Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation
Abstract
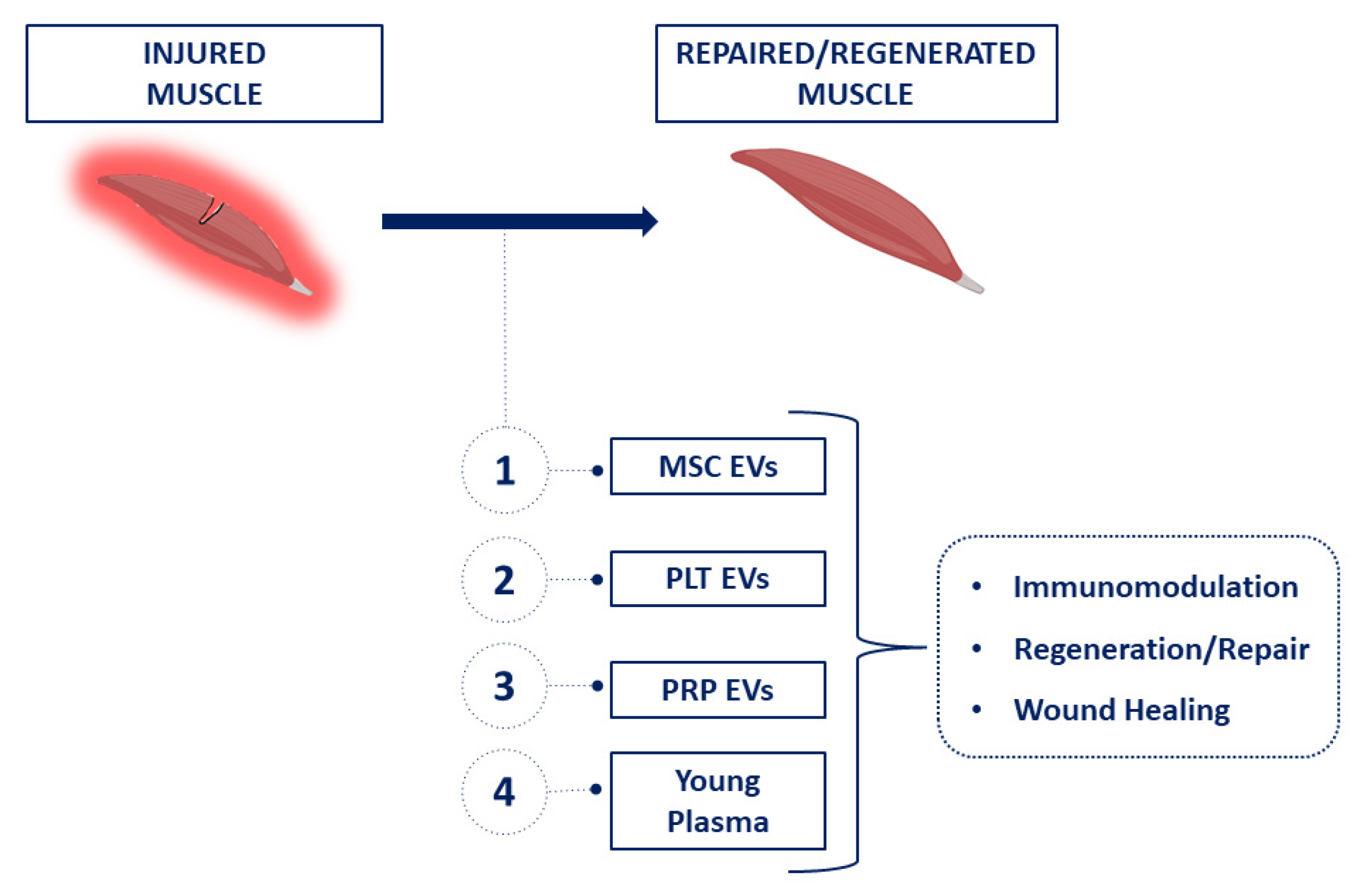
:1. Introduction
2. Results
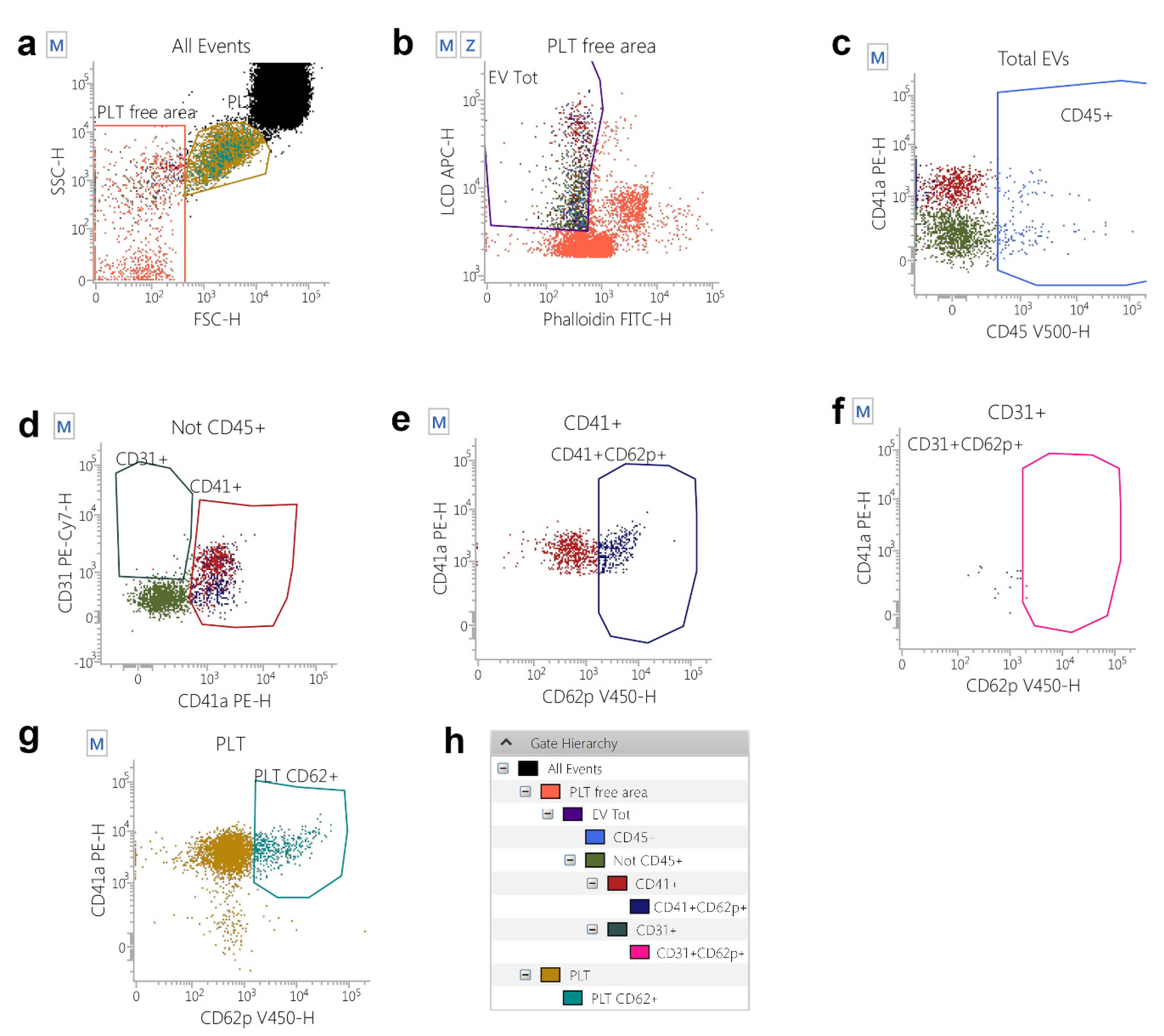
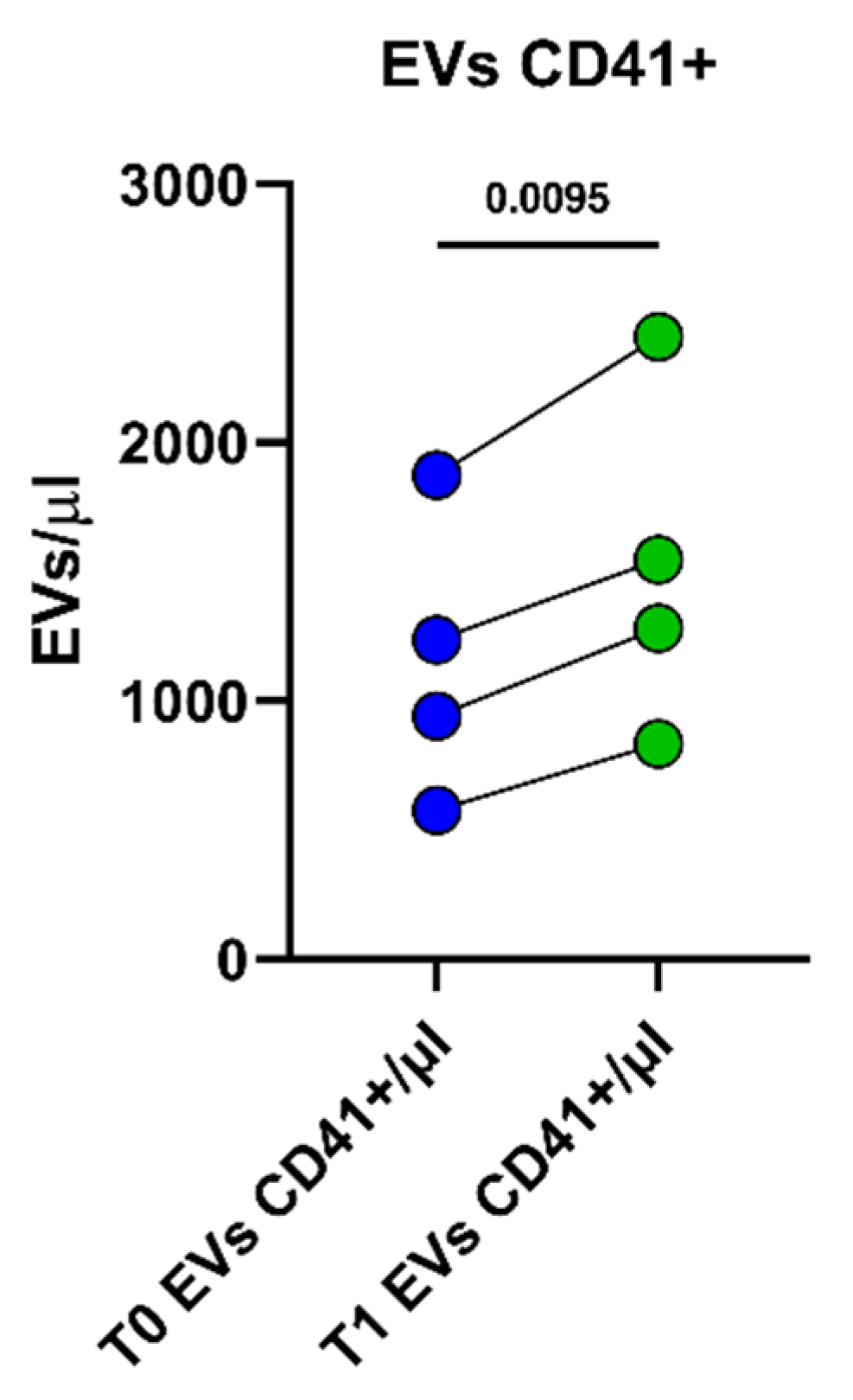
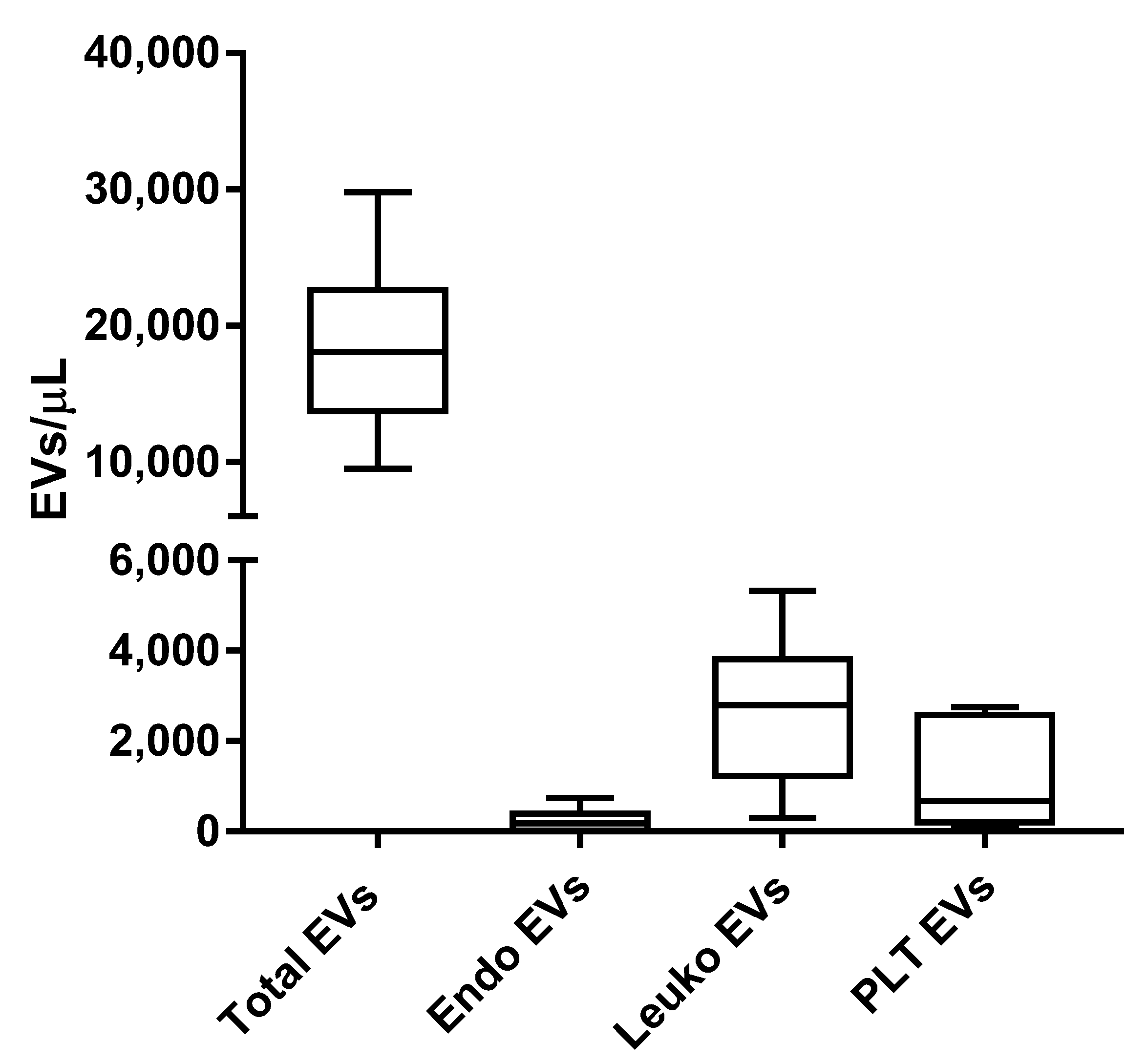
2.1. Peripheral Blood EV Identification and Count in Athletes Recovering from Muscle Injuries
2.2. Phenotypes of PRP-EVs
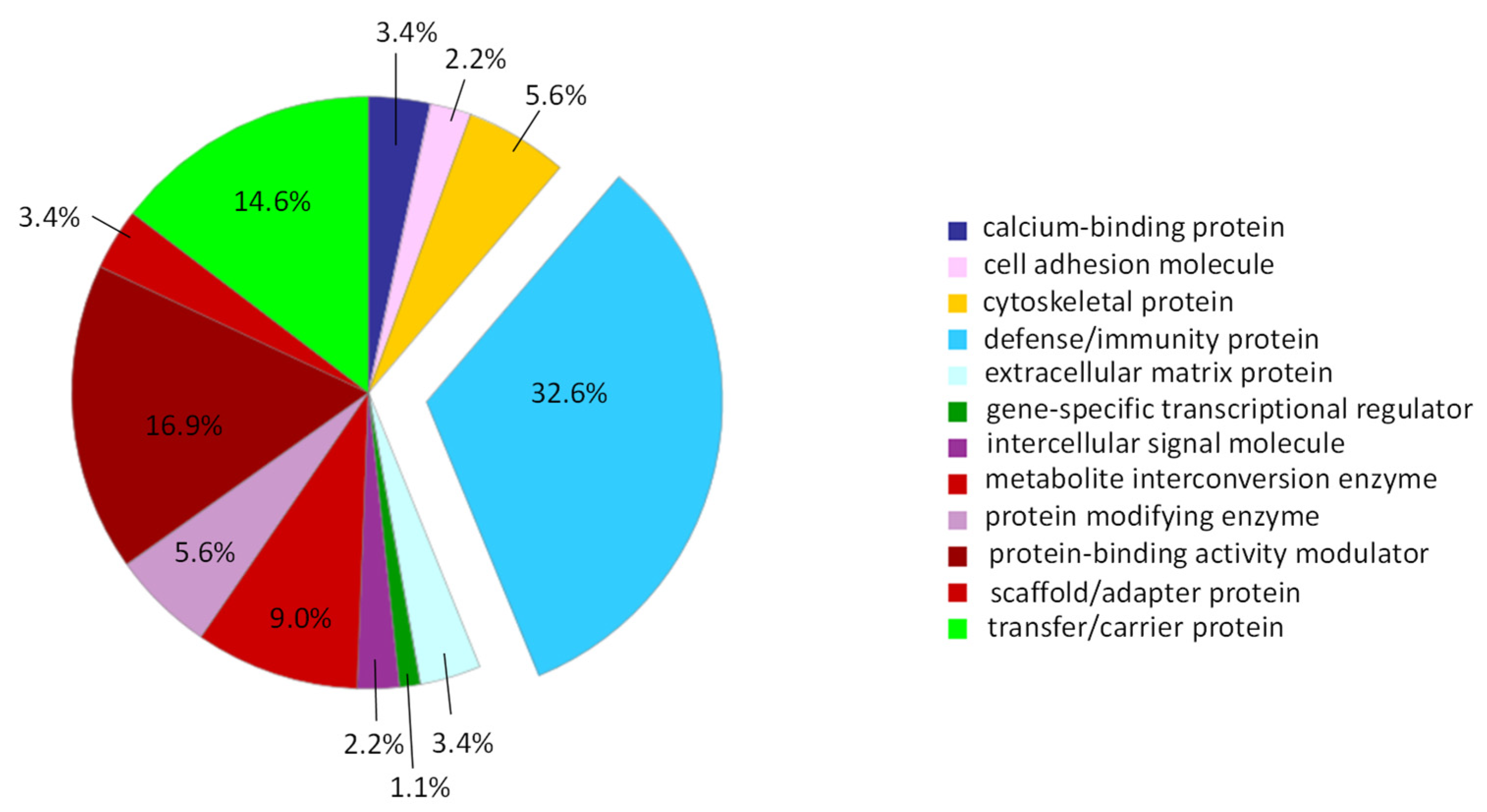
2.3. Proteomic Characterization of the PRP-EV Cargo
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Flow Cytometry Identification, Count, and Subtyping of EVs from Peripheral Blood Samples
4.3. Flow Cytometry Gating Strategy
4.4. Platelet-Enriched Plasma Preparation
4.5. EV Separation by Fluorescence-Activated Cell Sorting
4.6. EV Protein Cargo Detection by Label-Free Proteomics
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef]
- Patel, A.N.; Selzman, C.H.; Kumpati, G.S.; McKellar, S.H.; Bull, D.A. Evaluation of autologous platelet rich plasma for cardiac surgery: Outcome analysis of 2000 patients. J. Cardiothorac. Surg. 2016, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Muchedzi, T.A.; Roberts, S.B. A systematic review of the effects of platelet rich plasma on outcomes for patients with knee osteoarthritis and following total knee arthroplasty. Surgeon 2018, 16, 250–258. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Ther. Lett. 2019, 24, 1–6. [Google Scholar]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles. Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglìo, S.R.; Baldini, N. Exosomes: Novel effectors of human platelet lysate activity. Eur. Cells Mater. 2014, 28, 137–151; discussion 151. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell. Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Lanuti, P.; Marchisio, M.; De Laurenzi, V.; Del Boccio, P.; Pieragostino, D. Multi-Omics Approach for Studying Tears in Treatment-Naïve Glaucoma Patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef] [Green Version]
