Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro
Abstract
:1. Introduction
2. Results
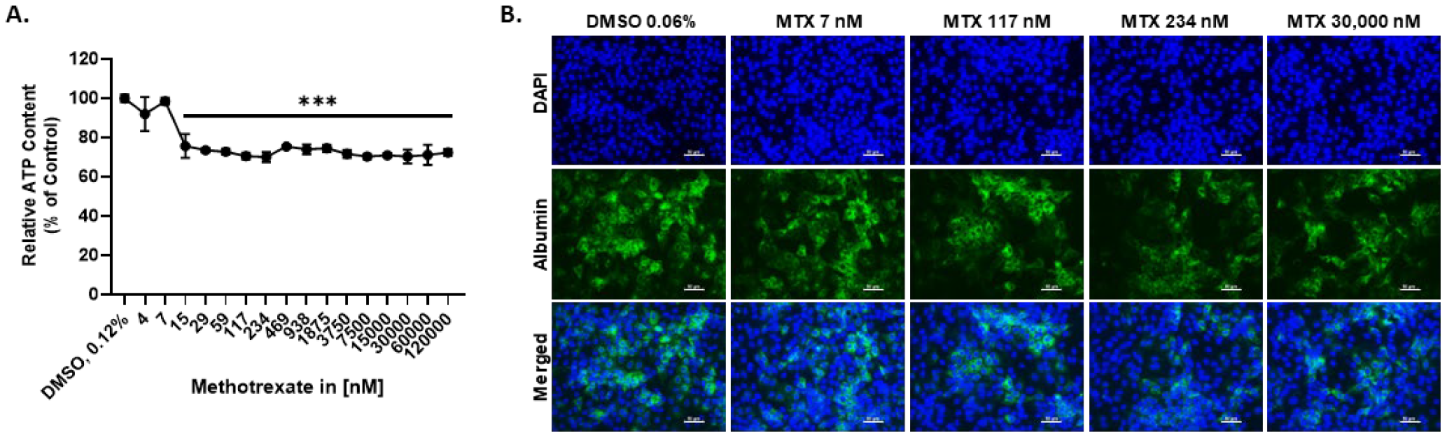
2.1. MTX Causes a Slight Reduction in the Viability of HepaRG at Therapeutic-Relevant Concentrations
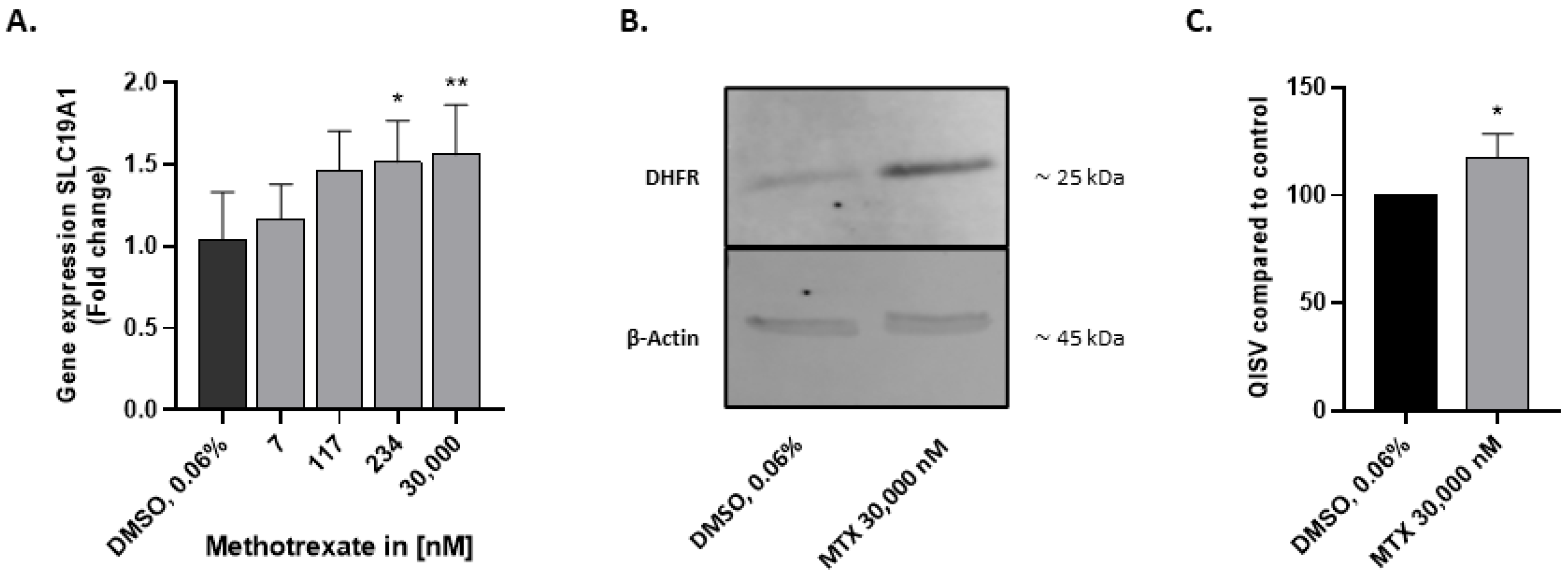
2.2. HepaRG Express Folate Transporter SLC19A1 and DHFR
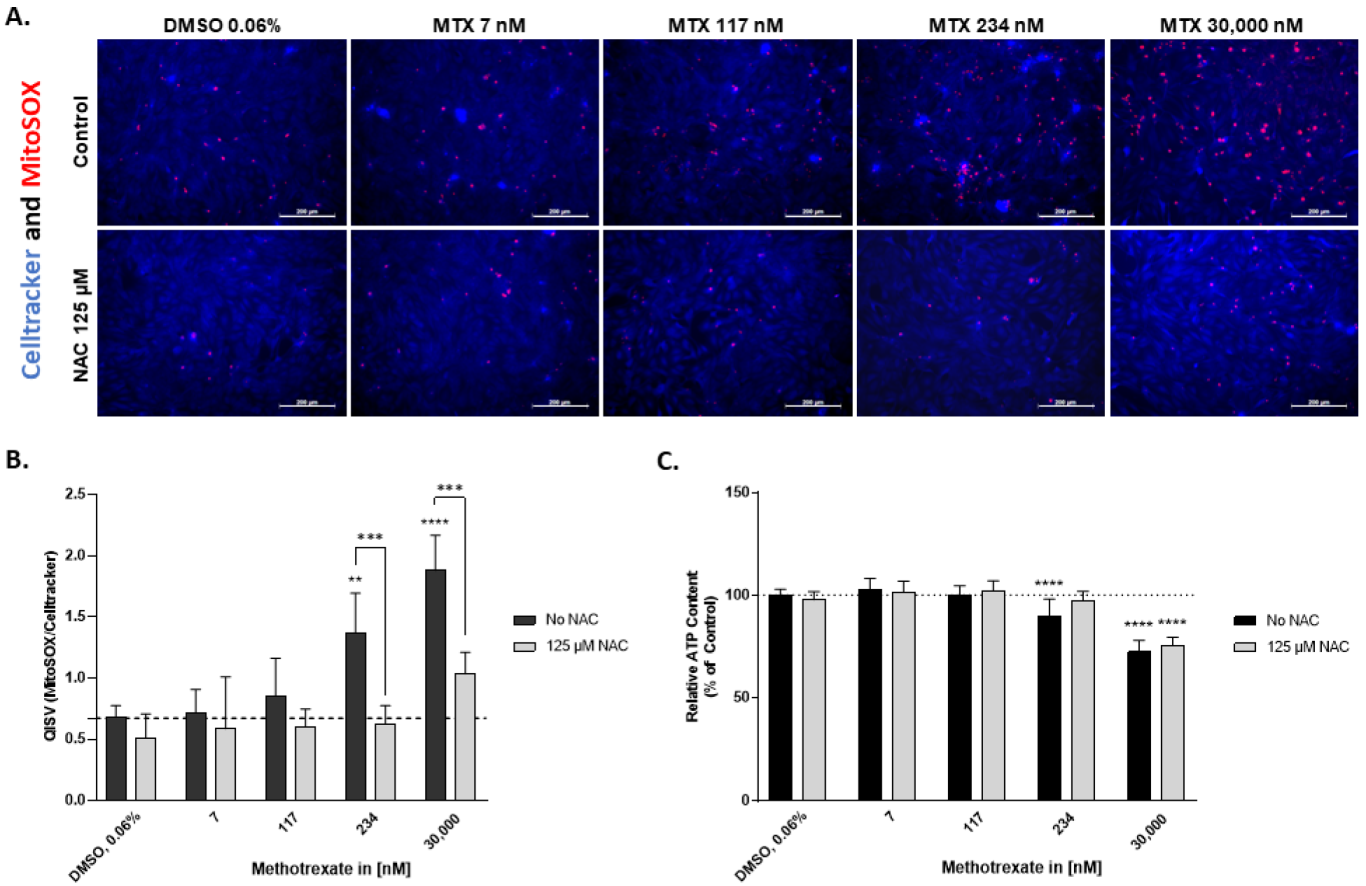
2.3. N-Acetylcysteine Alleviates Superoxide Formation Induced by MTX in HepaRG
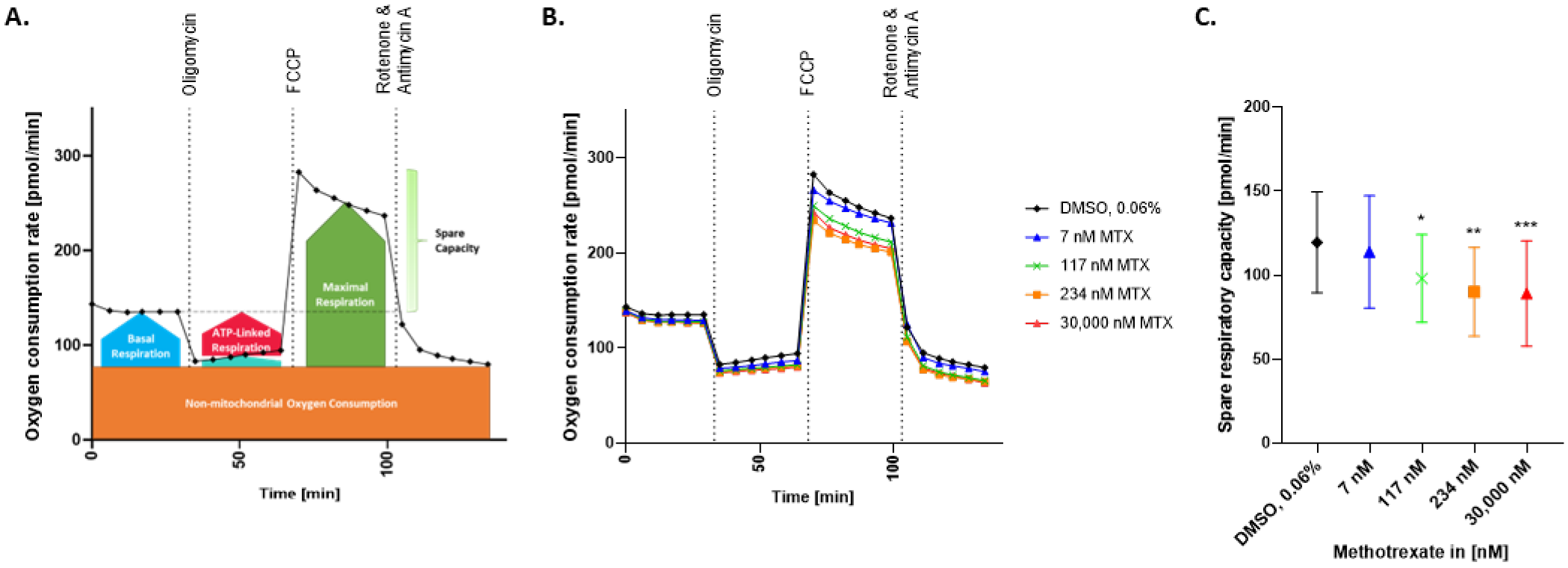
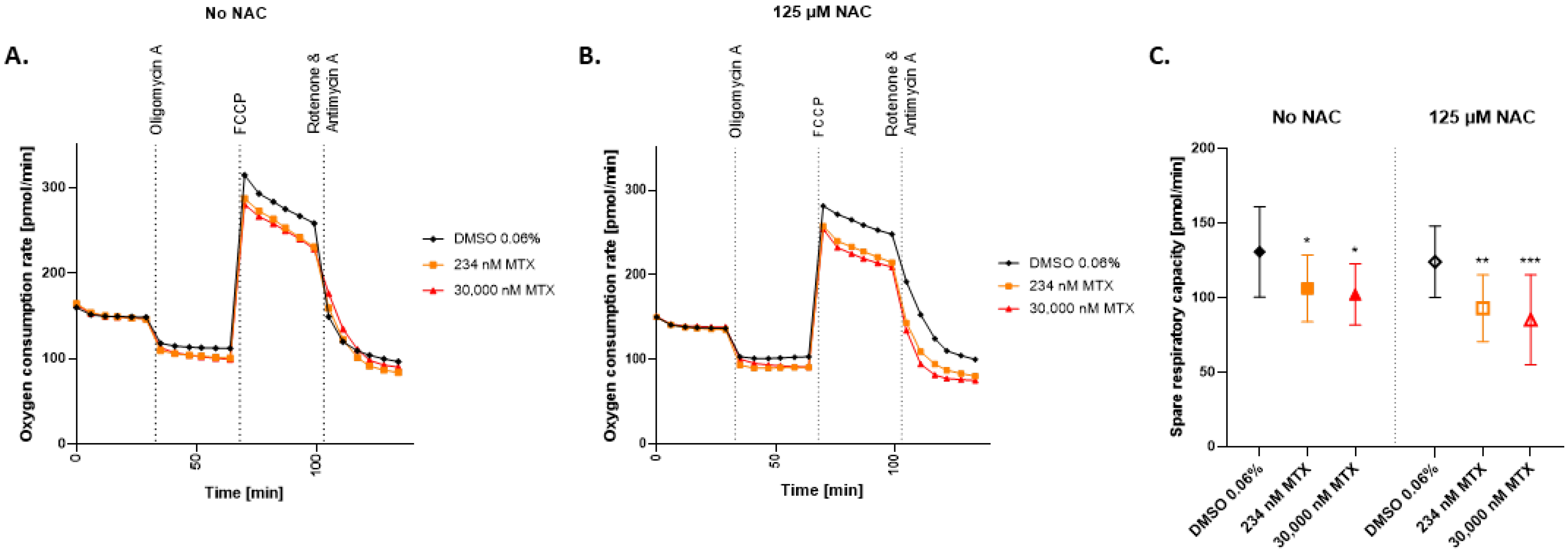
2.4. Spare Respiratory Capacity Is Reduced in HepaRG Exposed to MTX
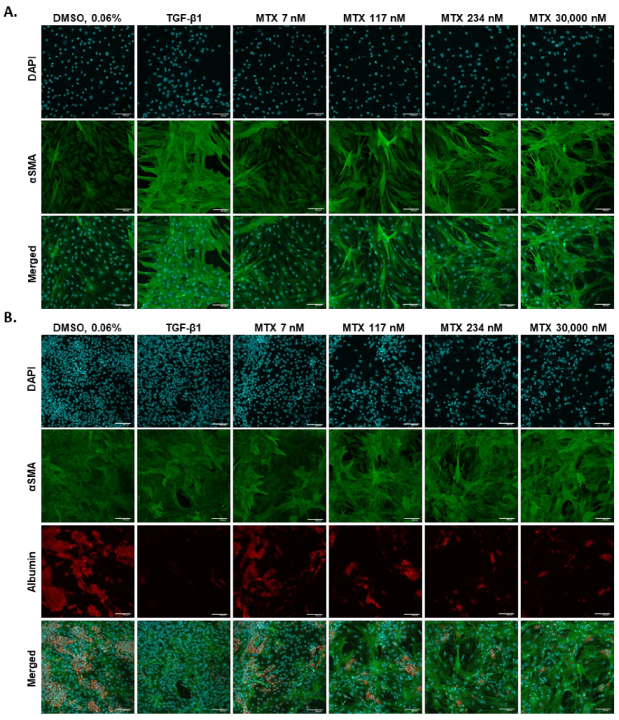
2.5. 2D hTERT-HSC Are Activated by MTX Treatment in Mono- and Co-Cultures with HepaRG
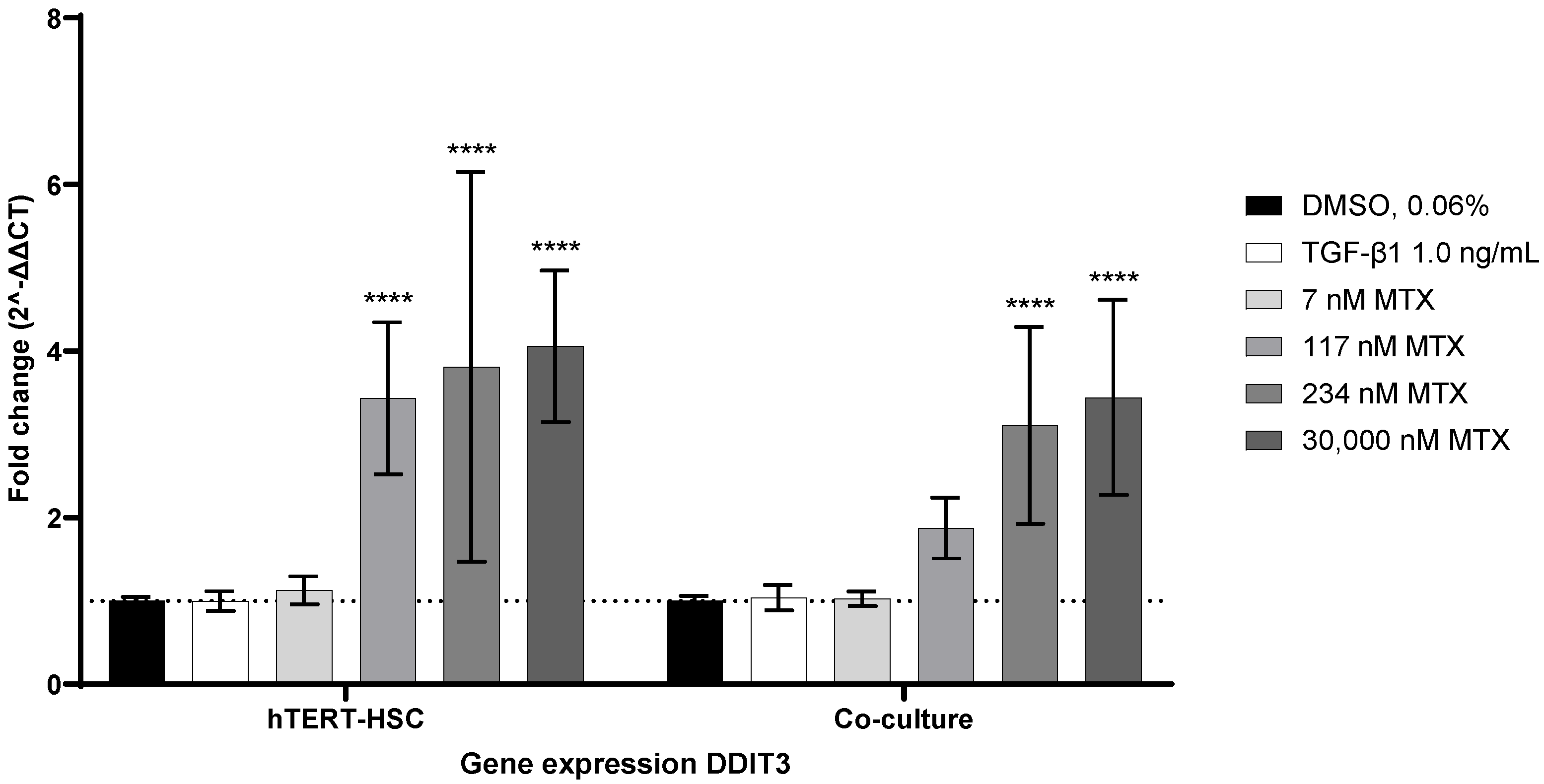
2.6. MTX-Induced ER-Stress as a Possible Activator of hTERT-HSC
3. Discussion
4. Materials and Methods
4.1. Human Cell Lines and Cell Seeding
4.2. Cell Treatments
4.3. Cell Viability Assays
4.4. Immunohistochemistry
4.5. Gene Expression Analysis
4.6. Western Blot Analysis
4.7. MitoSOXTM Red Mitochondrial Superoxide Indicator
4.8. Seahorse XF96 Analyzer
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babai, S.; Auclert, L.; Le-Louët, H. Safety data and withdrawal of hepatotoxic drugs. Therapies 2021, 76, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Alarcóan, G.S.; Tracy, I.C.; Blackburn, W.D., Jr. Methotrexate in rheumatoid arthritis. Toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum. 1989, 32, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Thorn, C.F.; Yang, J.J.; Ulrich, C.M.; French, D.; Zaza, G.; Dunnenberger, H.M.; Marsh, S.; McLeod, H.L.; Giacomini, K.; et al. PharmGKB summary: Methotrexate pathway. Pharmacogenet. Genom. 2011, 21, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bywater, M.J.; Poortinga, G.; Sanij, E.; Hein, N.; Peck, A.; Cullinane, C.; Wall, M.; Cluse, L.; Drygin, D.; Anderes, K.; et al. Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation of p53. Cancer Cell 2012, 22, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Berdis, A.J. Inhibiting DNA Polymerases as a Therapeutic Intervention against Cancer. Front. Mol. Biosci. 