1. Introduction
It has been established that feto-placental development and functioning are vulnerable to maternal risk factors (MRFs) such as diabetes, obesity, and hypertension. Impaired maternal -fetal environment, linked to these MRFs, has been shown to be associated with placental vascular malformations and dysfunction [
1,
2,
3].
This in turn creates an unfavorable environment for healthy fetal development. Poor neurological outcomes have been reported in offspring with prenatal MRFs [
4,
5,
6]. However, there is very scant evidence on relationship between MRFs, placental function and brain development during the critical fetal period.
In parallel, recent histopathological evidence has reported that placentas, in CHD, show a spectrum of pathological structural adaptations such as infarctions, chorangiosis, thrombosis and hypomature villi, suggesting vascular-related vulnerability and have been linked to the presence of postnatal brain injury, particularly in left ventricular outflow track obstruction heart lesion subtypes [
7,
8,
9,
10,
11]. While these placental pathologies have been shown to correlate with newborn outcomes such as birthweight [
8] and acquired postnatal brain injury, few direct associations have been found between placental abnormalities and neurodevelopmental outcomes in CHD. Russel and Gaynor [
12,
13] et al. recently demonstrated that damaging variants in proangiogenic genes may impact placental function and are associated with impaired fetal growth in CHD pregnancies. There is also recent evidence of overall dysregulation of placental and fetal brain angiogenesis in CHD, suggesting that these fetuses may have an intrinsic placental angiogenic impairment that could contribute to impaired functional brain development [
14]. There is, also, emerging evidence that CHD together with MRF is associated with impaired placental vasculature and substantially increased risk of mortality after cardiac surgery [
15,
16,
17,
18]. Taken together, these studies support the overarching hypothesis that MRFs and CHD would alter intrinsic placental and brain function.
MRI studies of placenta function have reported altered placental response in CHD pregnancies when the mother is subjected to external hyperoxia stimuli. More studies using hyperoxia stimuli have noted global reductions in perfusion and T2* in CHD placentas [
19,
20]. However, hyperoxygenation-induced vasoconstriction has varying effects on the intrinsic vascular properties in the placenta and the fetus [
21] and therefore may mask the very differences we seek to measure. Studies using hyperoxygenation have shown no changes in fetal oxygen measurements. Models of placental oxygenation in these studies work under the assumption that there is no physiological response to hyperoxygenation in the maternal-fetal system [
22]. However, it is well-documented that hyperoxygenation elicits various physiological adaptations such as maternal blood redistribution, changes to fetal heart rate variability, vasoconstriction of fetal vessels, etc. It has also been reported that these responses are variable based on multiple clinical and sub-clinical factors [
23,
24]. These findings suggest that hyperoxygenation may have multiple physiological effects on the fetal-maternal system. Placental measures using intrinsic BOLD measurements under norm-oxy conditions are not likely to be impacted by measurement errors from these confounding physiological alterations. Moreover, it has been shown that baseline T2* measurement are predictors of hyper-oxygenated responses, suggesting that baseline intrinsic BOLD properties are likely to harbor important physiological characteristics [
25]. Additionally, these hyperoxygenation methods are very susceptible to motion artifacts that are inevitable in fetal imaging, require higher degrees of logistical and technological expertise making them non-ideal for translation across large scale studies. Intrinsic BOLD, which has the potential of overcoming some of these limitations, may be a physiological surrogate for oxygenation and vascularity in the placenta and the fetal brain. However, to date, few studies have captured intrinsic BOLD signal properties of placenta and fetal brain in CHD pregnancies.
To address this knowledge gap, we quantitated, intrinsic placental and fetal brain BOLD signal properties (without the use of maternal hyperoxygenation) in prospectively recruited mothers-fetal dyads with variable MRF from a major referral fetal cardiology center. We developed an innovative acquisition and post-processing technique by leveraging previously validated quantitative metrics to capture both temporal (temporal variance) and spatial (spatial variance) properties of intrinsic BOLD signal in placenta and fetal brain. We recruited a sample of both CHD and non-CHD patients with varying levels of MRF. Our primary purpose was to determine the effect of MRF and CHD on intrinsic BOLD signal in the placenta and fetal brain.