- Falasca, K.; Lanuti, P.; Ucciferri, C.; Pieragostino, D.; Cufaro, M.C.; Bologna, G.; Federici, L.; Miscia, S.; Pontolillo, M.; Auricchio, A.; et al. Circulating extracellular vesicles as new inflammation marker in HIV infection. AIDS 2021, 35, 595–604. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Pieragostino, D.; Cufaro, M.C.; Simeone, P.; Bologna, G.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; Irtelli, L.; et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers 2021, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocco, D.; Lanuti, P.; Simeone, P.; Bologna, G.; Pieragostino, D.; Cufaro, M.C.; Graziano, V.; Peri, M.; Di Marino, P.; De Tursi, M.; et al. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J. Oncol. 2019, 2019, 5879616. [Google Scholar] [CrossRef] [PubMed]
- Cela, I.; Cufaro, M.C.; Fucito, M.; Pieragostino, D.; Lanuti, P.; Sallese, M.; Del Boccio, P.; Di Matteo, A.; Allocati, N.; De Laurenzi, V.; et al. Proteomic Investigation of the Role of Nucleostemin in Nucleophosmin-Mutated OCI-AML 3 Cell Line. Int. J. Mol. Sci. 2022, 23, 7655. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef]
- Buca, D.; D’Antonio, F.; Buca, D.; Di Sebastiano, F.; Simeone, P.; Di Girolamo, R.; Bologna, G.; Vespa, S.; Catitti, G.; Liberati, M.; et al. Extracellular Vesicles in pregnancy: Their potential role as a liquid biopsy. J. Reprod. Immunol. 2022, 154, 103734. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Bittel, D.C.; Jaiswal, J.K. Contribution of Extracellular Vesicles in Rebuilding Injured Muscles. Front. Physiol. 2019, 10, 828. [Google Scholar] [CrossRef] [Green Version]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [Green Version]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Zordan, P.; Sciorati, C.; Rovere-Querini, P.; Brunelli, S. Macrophage plasticity in skeletal muscle repair. Biomed Res. Int. 2014, 2014, 560629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. [Google Scholar] [CrossRef]
- Vajen, T.; Mause, S.F.; Koenen, R.R. Microvesicles from platelets: Novel drivers of vascular inflammation. Thromb. Haemost. 2015, 114, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Berckmans, R.J.; Lacroix, R.; Hau, C.M.; Sturk, A.; Nieuwland, R. Extracellular vesicles and coagulation in blood from healthy humans revisited. J. Extracell. Vesicles 2019, 8, 1688936. [Google Scholar] [CrossRef]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Santilli, F.; Marchisio, M.; Lanuti, P.; Boccatonda, A.; Miscia, S.; Davì, G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb. Haemost. 