2017, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- Pivovarov, K.; Zipursky, J.S. Low-dose methotrexate toxicity. Can. Med. Assoc. J. 2019, 191, E423. [Google Scholar] [CrossRef] [Green Version]
- Howard, S.C.; McCormick, J.; Pui, C.-H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Rademaker, M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: Utility of transient elastography. Psoriasis Targets Ther. 2018, 8, 21–29. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.; Burden, A.D. Noninvasive monitoring for methotrexate hepatotoxicity. Br. J. Dermatol. 2005, 152, 405–408. [Google Scholar] [CrossRef]
- Lertnawapan, R.; Chonprasertsuk, S.; Siramolpiwat, S. Association between cumulative methotrexate dose, non-invasive scoring system and hepatic fibrosis detected by Fibroscan in rheumatoid arthritis patients receiving methotrexate. Int. J. Rheum. Dis. 2019, 22, 214–221. [Google Scholar] [CrossRef]
- Horvat, T.; Landesmann, B.; Lostia, A.; Vinken, M.; Munn, S.; Whelan, M. Adverse outcome pathway development from protein alkylation to liver fibrosis. Arch. Toxicol. 2017, 91, 1523–1543. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.-T.; Chen, Y.-H.; Lin, C.-H.; Chen, D.-Y. Methotrexate is not associated with increased liver cirrhosis in a population-based cohort of rheumatoid arthritis patients with chronic hepatitis C. Sci. Rep. 2016, 6, 33104. [Google Scholar] [CrossRef] [Green Version]
- Aithal, G.P.; Haugk, B.; Das, S.; Card, T.; Burt, A.D.; Record, C.O. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: Are serial liver biopsies justified? Aliment. Pharmacol. Ther. 2004, 19, 391–399. [Google Scholar] [CrossRef]