2. Methods
2.1. Subject Population
This prospective study, conducted between 2014 and 2018, was approved by the institutional review board at the University of Pittsburgh.
Table 1 shows the demographic characteristics of the participants in this study. Participants in the CHD group were enrolled from one of the following three pediatric clinics: (1) High risk pregnancy center at Magee Women’s Hospital (2) Fetal cardiology clinic at Children’s Hospital of Pittsburgh and (3) an affiliated fetal cardiology clinic (Wexford, PA, USA). Inclusion criteria for the CHD group were: (1) pregnant women whose fetuses were diagnosed with complex CHD based on fetal echocardiograms; (2) in their third trimester of pregnancy—27–42 gestational weeks (GW); and (3) English speaking (for obtaining informed consent). Participants in the non-CHD group were enrolled using a variety of methods including flyers, self-referral via a university-sponsored medical research participation database and referral from fetal cardiology clinic after negative echocardiogram. Inclusion criteria (2) and (3) are identical to the CHD group but non-CHD participants were required to have no documented history of CHD. Some enrolled non-CHD participants underwent fetal echocardiogram, which was normal, due to increased risk for CHD (e.g., positive family history). Exclusion criteria for both groups were: (1) Focal neurological abnormality on postnatal clinical follow up; (2) Chronic seizures post-birth; (3) Severe congenital brain malformation detected on prenatal imaging; (4) Significant chromosomal abnormality/syndrome; (5) Sepsis or other infection; (6) Significant birth trauma and/or hypoxic ischemic injury; (7) Contraindication to MRI (e.g., claustrophobia, pacemaker).
2.2. Clinical Data
The electronic medical record (EMR) was reviewed for all participants and selected demographic variables were extracted including maternal age, gestational age (GA) at the time of the MRI. CHD lesions were classified by an experienced pediatric cardiologist (JVS) after review of pre and postnatal echocardiogram data as: (a) one of 7 categories CHD lesions listed below; (b) single versus double ventricle cardiac anatomy; (c) cyanotic versus acyanotic lesions. The seven cardiac lesion categories included: Hypoplastic left heart syndrome, Transposition of the great arteries, Double outlet right ventricle, Tetralogy of Fallot, Complex single ventricle (TA, PA), Coarctation of the aorta and Other (AVC, VSD, ASD, APVR). Cyanotic lesions included CHD lesions that were characterized by postnatal mixing of pulmonary venous and systemic venous blood resulting in reduced oxygen saturation. CHD lesions such as transposition of the great arteries (TGA), single ventricles, tetralogy of Fallot (TOF), and total anomalous pulmonary venous return (TAPVR) were considered cyanotic types of CHD lesions. Coarctation or isolated shunt lesions were considered acyanotic types of CHD lesions. Distribution of cardiac lesion types among study participants are summarized in
Table 1.
2.3. Maternal Risk Factors
Maternal risk factor data (
Table 2) was collected from the Magee Obstetric Medical and Infant Database (MOMI) in conjunction with prospective data collection as part of the recruitment. In 1995, Magee-Womens Hospital established the MOMI database to integrate data on maternal, fetal, and neonatal outcomes. This data is available to researchers with institutional review board approval for secondary analyses. The Medical Records Department abstracts maternal information, and infant outcome information comes from the electronic medical records. International Classification of Diseases (ICD) 9 and 10 diagnostic information is recorded on discharge and these data are coded into the MOMI database. Clinical data specifically collected for this study included the pre-gravid weight/height, presence of diabetes, and hypertension. Patients with gestational diabetes, type 1 diabetes mellitus, and type 2 diabetes mellitus were grouped into a single “maternal diabetes” group. Patients with chronic hypertension and gestational hypertension (including pre-eclampsia) were grouped together as well. These risk factors were determined to be present if this diagnosis was indicated in the MOMI database and then checked for explicitly mentioning in a prenatal obstetrical visit note. Maternal obesity was defined as a BMI (kg/m
2) of greater than 30 based on height and weight measurements taken prior to pregnancy (within 1 year). Pregnancy and delivery characteristics of the cohort are shown in
Supplemental Table S1A. In addition, placental weight and other birth characteristics of the infant were collected from both the MOMI dataset and the Children’s Hospital medical records (
Table 3).