2016, 116, 220–234. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review-The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.C.; Sabeh Junior, N.; dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Sahu, A.; Clemens, Z.J.; Shinde, S.N.; Sivakumar, S.; Pius, A.; Bhatia, A.; Picciolini, S.; Carlomagno, C.; Gualerzi, A.; Bedoni, M.; et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat. Aging 2021, 1, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B. Extracellular vesicle microRNAs mediate skeletal muscle myogenesis and disease. Biomed. Rep. 2016, 5, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figliolini, F.; Ranghino, A.; Grange, C.; Cedrino, M.; Tapparo, M.; Cavallari, C.; Rossi, A.; Togliatto, G.; Femminò, S.; Gugliuzza, M.V.; et al. Extracellular Vesicles From Adipose Stem Cells Prevent Muscle Damage and Inflammation in a Mouse Model of Hind Limb Ischemia: Role of Neuregulin-1. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 239–254. [Google Scholar] [CrossRef]
- Vajen, T.; Benedikter, B.J.; Heinzmann, A.C.A.; Vasina, E.M.; Henskens, Y.; Parsons, M.; Maguire, P.B.; Stassen, F.R.; Heemskerk, J.W.M.; Schurgers, L.J.; et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles 2017, 6, 1322454. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn, R.F.; Otsuru, S.; Lovering, R.M. Exosomes Isolated From Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function after Muscle Injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, J.; Xu, J.; Dai, H.; Fan, Q.; Fei, Z.; Chu, J.; Yao, C.; Shi, H.; Zhou, X.; et al. Reshaping the Inflammatory Environment in Rheumatoid Arthritis Joints by Targeting Delivery of Berberine with Platelet-Derived Extracellular Vesicles. Adv. NanoBiomed Res. 2021, 1, 2100071. [Google Scholar] [CrossRef]
- Chandler, W.L. Microparticle counts in platelet-rich and platelet-free plasma, effect of centrifugation and sample-processing protocols. Blood Coagul. Fibrinolysis 2013, 24, 125–132. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Casolo, A.; Del Vecchio, A.; Balshaw, T.G.; Maeo, S.; Lanza, M.B.; Felici, F.; Folland, J.P.; Farina, D. Behavior of motor units during submaximal isometric contractions in chronically strength-trained individuals. J. Appl. Physiol. 