- Berends, M.A.M.; van Oijen, M.G.H.; Snoek, J.; van de Kerkhof, P.C.M.; Drenth, J.P.H.; Han van Krieken, J.; de Jong, E.M.G.J. Reliability of the Roenigk Classification of Liver Damage After Methotrexate Treatment for Psoriasis: A Clinicopathologic Study of 160 Liver Biopsy Specimens. Arch. Dermatol. 2007, 143, 1515–1519. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Williams, M.N.; Poe, M.; Greenfield, N.J.; Hirshfield, J.M.; Hoogsteen, K. Methotrexate Binding to Dihydrofolate Reductase from a Methotrexate-resistant Strain of Escherichia coli. J. Biol. Chem. 1973, 248, 6375–6379. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; Franca, M.L.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [Green Version]
- Haskó, G.; Cronstein, B. Regulation of Inflammation by Adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef] [Green Version]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef]
- Mehrzadi, S. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed. Pharmacother. 2018, 7, 233–239. [Google Scholar] [CrossRef]
- Kalantari, H.; Asadmasjedi, N.; Abyaz, M.R.; Mahdavinia, M.; Mohammadtaghvaei, N. Protective effect of inulin on methotrexate- induced liver toxicity in mice. Biomed. Pharmacother. 2019, 110, 943–950. [Google Scholar] [CrossRef]
- Bu, T. Hepatoprotective effect of rhein against methotrexate-induced liver toxicity. Eur. J. Pharmacol. 2018, 8, 266–273. [Google Scholar] [CrossRef]
- Kremer, J.M.; Galivan, J.; Streckfuss, A.; Kamen, B. Methotrexate Metabolism Analysis in Blood and Liver of Rheumatoid Arthritis PatientS Association with Hepatic Folate Deficiency and Formation of Polyglutamates. Arthritis Rheum. 1986, 29, 832–835. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Ghafar, O.A.; Hassanein, E.H.M.; Ali, F.E.M.; Omar, Z.M.M.; Rashwan, E.K.; Mohammedsaleh, Z.M.; Sayed, A.M. Hepatoprotective effect of acetovanillone against methotrexate hepatotoxicity: Role of Keap-1/Nrf2/ARE, IL6/STAT-3, and NF-κB/AP-1 signaling pathways. Phytother. Res. 2022, 36, 488–505. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-Glycyrrhetinic acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef]