2.4. Placental Pathology
Placental histopathologic and gross examinations were collected for all deliveries at our institution for which such examination was indicated clinically. Pathology examinations were performed by dedicated perinatal pathologists according to the standard clinical protocol used at our institution. Pathology reports (
Supplemental Tables S1B and S2) were reviewed for (1) placental weight and (2) pathologic lesions including placental infarcts, vascular thrombosis, chorangiosis, and chorioamnionitis as previously described in the literature.
2.5. MRI Image Acquisition
Maternal subjects were imaged using a 3T Skyra (Siemens, Ehrlangen Germany) with an 18-channel receive-transmit phased body array. Patients were imaged supine, if tolerated and lateral decubitus positions, depending on comfort. All patients were imaged without contrast or sedation. Placental Imaging: T2 HASTE was obtained through the uterus, for anatomic localization, with the following scan parameters: TR = 1110, TE = 78, flip angle = 142 deg. Echo-planar imaging (EPI) BOLD images were obtained through the placenta with the following scan parameters: FOV = 300 mm TR = 2280, TE = 32, flip angle = 80 deg., with an in-plane resolution of 4.7 × 4.7 mm2 and slice thickness of 3 mm. 100-time frames were attempted on each mother over 3 min and 53 s. Placental BOLD sequences were repeated twice and the series with the least amount of motion observed was used for analysis.
2.6. Fetal Brain Imaging
Axial T2 HASTE was obtained through the fetal brain, for anatomic localization, with the following scan parameters: TR = 1110, TE = 78, flip angle = 142 deg. Echo-planar imaging (EPI) BOLD images were obtained through the fetal brain with the following scan parameters: FOV = 320 mm TR = 2280, TE = 32, flip angle = 80 deg., with an in-plane resolution of 5 × 5 mm2 and slice thickness of 3 mm. 100-time frames were attempted on each mother over 3 min and 53 s.
2.7. Image Analysis
2.7.1. Image Pre-Processing
Placental imaging requires extensive pre-processing before analysis because of the unique artifacts caused by spontaneous feto-maternal motion. To ensure fidelity of data across the dataset, each image was reviewed by senior radiology resident (AE) and research associates (JW, WR) with final image review by an attending pediatric radiologist with 20 years of fetal imaging experience (AP). The first step of image analysis involved manual review of each study for imaging artifacts. Imaging artifacts primarily included (1) excessive motion of the fetus, uterus and/or mother and (2) standing wave (“dielectric artifact”). Studies were excluded when there was excessive artifact such that post-processing techniques were likely to fail. To mitigate standing wave artifact, the placement of a saline filled pad on the anterior abdominal wall was attempted in an initial subset of patients. Ultimately, saline bag placement did not significantly affect our rate of standing wave artifact, thus it was discontinued after the first 21 patients. Next, in each image, placental region was manually extracted in the first frame of the BOLD data using Medical Image Processing, Analysis, and Visualization (MIPAV version 7.1.1; National Institutes of Health, Bethesda, MD, USA). This region was then propagated to subsequent frames to create a volume of interest (VOI) and manually assessed for fidelity. For patients in whom the entire placenta was not covered during imaging, a VOI was placed on 5 consecutive slices of the subjectively largest portion of the placenta in the axial imaging plane. The VOI of the fetal brain was also manually extracted from the first frame of the BOLD image and propagated to subsequent frames. Each image was then manually inspected for fidelity. Following extraction of VOIs, BOLD data, were motion-corrected using an inter-slice, rigid body registration with normalized cross-correlation cost function (implemented in Python and FSL). Then, motion corrected BOLD data were corrected for noise using a combination of filtering and controlling for signal in amniotic fluid using FSL.