2021, 131, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Casolo, A.; Farina, D.; Falla, D.; Bazzucchi, I.; Felici, F.; Del Vecchio, A. Strength Training Increases Conduction Velocity of High-Threshold Motor Units. Med. Sci. Sports Exerc. 2020, 52, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2020, 22, 48. [Google Scholar] [CrossRef]
- Brocco, D.; Simeone, P.; Buca, D.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; De Lellis, L.; Martino, M.T.; Veschi, S.; Iezzi, M.; et al. Blood Circulating CD133+ Extracellular Vesicles Predict Clinical Outcomes in Patients with Metastatic Colorectal Cancer. Cancers 2022, 14, 1357. [Google Scholar] [CrossRef] [PubMed]
- Buca, D.; Bologna, G.; D’Amico, A.; Cugini, S.; Musca, F.; Febbo, M.; D’Arcangelo, D.; Buca, D.; Simeone, P.; Liberati, M.; et al. Extracellular Vesicles in Feto-Maternal Crosstalk and Pregnancy Disorders. Int. J. Mol. Sci. 2020, 21, 2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeone, P.; Celia, C.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Cilurzo, F.; Grande, R.; Diomede, F.; Vespa, S.; Canonico, B.; et al. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 7885. [Google Scholar] [CrossRef]
- Lanuti, P.; Ciccocioppo, F.; Bonanni, L.; Marchisio, M.; Lachmann, R.; Tabet, N.; Pierdomenico, L.; Santavenere, E.; Catinella, V.; Iacone, A.; et al. Amyloid-specific T-cells differentiate Alzheimer’s disease from Lewy body dementia. Neurobiol. Aging 2012, 33, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Simeone, P.; Rotta, G.; Almici, C.; Avvisati, G.; Azzaro, R.; Bologna, G.; Budillon, A.; Di Cerbo, M.; Di Gennaro, E.; et al. A standardized flow cytometry network study for the assessment of circulating endothelial cell physiological ranges. Sci. Rep. 2018, 8, 5823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef] [PubMed]
- Potenza, F.; Cufaro, M.C.; Di Biase, L.; Panella, V.; Di Campli, A.; Ruggieri, A.G.; Dufrusine, B.; Restelli, E.; Pietrangelo, L.; Protasi, F.; et al. Proteomic Analysis of Marinesco-Sjogren Syndrome Fibroblasts Indicates Pro-Survival Metabolic Adaptation to SIL1 Loss. Int. J. Mol. Sci. 2021, 22, 12449. [Google Scholar] [CrossRef] [PubMed]

| Protein UniProt Code | Gene Name | Protein Name | Protein Class | Functional Network Interaction |
