray, S. Nitric oxide resets kisspeptin excited GnRH neurons via PIP2 Replenishment. Proc. Natl. Acad. Sci. USA 2021, 118, e2012339118. [Google Scholar] [CrossRef] [PubMed]
- Watanobe, H.; Schiöth, H.B. Nitric oxide mediates leptin-induced preovulatory luteinzing hormone and prolactin surges in rats. Brain Res. 2021, 923, 193–197. [Google Scholar] [CrossRef]
- Jablonka-Shariff, A.; Olson, L.M. The role of nitric oxide in oocyte meiotic maturation and ovulation: Metiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes. Endocrinology 1998, 139, 2944–2954. [Google Scholar] [CrossRef]
- Jablonka-Shariff, A.; Olson, L.M. Nitric oxide is essential for optimal meiotic maturation of murine cumulus-oocyte complexes in vitro. Mol. Reprod. Dev. 2000, 55, 412–420. [Google Scholar] [CrossRef]
- Tranguch, S.; Steuerwald, N.; Huet-Hudson, Y.M. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol. Reprod. 1998, 58, 875–879. [Google Scholar] [CrossRef]
- Darzen, D.L.; Klein, S.L.; Burnett, A.L.; Wallach, E.E.; Crone, J.K.; Huang, P.L.; Melson, R.J. Reproductive function in female mice lacking the gene for endothelial nitric oxide synthase. Nitric Oxide 1999, 3, 366–374. [Google Scholar] [CrossRef][Green Version]
- Huang, P.L. Lessons learned from nitric oxide synthase knockout animals. Semin. Perinatol. 2000, 24, 87–90. [Google Scholar] [CrossRef]
- Sengoku, K.; Takuma, N.; Horikawa, M.; Tsuchia, K.; Komori, H.; Sharifa, D.; Tamate, K.; Ishikawa, M. Requirement of nitric oxide for murine oocyte maturation, embryo development and trophoblast outgrowth in vitro. Mol. Reprod. Dev. 2001, 58, 262–268. [Google Scholar] [CrossRef]
- Gouge, R.C.; Marshburn, P.; Gordon, B.E.; Nunley, W.; Huet-Hudson, Y.M. Nitric oxide as a regulator of embryonic development. Biol. Reprod. 1998, 58, 875–879. [Google Scholar] [CrossRef]
- Chen, H.W.; Jiang, W.S.; Tzeng, R.C. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil. Steril. 2001, 75, 1163–1171. [Google Scholar] [CrossRef]
- Zullino, S.; Buzzella, F.; Simoncini, T. Nitric oxide and the biology of pregnancy. Vasc. Pharmacol. 2018, 110, 71–74. [Google Scholar] [CrossRef]
- Petterson, A.; Hedner, T.; Milson, I. Increased circulatory an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998, 77, 808–813. [Google Scholar] [CrossRef]
- Fickling, S.A.; Williams, D.; Vallace, P.; Nussey, S.S.; Whitley, G.S. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet 1993, 342, 242–243. [Google Scholar] [CrossRef]
- Holden, D.P.; Fickling, S.A.; Whitley, G.S.; Nussey, S.S. Plasma concentration of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and pre-eclampsia. Am. J. Obstet. Gynecol. 1998, 178, 551–556. [Google Scholar] [CrossRef]
- Akbar, F.; Heinonen, S.; Pirskanen, M. Uimari, P.; Tuomainen, T.P.; Salonen, I.T. Haplotypic association of DDAHI with susceptibility to pre-eclampsia. Mol. Hum. Reprod. 2004, 11, 73–77. [Google Scholar] [CrossRef]
- Tamás, P.; Bódis, J.; Sulyok, E.; Kovács, L.G.; Hantosi, E.; Molnár, G.; Martens-Lobenhoffer, J.; Bode-Böger, S. L-arginine metabolism in early-onset and late-onset. Scand. J. Clin. Lab. Investig. 2013, 83, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M. Nitric oxide and reproduction. Mol. Hum. Reprod. 1997, 8, 639–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox consifderation in female reproductive function and assisted reproduction. From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Várnagy, Á.; Kőszegi, T.; Györgyi, E.; Szegedi, S.; Sulyok, E.; Prémusz, V.; Bódis, J. Levels of total antioxidant capacity and 8-hydroxy-2′-dehydroxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: Focusing on endometriosis. Hum. Fertil. 2020, 23, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Babu, K.N.; Chattopadhyay, R.; Chakovarty, B.; Chaudhury, K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favourable. Reprod. Toxicol. 2010, 29, 447–451. [Google Scholar] [CrossRef]