2.7.2. Computation of Temporal Variance
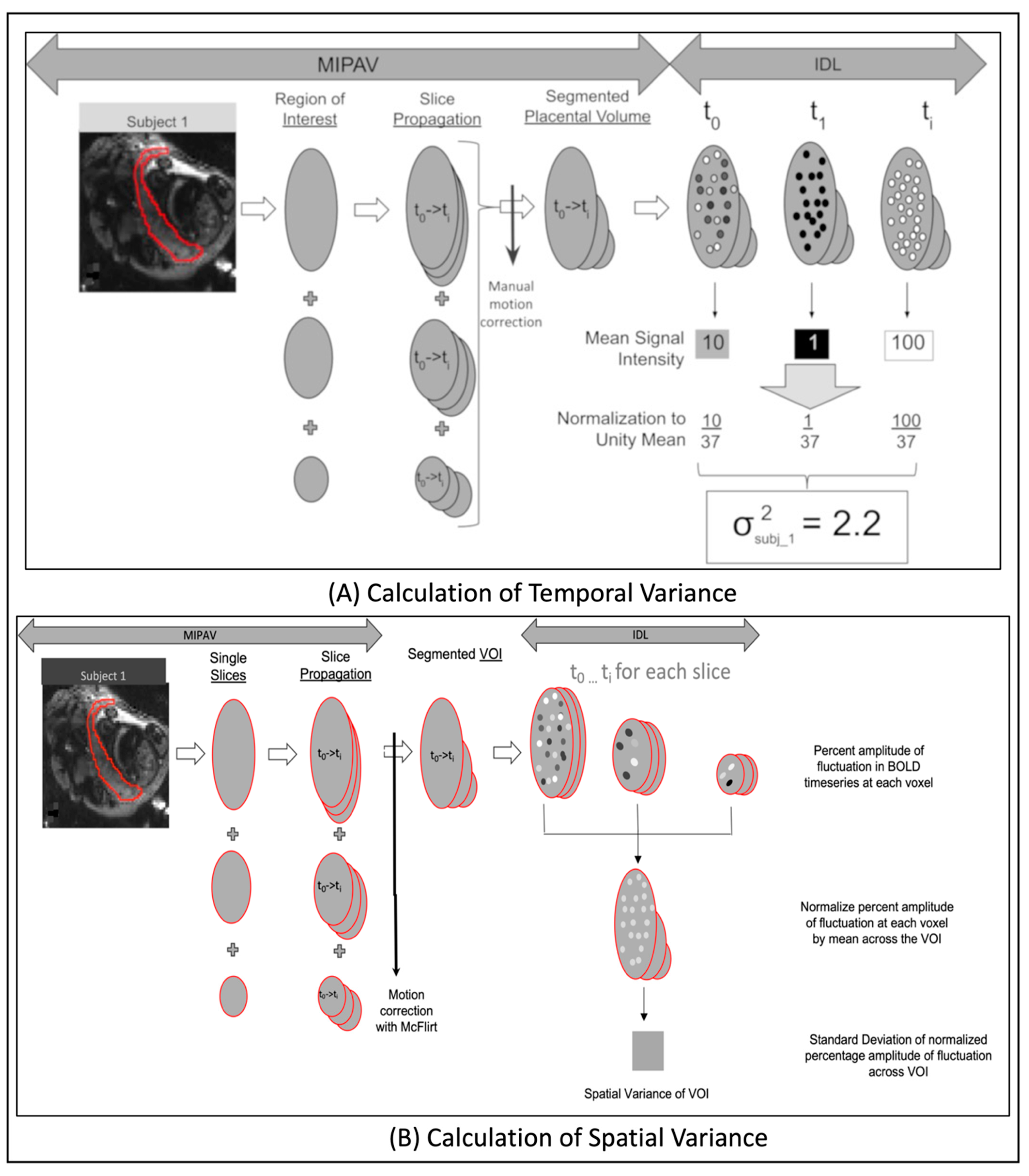
A custom IDL (interactive date language) program was used to calculate placental BOLD MRI temporal variance for each patient. Temporal variance was calculated by taking the average signal intensity over the placental volume for each time point and computing the standard deviation over the entire acquisition time period (
Figure 1A). The values for each time point were normalized to the unity mean (mean of 1) to non-CHD for effects such as receiver gain, since MRI values are fundamentally arbitrary up to a scaling constant. Temporal variation in pBOLD is due to cyclical variations in inflow of maternal blood. During part of the cycle when maternal inflow is high, more fully oxygenated blood enters the placenta causing oxygen to be higher and lower when inflow is lower. Temporal variance is also moderated by fetal hemodynamics where oxygenated blood is flowing away from the placenta while deoxygenated blood is flowing into it lessening the effect. Thus, greater intrinsic pBOLD temporal variance is expected when oxygenation demand should be the same (fetuses of a certain GA) but vasculature is different such that inflow to the fetus is less (like in a fetus with CHD). See
Supplemental Methods for further details.