|---|---|---|---|---|
| P01834 | IGKC | Immunoglobulin kappa constant | defense/immunity protein | - |
| P01860 | IGHG3 | Immunoglobulin heavy constant gamma 3 | defense/immunity protein | - |
| A0A0C4DH42 | IGHV3–66 | Immunoglobulin heavy variable 3–66 | defense/immunity protein | - |
| P0DOY3 | IGLC3 | Immunoglobulin lambda constant 3 | defense/immunity protein | - |
| P0DP04 | IGHV3–43D | Immunoglobulin heavy variable 3–43D | defense/immunity protein | VT |
| P01857 | IGHG1 | Immunoglobulin heavy constant gamma 1 | defense/immunity protein | - |
| P02749 | APOH | Beta-2-glycoprotein 1 | defense/immunity protein | VT |
| P01876 | IGHA1 | Immunoglobulin heavy constant alpha 1 | defense/immunity protein | - |
| P0DP01 | IGHV1–8 | Immunoglobulin heavy variable 1–8 | defense/immunity protein | - |
| P25311 | AZGP1 | Zinc-alpha-2-glycoprotein | defense/immunity protein | - |
| A0A075B7B8 | IGHV3OR16–12 | Immunoglobulin heavy variable 3/OR16–12 (non-functional) | defense/immunity protein | - |
| P01871 | IGHM | Immunoglobulin heavy constant mu | defense/immunity protein | - |
| A0A0C4DH31 | HV1–18 | Immunoglobulin heavy variable 1–18 | defense/immunity protein | - |
| P08603 | CFH | Complement factor H | defense/immunity protein | - |
| P0DP08 | IGHV4–38–2 | Immunoglobulin heavy variable 4–38–2 | defense/immunity protein | VT |
| P0DP03 | HV3–30–5 | Immunoglobulin heavy variable 3–30–5 | defense/immunity protein | - |
| P01591 | IGJ | Immunoglobulin J chain | defense/immunity protein | VT |
| P04003 | C4BPA | C4b-binding protein alpha chain | defense/immunity protein | VT |
| A2NJV5 | KV2–29 | Immunoglobulin kappa variable 2–29 | defense/immunity protein | - |
| P0CG04 | IGLL5 | Immunoglobulin lambda like polypeptide 5 | defense/immunity protein | VT |
| P01861 | IGHG4 | Immunoglobulin heavy constant gamma 4 | defense/immunity protein | - |
| P01619 | KV320 | Immunoglobulin kappa variable 3–20 | defense/immunity protein | - |
| A0A0B4J1V6 | HV373 | Immunoglobulin heavy variable 3–73 | defense/immunity protein | - |
| P07357 | C8A | Complement component C8 alpha chain | defense/immunity protein | - |
| Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | defense/immunity protein | - |