- Elango, T.; Dayalan, H.; Gnanaraj, P.; Malligarjunan, H.; Subramanian, S. Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin. Exp. Med. 2014, 14, 431–437. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, X.; Wang, J.; Miao, Y.; Lu, Y.; Li, B. The HepaRG cell line, a superior in vitro model to L-02, HepG2 and hiHeps cell lines for assessing drug-induced liver injury. Cell Biol. Toxicol. 2016, 32, 37–59. [Google Scholar] [CrossRef]
- Rubin, K.; Janefeldt, A.; Andersson, L.; Berke, Z.; Grime, K.; Andersson, T.B. HepaRG Cells as Human-Relevant In Vitro Model to Study the Effects of Inflammatory Stimuli on Cytochrome P450 Isoenzymes. Drug Metab. Dispos. 2015, 43, 119–125. [Google Scholar] [CrossRef]
- Schnabl, B.; Choi, Y.H.; Olsen, J.C.; Hagedorn, C.H.; Brenner, D.A. Immortal Activated Human Hepatic Stellate Cells Generated by Ectopic Telomerase Expression. Lab. Investig. 2002, 82, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestigiacomo, V.; Weston, A.; Messner, S.; Lampart, F.; Suter-Dick, L. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and Nrf2 activation in a human 3D-multicellular model of liver fibrosis. PLoS ONE 2017, 12, e0179995. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M. Adverse Outcome Pathways and Drug-Induced Liver Injury Testing. Chem. Res. Toxicol. 2015, 28, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis: ACR RA Treatment Recommendations. Arthritis Care Res. 2016, 68, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Wippel, B.; Gundle, K.R.; Dang, T.; Paxton, J.; Bubalo, J.; Stork, L.; Fu, R.; Ryan, C.W.; Davis, L.E. Safety and efficacy of high-dose methotrexate for osteosarcoma in adolescents compared with young adults. Cancer Med. 2019, 8, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Herman, R.A.; Veng-Pedersen, P.; Hoffman, J.; Koehnke, R.; Furst, D.E. Pharmacokinetics of Low-Dose Methotrexate in Rheumatoid Arthritis Patients. J. Pharm. Sci. 1989, 78, 165–171. [Google Scholar] [CrossRef]
- Winick, N.; Shuster, J.J.; Bowman, W.P.; Borowitz, M.; Farrow, A.; Jacaruso, D.; Buchanan, G.R.; Kamen, B.A. Intensive oral methotrexate protects against lymphoid marrow relapse in childhood B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 1996, 14, 2803–2811. [Google Scholar] [CrossRef]