2.7.3. Computation of Spatial Variance
For each subject, we computed the percentage amplitude fluctuation of the BOLD time-series [
26] at each voxel within the VOI. The values at each voxel were normalized by the mean of percent amplitude fluctuation across all voxels in the VOI. The standard deviation of the normalized percentage amplitude in fluctuation, across all voxels [
27], corresponds to spatial variance in spontaneous BOLD fluctuations of the VOI (
Figure 1B). Spatial variance is scale-independent can be used to directly compared across various subjects. It is known that placental blood volume flow increased with GA to meet the increasing demands of a growing fetus [
28]. In healthy placentas, spatial variance will increase with increasing GA as placental blood volume flow increases across the multiple cotelydons (See
Supplemental Methods for further details.). In the placenta, spatial variance characterizes the overall vascular health/integrity of the organ despite the differing number of cotyledons in any placenta. It can also be used to localize pathological anatomical areas within the placenta. In the brain, spatial variance is commonly removed from the BOLD signal because it’s considered to be of non-neural origins. However, differences in brain spatial variance, from normative brains, have been reported in diseases such as schizophrenia and brain tumors [
29,
30].
2.8. Statistical Analysis
For this study, our null hypotheses are: (1) MRF will decrease spatiotemporal variance of intrinsic BOLD signal in the placental and fetal brain; (2) Spatiotemporal variance of intrinsic BOLD signal in placenta and fetal brain will be altered in CHD compared to non-CHD.
Clinical and Demographic variables were compared between the CHD and non-CHD groups using the Student’s
t-test. Similarly, placental pathology incidence were also compared between the CHD and non-CHD groups using the Student’s
t-test. Statistical analysis was performed using bespoke pipelines developed in Interactive Data Language (IDL). Influence of Maternal Risk Factors: Regression analysis, using a wild bootstrap (5000 repetitions) suitable for heteroskedastic regression [
31], was used to model the relationship between MRFs (independent variable) and placental temporal variance (dependent variable) after adjusting for gestational age. This model was then expanded to include CHD status (presence or absence) as an interaction variable with MRFs. Individual regression models were used to study the relationship between MRFs (independent variable) and each of the following dependent variables (after adjusting for gestational age): fetal brain temporal variance, placental spatial variance, and fetal brain spatial variance. Differences between CHD and non-CHD groups: Differences in the distribution of temporal variance of the placenta between CHD and non-CHD groups was calculated using Kolmogorov–Smirnov test after adjusting for gestational age. Differences in histogram measures such as variance, kurtosis and skewness were compared using the F-test. Groupwise differences in histogram properties, between CHD and non-CHD, were also calculated for fetal brain temporal variance, placental spatial variance, and fetal brain spatial variance between CHD and non-CHD groups. Since anatomical properties of the placenta and fetal brain change over the gestation, we also used regression analysis to investigate the interactive effect of CHD status on the relationship between gestational age (independent variable) and placental spatial variance. This analysis was repeated with fetal brain spatial variance as the dependent variable.
4. Discussion
In this study, our results show that MRFs are associated with reduced spatiotemporal variance of intrinsic BOLD signal in the placenta and brain in fetuses with and without CHD. Secondly, we show that fetal brain temporal variance is reduced in fetuses with CHD compared to those without. Finally, we show that interaction of MRF and CHD was associated with reduced temporal variance of intrinsic placental BOLD but not the fetal brain.