| P01877 | IGHA2 | Immunoglobulin heavy constant alpha 2 | defense/immunity protein | - |
| P01859 | IGHG2 | Immunoglobulin heavy constant gamma 2 | defense/immunity protein | - |
| Q0VDD8 | DNAH14 | Dynein axonemal heavy chain 14 | cytoskeletal protein | - |
| Q8TF72 | SHROOM3 | Protein Shroom3 | cytoskeletal protein | - |
| Q13835 | PKP1 | Plakophilin-1 | cytoskeletal protein | VT |
| P15924 | DSP | Desmoplakin | cytoskeletal protein | WH, VT |
| P63261 | ACTG1 | Actin, cytoplasmic 2 | cytoskeletal protein | WH, VT |
| P69905 | HBA2 | Hemoglobin subunit alpha 2 | transfer/carrier protein | VT |
| P68871 | HBB | Hemoglobin subunit beta | transfer/carrier protein | WH, VT |
| P02787 | TF | Serotransferrin | transfer/carrier protein | VT |
| P02042 | HBD | Hemoglobin subunit delta | transfer/carrier protein | WH |
| P02656 | APOC3 | Apolipoprotein C-III | transfer/carrier protein | - |
| P02774 | GC | Vitamin D-binding protein | transfer/carrier protein | - |
| P04114 | APOB | Apolipoprotein B-100 | transfer/carrier protein | VT |
| P05090 | APOD | Apolipoprotein D | transfer/carrier protein | - |
| P43652 | AFM | Afamin | transfer/carrier protein | - |
| P06727 | APOA4 | Apolipoprotein A-IV | transfer/carrier protein | - |
| P02649 | APOE | Apolipoprotein E | transfer/carrier protein | WH, VT |
| P02652 | APOA2 | Apolipoprotein A-II | transfer/carrier protein | - |
| P02647 | APOA1 | Apolipoprotein A-I | transfer/carrier protein | VT |
| P00738 | HP | Haptoglobin | protein-modifying enzyme | VT |
| P00734 | F2 | Prothrombin | protein-modifying enzyme | WH, VT |
| P00747 | PLG | Plasminogen | protein-modifying enzyme | WH, VT |
| P31944 | CASP14 | Caspase-14 | protein-modifying enzyme | - |
| P02790 | HPX | Hemopexin | protein-modifying enzyme | VT |
| P02675 | FGB | Fibrinogen beta chain | intercellular signal molecules | WH, VT |
| P02679 | FGG | Fibrinogen gamma chain | intercellular signal molecules | WH, VT |
| P01024 | C3 | Complement C3 | protein-binding activity modulator | VT |
| P01009 | SERPINA1 | Alpha-1-antitrypsin | protein-binding activity modulator | WH, VT |
| P19827 | ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 | protein-binding activity modulator | - |
| P01011 | GIG25 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | protein-binding activity modulator | VT |