- Sinnett, M.; Groff, G.; Raddatz, D.; Franck, W.; Bertino, J., Jr. Methotrexate pharmacokinetics in patients with rheumatoid arthritis. J. Rheumatol. 1989, 16, 745–748. [Google Scholar]
- Zelcer, S.; Kellick, M.; Wexler, L.H.; Shi, W.; Sankaran, M.; Lo, S.; Healey, J.; Huvos, A.G.; Meyers, P.A.; Gorlick, R. Methotrexate levels and outcome in osteosarcoma: Methotrexate Levels and Osteosarcoma Outcome. Pediatr. Blood Cancer 2005, 44, 638–642. [Google Scholar] [CrossRef]
- De Vries, H.S.; de Heij, T.; Roelofs, H.M.J.; te Morsche, R.H.M.; Peters, W.H.M.; de Jong, D.J. Infliximab Exerts No Direct Hepatotoxic Effect on HepG2 Cells In Vitro. Dig. Dis. Sci. 2012, 57, 1604–1608. [Google Scholar] [CrossRef] [Green Version]
- Gawandi, S.J.; Desai, V.G.; Joshi, S.; Shingade, S.; Pissurlenkar, R.R. Assessment of elementary derivatives of 1,5-benzodiazepine as anticancer agents with synergy potential. Bioorg. Chem. 2021, 117, 105331. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Abdollah, M.R.A.; Elmazar, M.M.; El-Fawal, H.A.N.; Abdelnaser, A. Effects of Metformin Combined with Antifolates on HepG2 Cell Metabolism and Cellular Proliferation. Front. Oncol. 2022, 12, 828988. [Google Scholar] [CrossRef]
- Allard, J.; Bucher, S.; Massart, J.; Ferron, P.-J.; Le Guillou, D.; Loyant, R.; Daniel, Y.; Launay, Y.; Buron, N.; Begriche, K.; et al. Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in HepaRG cells: Proof of multiple mechanism-based toxicity. Cell Biol. Toxicol. 2021, 37, 151–175. [Google Scholar] [CrossRef]
- Messner, C.J.; Schmidt, S.; Özkul, D.; Gaiser, C.; Terracciano, L.; Krähenbühl, S.; Suter-Dick, L. Identification of miR-199a-5p, miR-214-3p and miR-99b-5p as Fibrosis-Specific Extracellular Biomarkers and Promoters of HSC Activation. Int. J. Mol. Sci. 2021, 22, 9799. [Google Scholar] [CrossRef]
- Tag, H.M. Hepatoprotective effect of mulberry (Morus nigra) leaves extract against methotrexate induced hepatotoxicity in male albino rat. BMC Complement. Altern. Med. 2015, 15, 252. [Google Scholar] [CrossRef] [Green Version]