MRFs (particularly diabetes, obesity, and hypertension) are associated with increased incidence of placental impairments. Maternal diabetes has been shown to increase the incidence of placental vascularization and decreased fetal oxygenation and reduced feto-placental transfer [
32,
33]. Maternal obesity, which often occurs in conjunction with maternal diabetes, is associated with reduced vascularity and blood flow in the placenta [
1,
34]. Maternal hypertension is associated with reduced spiral artery remodeling, decreased utero-placental perfusion and placental insufficiency [
35,
36,
37]. In line with these published findings, our results also show that, in the presence of these MRFs, both spatial and temporal variance is reduced in placentas regardless of CHD status.
There is a wide body of evidence showing a higher incidence of neurodevelopmental and neuropsychiatric disorders in children and adults exposed MRFs in utero [
38,
39,
40,
41]. However, little is known about the direct impact of MRFs on the fetal brain. Our findings show a global reduction in spatiotemporal variance in the fetal brain in the presence of MRFs. These findings are consistent with other published work, using indirect measures of fetal brain function, demonstrating altered functional properties of fetal brains in the presence of MRFs [
42,
43]. Fetal brain function measured using fetal magnetic encephalography showed that maternal diabetes was associated with reduced brain function as evidenced by a slower fetal response to auditory stimuli [
36]. Similarly, fetuses of hypertensive mothers showed delayed sensorineural responses to audio signals compared to fetuses of healthy mothers which have been attributed to functional differences or maturational delays in the fetal brain [
42,
43]. These findings together suggest that the presence of MRFs is associated with altered brain organization during a critical period of development which may manifest as the spectrum of neurocognitive and/or psychiatric disorders observed in offspring across their lifespan.
Significant changes to both spatial and temporal variance of intrinsic pBOLD is representative of abnormal placental vascular characteristics in CHD compared to non-CHDs. The multiple circulatory units, i.e., cotyledons, in the placenta need hemodynamic cohesion to act as an effective oxygenator meeting the increasing demands of the growing fetus. At any given GA, blood volume and flow rate are uniform in a healthy placenta. Therefore, intrinsic pBOLD signal fluctuations largely reflect changes in placental blood oxygen content. Therefore, higher pBOLD temporal variance could correspond to a less cohesive hemodynamic response among the various placental cotyledons in CHD compared to non-CHD. This could explain varying response times of CHD placentas to hyperoxygenation stimulus [
41]. Differences in spatial variance, seen in
Figure 2, based on CHD status, may correspond to the prevalence of regional vascular impairments in each of the cotelydons. Similar regional variations in placental perfusion in CHD were also reported by Zun [
44] et al. Taking our findings together with known histopathological findings in CHD placentas [
11,
45,
46], we postulate that maladaptive placental vascular development results in placental dysfunction in CHD. This alludes to the cumulative effect of CHD and increased maternal risk exacerbates feto-maternal dysfunction constituting a “multi-hits scenario [
47,
48]” as suggested by some authors.
Impaired cerebral oxygenation due to intracardiac mixing is implicated as a major contributor of deficient neurodevelopment in fetuses with CHD [
49,
50,
51,
52]. In the fetal brain, decrease in temporal variance in the CHD group is indicative of delays in the emerging functional connectivity [
53,
54] and is consistent with a recent study that reported delay brain function in neonates with CHD [
55]. Multiple in utero factors are likely to influence fetal brain dysmaturation and long term sequalae of neurodevelopmental impairment in CHD, including deleterious gene variants, environmental factors, fetal circulatory disturbances (reduced substrate delivery including oxygen, glucose and other nutrients). Recently, placental abnormalities have been shown to play an important role in fetal brain dysmaturation in CHD patients using structural placental MRI. Andescavage et al. showed that placental volume was positively interacted with birth weight and it increased more steeply CHD-affected fetuses compared to non-CHDs [
8]. Importantly, Seed et al. [
52] demonstrated that decreased oxygen saturation in the umbilical venous blood, which goes from the placenta to the fetus, was associated with reduced cerebral oxygenation and impaired brain growth in fetuses with CHD compared with non-CHDs. Taken together, these studies support the concept that placental dysfunction is an important link between abnormal fetal hemodynamics and abnormal brain function in fetuses with CHD.