| P01042 | KNG1 | Kininogen-1 | protein-binding activity modulator | WH, VT |
| P01008 | SERPINC1 | Antithrombin-III | protein-binding activity modulator | WH |
| P19823 | ITIH2 | Inter-alpha-trypsin inhibitor heavy chain H2 | protein-binding activity modulator | - |
| P01019 | AGT | Angiotensinogen | protein-binding activity modulator | - |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | protein-binding activity modulator | WH, VT |
| P0C0L5 | C4B | Complement C4-B | protein-binding activity modulator | - |
| P01031 | C5 | Complement C5 | protein-binding activity modulator | - |
| Q14624 | ITIH4 | Inter-alpha-trypsin inhibitor heavy chain H4 | protein-binding activity modulator | VT |
| P05546 | SERPIND1 | Heparin cofactor 2 | protein-binding activity modulator | WH |
| P08185 | SERPINA6 | Corticosteroid-binding globulin | protein-binding activity modulator | - |
| P01023 | A2M | Alpha-2-macroglobulin | protein-binding activity modulator | WH, VT |
| P02452 | COL1A1 | Collagen alpha-1(I) chain | extracellular matrix protein | WH, VT |
| P02751 | FN1 | Fibronectin type III domain containing | extracellular matrix protein | VT |
| P08123 | COL1A2 | Collagen alpha-2(I) chain | extracellular matrix protein | WH, VT |
| P06702 | S100A9 | Protein S100-A9 | calcium-binding protein | VT |
| P07355 | ANXA2 | Annexin A2 | calcium-binding protein | WH, VT |
| P05109 | S100A8 | Protein S100-A8 | calcium-binding protein | WH, VT |
| P00915 | CA1 | Carbonic anhydrase 1 | metabolite interconversion enzyme | - |
| P00918 | CA2 | Carbonic anhydrase 2 | metabolite interconversion enzyme | - |
| P02766 | TTR | Transthyretin | metabolite interconversion enzyme | VT |
| P32119 | PRDX2 | Peroxiredoxin-2 | metabolite interconversion enzyme | WH |
| P04406 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | metabolite interconversion enzyme | - |
| P04040 | CAT | Catalase | metabolite interconversion enzyme | VT |
| Q08188 | TGM3 | Protein-glutamine gamma-glutamyltransferase E | metabolite interconversion enzyme | - |
| P00450 | CP | Ceruloplasmin | metabolite interconversion enzyme | - |
| P02747 | C1QC | Complement C1q subcomponent subunit C | scaffold/adaptor protein | - |