- Shergy, W.; Pisetsky, D. Methotrexate-Associated Hepatotoxicity: Retrospective Analysis of 210 Patients with Rheumatoid Arthritis. Am. J. Med. 1988, 85, 771–774. [Google Scholar] [CrossRef]
- Matherly, L.H.; Hou, Z.; Deng, Y. Human reduced folate carrier: Translation of basic biology to cancer etiology and therapy. Cancer Metastas. Rev. 2007, 26, 111–128. [Google Scholar] [CrossRef]
- O’Connor, C.; Wallace-Povirk, A.; Ning, C.; Frühauf, J.; Tong, N.; Gangjee, A.; Matherly, L.H.; Hou, Z. Folate transporter dynamics and therapy with classic and tumor-targeted antifolates. Sci. Rep. 2021, 11, 6389. [Google Scholar] [CrossRef]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takemura, Y.; Ohnuma, T. Variable expression of RFC1 in human leukemia cell lines resistant to antifolates. Cancer Lett. 1998, 124, 135–142. [Google Scholar] [CrossRef]
- Rose, S.; Cuvellier, M.; Ezan, F.; Carteret, J.; Bruyère, A.; Legagneux, V.; Nesslany, F.; Baffet, G.; Langouët, S. DMSO-free highly differentiated HepaRG spheroids for chronic toxicity, liver functions and genotoxicity studies. Arch. Toxicol. 2022, 96, 243–258. [Google Scholar] [CrossRef]
- Galbiatti, A.L.S.; Castro, R.; Caldas, H.C.; Padovani, J.A.; Pavarino, É.C.; Goloni-Bertollo, E.M. Alterations in the expression pattern of MTHFR, DHFR, TYMS, and SLC19A1 genes after treatment of laryngeal cancer cells with high and low doses of methotrexate. Tumor Biol. 2013, 34, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Obuchi, W.; Ohtsuki, S.; Uchida, Y.; Ohmine, K.; Yamori, T.; Terasaki, T. Identification of Transporters Associated with Etoposide Sensitivity of Stomach Cancer Cell Lines and Methotrexate Sensitivity of Breast Cancer Cell Lines by Quantitative Targeted Absolute Proteomics. Mol. Pharmacol. 