Our findings are consistent with the hypothesis that CHD is associated with a genetic and/or environmental background of developmental brain differences with superimposed effects of MRFs. In the fetal brain, differences in temporal characteristics between non-CHDs and CHD are reflective of alterations to the emerging functional connectivity in the brain [
53,
54]. Our results are consistent with what one would expect with fetal brain dysmaturation in CHD which has recently been associated with poor early neurodevelopmental outcomes [
56]. However, our results further highlight the complexity of determining long-term neurodevelopmental outcomes in CHD. Further research is needed to disentangle the impact of individual MRFs on placenta and brain impairments in CHD.
Intrinsic BOLD provides a comprehensive and dynamic measure of functional characteristics in the placenta and the fetal brain. It is also more robust to motion artifacts inevitable in fetal imaging. Histopathological evidence, while useful, are subject to sampling error, do not include a measure of the total functional tissue, and occur only at a single, postnatal timepoint when functional characteristics cannot be measured. Previous examinations of functional placental and fetal brain properties have used maternal hyperoxia challenges and investigated differences in transversal relaxation time constant between the two conditions. However, placental and/or brain responses to hyperoxia challenges may not be reflective of normal function under normoxic conditions, limiting the interpretation of these type of studies. It is well-documented that T2* measurements are susceptible to motion. Maternal respiration and spontaneous uterine contractions are known to impact T2* measurements. This motion when coupled with regional variations in paramagnetic properties (due to varying concentration of deoxygenated blood) creates non-static changes to the magnetic field. This results in inconsistent T2* values in the placenta which are more difficult to correct compared to intrinsic BOLD signal in which more robust algorithm and criteria have been developed to mitigate motion corruptions, particularly in the brain [
57]. More research to directly apply this motion correction technique for placental BOLD intrinsic signal is needed.
5. Limitation
Our current study did not investigate the effect of various cardiac lesion types on pBOLD characteristics due to insufficient sample size. Future multi-site studies, with larger sample sizes, can further delineate how cardiac lesion type interacts with placenta and brain BOLD characteristics in CHD. This is a cross-sectional, observational study and cannot investigate mechanisms by which MRF and CHD impair placenta and fetal brain functional characteristics.
There are a couple of reasons why pre-gravid obesity may reduce spatial variance in the placental compared to gestational diabetes mellitus. The magnitude and timing of exposure of pre-gravid obesity and gestational diabetes mellitus relative to placental function have been linked to different and similar mechanism from preclinical studies. Maternal obesity is likely to have a deleterious impact on chronic inflammation and pre-programmed chronic inflammatory pathways and metabolism of the placental compared to gestational DM. Adverse effects of obesity on maternal and fetal health are mediated by complex interactions between metabolic, inflammatory, and oxidative stress signaling in the placenta [
58]. However, there are overlapping mechanisms of the impact of maternal obesity and diabetes on the placenta. Many of the placental alterations seen at term of pregnancy are the result of fetoplacental interactions often driven by fetal signals associated with maternal diabetes or obesity. These alterations can be regarded as adaptations to maintain homeostasis at the fetoplacental interface and, thus, to protect the fetus [
59]. However, the magnitude of these exposures such as poorly controlled diabetes or pronounced obesity may exceed placental homeostatic capacity, with potentially adverse consequences for the fetus. Thus, in late pregnancy, placenta function can be impacted by environmental perturbations which can exceed placental capacity for mounting adaptive responses. There is significant overlap between these risk factors both in the non-CHD and CHD groups. Nearly 1/3 of the control cohort and ½ of the CHD cohort in our sample had 2 or more maternal risk factors which could also explain the heterogeneity of our results. Future large, sampled studies are needed to be able to adequately parse the individual and compounded effect of these MRFs on placental function in controls and CHD cohorts.