- Gardner, D.K.; Wale, P.L. Analysis of metabolism to select viable human embryos for transfer. Fertil. Steril. 2013, 99, 1062–1072. [Google Scholar] [CrossRef]
- Sydow, K.; Münzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Kalyanaraman, B.; Martásek, P.; Hogg, N.; Masters, B.S.; Karoui, H.; Tordo, P.; Pritchard, K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA 1998, 95, 9220–9225. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase a critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharm. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef]
- Keskin, U.; Göktolga, Ü.; Cakir, E.; Ceyhan, S.T.; Ercan, C.M.; Baser, İ. Assymetrical dimethylarginine levels on the implantation success of in vitro fertilization and embryo transfer. Gynecol. Endocrinol. 2012, 28, 805–808. [Google Scholar] [CrossRef]
- Böger, R.H. The Pharmacodynamics of L-Arginine. J. Nutr. 2007, 137, 1650S–1655S. [Google Scholar] [CrossRef]
- Vukosavljevic, N.; Jaron, D.; Barbee, K.A.; Buerk, D.G. Quantifying the L-arginine paradox in vivo. Microvasc. Res. 2006, 71, 48–54. [Google Scholar] [CrossRef]
- Redel, B.K.; Tessane, K.J.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase-dimethylarginine dimethylamino hydrolase-nitric oxide axis. Reprod. Fertil. Develop. 2015, 27, 655–666. [Google Scholar] [CrossRef]
- Santana, P.D.i.B.; Guimaraes, S.; da Costa, N.N.; da Silva, B.B.; Carter, T.F.; Cordeiro, M.D.; da Silva, B.J.; Santos, S.D.; Herculano, A.M.; Adona, P.R.; et al. Supplementation of ovine embryo culture medium with l-arginine improves embryo quality via nitric oxide production. Mol. Reprod. Dev. 2014, 85, 918–927. [Google Scholar] [CrossRef]
- Battaglia, C.; Salvatori, M.; Maxia, N.; Petraglia, F.; Facchinetti, F.; Volpe, A. Adjuvant L-arginine treatment for in-vitro fertilization in poor- responder patients. Hum. Reprod. 1999, 14, 1690–1697. [Google Scholar] [CrossRef][Green Version]
- Castillo-Castrejon, M.; Migoya-Grane, A.; Meraz-Cruz, N.; Vadillo-Ortega, F. The role of l-arginine in human pregnancy. Agro Food Ind. Hi Tech 2011, 22, 26–28. [Google Scholar]
- Greene, J.M.; Feugang, J.M.; Pfeiffer, K.E.; Stokes, J.V.; Bowers, S.D.; Ryan, P.L. L-arginine enhances cell proliferation and reduces apoptosis in human endometrial RL95-2 cells. Reprod. Biol. Endocrinol. 2013, 11. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodis, J.; Farkas, B.; Nagy, B.; Kovacs, K.; Sulyok, E. The Role of L-Arginine-NO System in Female Reproduction: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 14908. https://doi.org/10.3390/ijms232314908
Bodis J, Farkas B, Nagy B, Kovacs K, Sulyok E. The Role of L-Arginine-NO System in Female Reproduction: A Narrative Review. International Journal of Molecular Sciences. 2022; 23(23):14908. https://doi.org/10.3390/ijms232314908
Chicago/Turabian StyleBodis, Jozsef, Balint Farkas, Bernadett Nagy, Kalman Kovacs, and Endre Sulyok. 2022. "The Role of L-Arginine-NO System in Female Reproduction: A Narrative Review" International Journal of Molecular Sciences 23, no. 23: 14908. https://doi.org/10.3390/ijms232314908
APA StyleBodis, J., Farkas, B., Nagy, B., Kovacs, K., & Sulyok, E. (2022). The Role of L-Arginine-NO System in Female Reproduction: A Narrative Review. International Journal of Molecular Sciences, 23(23), 14908. https://doi.org/10.3390/ijms232314908