2013, 83, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skacel, N.; Menon, L.G.; Mishra, P.J.; Peters, R.; Banerjee, D.; Bertino, J.R.; Abali, E.E. Identification of Amino Acids Required for the Functional Up-regulation of Human Dihydrofolate Reductase Protein in Response to Antifolate Treatment. J. Biol. Chem. 2005, 280, 22721–22731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertino, J.R.; Donohue, D.R.; Gabrio, B.W.; Silber, R.; Alenty, A.; Meyer, M.; Huennekens, F.M. Increased Level of Dihydrofolic Reductase in Leucocytes of Patients Treated with Amethopterin. Nature 1962, 193, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Abali, E.E.; Skacel, N.E.; Celikkaya, H.; Hsieh, Y. Chapter 9 Regulation of Human Dihydrofolate Reductase Activity and Expression. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2008; Volume 79, pp. 267–292. ISBN 978-0-12-374232-2. [Google Scholar]
- Scionti, I.; Michelacci, F.; Pasello, M.; Hattinger, C.M.; Alberghini, M.; Manara, M.C.; Bacci, G.; Ferrari, S.; Scotlandi, K.; Picci, P.; et al. Clinical impact of the methotrexate resistance-associated genes C-MYC and dihydrofolate reductase (DHFR) in high-grade osteosarcoma. Ann. Oncol. 2008, 19, 1500–1508. [Google Scholar] [CrossRef]
- Rodenhuis, S.; Kremer, J.M.; Bertino, J.R. Increase of dihydrofolate reductase in peripheral blood lymphocytes of rheumatoid arthritis patients treated with low-dose oral methotrexate. Arthritis Rheum. 1987, 30, 369–374. [Google Scholar] [CrossRef]
- Hall, M.J.; Lawrence, D.A.; Lansiedel, J.C.; Walsh, A.C.; Comstock, L.L.; Kremer, J.M. Long-term exposure to methotrexate induces immunophenotypic changes, decreased methotrexate uptake and increased dihydrofolate gene copy number in jurkat T cells. Int. J. Immunopharmacol. 1998, 19, 709–720. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Yépez, V.A.; Kremer, L.S.; Iuso, A.; Gusic, M.; Kopajtich, R.; Koňaříková, E.; Nadel, A.; Wachutka, L.; Prokisch, H.; Gagneur, J. OCR-Stats: Robust estimation and statistical testing of mitochondrial respiration activities using Seahorse XF Analyzer. PLoS ONE 2018, 13, e0199938. [Google Scholar] [CrossRef] [Green Version]
- Seahorse XF Cell Mito Stress Test Kit User Guide; Agilent Technologies: Santa Clara, CA, USA, 2019.