| P31947 | SFN | 14–3–3 protein sigma | scaffold/adaptor protein | VT |
| P63104 | YWHAZ | 14–3–3 protein zeta/delta | scaffold/adaptor protein | WH, VT |
| O75882 | ATRN | Attractin | gene-specific transcriptional regulator | - |
| Q08554 | DSC1 | Desmocollin-1 | cell adhesion molecule | VT |
| Q02413 | DSG1 | Desmoglein-1 | cell adhesion molecule | VT |
| P12273 | PIP | Prolactin induced protein | - | - |
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 | - | VT |
| P02671 | FGA | Fibrinogen alpha chain | - | WH, VT |
| S4R460 | ENSG00000259680 | Uncharacterized protein | - | - |
| P81605 | DCD | Dermcidin | - | - |
| P10909 | CLU | Clusterin | - | VT |
| P02760 | AMBP | Protein AMBP | - | VT |
| P04217 | A1BG | Alpha-1B-glycoprotein | - | VT |
| P27169 | PON1 | Serum paraoxonase/arylesterase 1 | - | - |
| O43866 | CD5L | CD5 antigen-like | - | VT |
| P04004 | VTN | Vitronectin | - | WH, VT |
| P62987 | UBA52 | Ubiquitin-60S ribosomal protein L40 | - | VT |
| P00751 | CFB | Complement factor B | - | - |
| O75223 | GGCT | Gamma-glutamylcyclotransferase | - | - |
| P14923 | JUP | Junction plakoglobin | - | VT |
| Q32MH5 | FAM214A | Protein FAM214A | - | - |
| Q5T749 | KPRP | Keratinocyte proline rich protein | - | - |
| Q5TA81 | LCE2C | Late cornified envelope protein 2C | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catitti, G.; Cufaro, M.C.; De Bellis, D.; Cicalini, I.; Vespa, S.; Tonelli, F.; Miscia, G.; Secondi, L.; Simeone, P.; De Laurenzi, V.; et al. Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation. Int. J. Mol. Sci. 2022, 23, 14913. https://doi.org/10.3390/ijms232314913
Catitti G, Cufaro MC, De Bellis D, Cicalini I, Vespa S, Tonelli F, Miscia G, Secondi L, Simeone P, De Laurenzi V, et al. Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation. International Journal of Molecular Sciences. 2022; 23(23):14913. https://doi.org/10.3390/ijms232314913
Chicago/Turabian StyleCatitti, Giulia, Maria Concetta Cufaro, Domenico De Bellis, Ilaria Cicalini, Simone Vespa, Federico Tonelli, Giulia Miscia, Lorenzo Secondi, Pasquale Simeone, Vincenzo De Laurenzi, and et al. 2022. "Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation" International Journal of Molecular Sciences 23, no. 23: 14913. https://doi.org/10.3390/ijms232314913