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Sanz-García, C.; Fernández-Iglesias, A.; Gracia-Sancho, J.; Arráez-Aybar, L.A.; Nevzorova, Y.A.; Cubero, F.J. The Space of Disse: The Liver Hub in Health and Disease. Livers 2021, 1, 3–26. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Chan, E.S.L.; Cronstein, B.N. Adenosine in fibrosis. Mod. Rheumatol. 2010, 20, 114–122. [Google Scholar] [CrossRef]
- An, J. The acitretin and methotrexate combination therapy for psoriasis vulgaris achieves higher effectiveness and less liver fibrosis. Pharmacol. Res. 2017, 121, 158–168. [Google Scholar] [CrossRef]
- Brovold, M.; Keller, D.; Soker, S. Differential fibrotic phenotypes of hepatic stellate cells within 3D liver organoids. Biotechnol. Bioeng. 2020, 117, 2516–2526. [Google Scholar] [CrossRef]
- Koo, J.H.; Lee, H.J.; Kim, W.; Kim, S.G. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology 2016, 150, 181–193.e8. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Liu, B.; Liu, R.; Chen, Y.; Li, C.; Liu, G.; Song, Z.; Lu, C.; Lu, A.; et al. Interaction of nobiletin with methotrexate ameliorates 7-OH methotrexate-induced nephrotoxicity through endoplasmic reticulum stress-dependent PERK/CHOP signaling pathway. Pharmacol. Res. 2021, 165, 105371. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Wink, S.; Herpers, B.; Benedetti, G.; Hadi, M.; de Bont, H.; Groothuis, G.; Luijten, M.; Danen, E.; de Graauw, M.; et al. Drug-Induced Endoplasmic Reticulum and Oxidative Stress Responses Independently Sensitize Toward TNFα-Mediated Hepatotoxicity. Toxicol. Sci. 2014, 140, 144–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Sun, W.; Jing, X.; Zhang, Q.; Huang, H.; Xu, Z. Endoplasmic reticulum stress modulates the fate of lung resident mesenchymal stem cell to myofibroblast via C/EBP homologous protein during pulmonary fibrosis. Stem Cell Res. Ther. 2022, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
| Protein of Interest | Primary Antibody | Secondary Antibody |
|---|---|---|
| αSMA | Mouse polyclonal antibody (Sigma, Taufkirchen, Germany, A5228)/1:400 | Goat anti-mouse Alexafluor 488 (Sigma, Taufkirchen, Germany, A-11017)/1:1000 |
| Albumin | Rabbit monoclonal antibody (Abcam, Cambridge, United Kingdom, ab207327)/1:800 | Goat anti-rabbit Alexafluor 546 (Sigma, Taufkirchen, Germany, A-11071)/1:1000 |
| Gene of Interest | Abbreviation | Ref. Nr |
|---|---|---|
| Beta-2-Microglobulin | B2M | Hs00187842_m1 |
| Solute Carrier Family 19 Member 1 (folate transporter 1) | SLC19A1 | Hs00953344_m1 |
| DNA Damage Inducible Transcript 3 | DDIT3 | Hs00358796_g1 |
| Protein of Interest | Primary Antibody | Secondary Antibody |
|---|---|---|
| Dihydrofolate reductase (DHFR) | Rabbit polyclonal antibody (ThermoFisherScientific, Reinach, Switzerland, PA5-90913)/1:400 | Donkey anti-rabbit IRDye® 680RD (LI-COR Biosciences, Bad Homburg, Germany, 926-68073)/1:20,000 |
| Beta-actin (β-actin) | Mouse monoclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany, sc-47778)/1:1000 | Goat anti-mouse IRDye® 800CW (LI-COR Biosciences, Bad Homburg, Germany, 926-32210)/1:20,000 |
| Condition | Measurement Timeline (min) | Electron Transport Chain Target | Effect on OCR |
|---|---|---|---|
| Basal OCR | 0, 6, 12, 17, 23, 29 | ||
| Oligomycin 1 µM | 35, 41, 47, 52, 58, 64 | ATP Synthase (Complex V) | Decrease |
| Cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) 2.5 µM | 70, 76, 82, 87, 93, 99 | Inner mitochondrial membrane | Increase |
| Rotenone 0.5 µM + Antimycin A 0.5 µM | 105, 111, 117, 123, 128, 134 | Complex I and III (respectively) | Decrease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 15116. https://doi.org/10.3390/ijms232315116
Schmidt S, Messner CJ, Gaiser C, Hämmerli C, Suter-Dick L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. International Journal of Molecular Sciences. 2022; 23(23):15116. https://doi.org/10.3390/ijms232315116
Chicago/Turabian StyleSchmidt, Saskia, Catherine Jane Messner, Carine Gaiser, Carina Hämmerli, and Laura Suter-Dick. 2022. "Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro" International Journal of Molecular Sciences 23, no. 23: 15116. https://doi.org/10.3390/ijms232315116
APA StyleSchmidt, S., Messner, C. J., Gaiser, C., Hämmerli, C., & Suter-Dick, L. (2022). Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. International Journal of Molecular Sciences, 23(23), 15116. https://doi.org/10.3390/ijms232315116






