PP1, PP2A and PP2B Interplay in the Regulation of Sperm Motility: Lessons from Protein Phosphatase Inhibitors
Abstract
1. Introduction
2. Activated and Hyperactivated Sperm Motility
3. Protein Phosphatases and Their Role in Spermatozoa Function
3.1. Protein Phosphatase Type 1 (PP1)
Protein Phosphatase 1 Gamma 2 (PP1γ2)
3.2. Protein Phosphatase Type 2A (PP2A)
3.3. Phosphoprotein Phosphatase Type 2B (PP2B)
3.4. PP1γ2, PP2A and PP2B Interplay in the Regulation of Sperm Motility
4. PP1, PP2A and PP2B Inhibition in Spermatozoa
4.1. Calyculin A (CA)
4.2. Okadaic Acid (OA)
4.3. Cyclosporin A (CsA)
4.4. Deltamethrin (DEL)
4.5. Endothall (E)
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vadnais, M.L.; Aghajanian, H.K.; Lin, A.; Gerton, G.L. Signaling in Sperm: Toward a Molecular Understanding of the Acquisition of Sperm Motility in the Mouse Epididymis. Biol. Reprod. 2013, 89, 127. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular Changes and Signaling Events Occurring in Spermatozoa during Epididymal Maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M. Moving to the Beat: A Review of Mammalian Sperm Motility Regulation. Reprod. Fertil. Dev. 2006, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling Mechanisms in Mammalian Sperm Motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Dcunha, R.; Hussein, R.; Ananda, H.; Kumari, S.; Adiga, S.; Kannan, N.; Zhao, Y.; Kalthur, G. Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod. Sci. 2020, 29, 7–25. [Google Scholar] [CrossRef]
- Baldi, E.; Luconi, M.; Bonaccorsi, L.; Forti, G. Signal Transduction Pathways in Human Spermatozoa. J. Reprod. Immunol. 2002, 53, 121–131. [Google Scholar] [CrossRef]
- Fardilha, M.; Esteves, S.L.C.; Korrodi-Gregório, L.; Pelech, S.; da Cruz e Silva, O.A.B.; da Cruz e Silva, E. Protein Phosphatase 1 Complexes Modulate Sperm Motility and Present Novel Targets for Male Infertility. Mol. Hum. Reprod. 2011, 17, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Wolf, D.P.; Trautman, K.; Cruz e Silva, E.F.; Greengard, P.; Srinivasan, V. Primate Sperm Contain Protein Phosphatase 1, a Biochemical Mediator of Motility. Biol. Reprod. 1996, 54, 719–727. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Stephens, D.; Trautman, K.; Smith, G.; Khatra, B.; Cruz e Silva, E.F.; Greengard, P. Sperm Motility Development in the Epididymis Is Associated with Decreased Glycogen Synthase Kinase-3 and Protein Phosphatase 1 Activity. Biol. Reprod. 1996, 54, 709–718. [Google Scholar] [CrossRef]
- Fardilha, M.; Esteves, S.; Korrodi-Gregório, L.; Vintém, A.; Domingues, S.; Rebelo, S.; Morrice, N.; Cohen, P.; da Cruz e Silva, O.; da Cruz e Silva, E. Identification of the Human Testis Protein Phosphatase 1 Interactome. Biochem. Pharmacol. 2011, 82, 1403–1415. [Google Scholar] [CrossRef]
- Fardilha, M.; Esteves, S.L.C.; Korrodi-Gregorio, L.; da Cruz e Silva, O.A.B.; da Cruz e Silva, E.F. The Physiological Relevance of Protein Phosphatase 1 and Its Interacting Proteins to Health and Disease. Curr. Med. Chem. 2010, 17, 3996–4017. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Vasconcelos, V.M.; Antunes, A. The Phosphoprotein Phosphatase Family of Ser/Thr Phosphatases as Principal Targets of Naturally Occurring Toxins. Crit. Rev. Toxicol. 2011, 41, 83–110. [Google Scholar] [CrossRef]
- Swingle, M.; Ni, L.; Honkanen, R.E. Small-Molecule Inhibitors of Ser/Thr Protein Phosphatases: Specificity, Use and Common Forms of Abuse. Methods Mol. Biol. 2007, 365, 23–38. [Google Scholar] [CrossRef]
- Honkanen, R.; Golden, T. Regulators of Serine / Threonine Protein Phosphatases at the Dawn of a Clinical Era? Curr. Med. Chem. 2002, 9, 2055–2075. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Fusetani, N.; Matsunaga, S.; Hashimoto, K.; Fujita, S.; Furuya, T. Calyculin A, a Novel Antitumor Metabolite from the Marine Sponge Discodermia Calyx1. J. Am. Chem. Soc. 1986, 108, 2780–2781. [Google Scholar] [CrossRef]
- Murakami, Y.; Oshima, Y.; Yasumoto, T. Identification of Okadaic Acid as a Toxic Component of a Marine Dinoflagellate Prorocentrum Lima. Bull. Jpn. Soc. Sci. Fish 1982, 48, 69–72. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial Membrane Potential Profile and Its Correlation with Increasing Sperm Motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Mikamo, K.; Suzuki, F.; Yanagimachi, H. Maturation of Spermatozoa in the Epididymis of the Chinese Hamster. Am. J. Anat. 1985, 172, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Yanagimachi, R. Fertility of Mammalian Spermatozoa: Its Development and Relativity. Zygote 1994, 2, 371–372. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm Transport in the Female Reproductive Tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Yang, W.X. Factors and Pathways Involved in Capacitation: How Are They Regulated? Oncotarget 2017, 8, 3600. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Suarez, S.S. Hyperactivation of Mammalian Spermatozoa: Function and Regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.L.; Razy-Faulkner, P. Control of Human Sperm Intracellular PH by Cholesterol and Its Relationship to the Response of the Acrosome to Progesterone. Biol. Reprod. 1997, 56, 1169–1174. [Google Scholar] [CrossRef]
- López-González, I.; Torres-Rodríguez, P.; Sánchez-Carranza, O.; Solís-López, A.; Santi, C.M.; Darszon, A.; Treviño, C.L. Membrane Hyperpolarization during Human Sperm Capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef]
- Baldi, E.; Casano, R.; Falsetti, C.; Krausz, C.; Maggi, M.; Forti, G. Intracellular Calcium Accumulation and Responsiveness to Progesterone in Capacitating Human Spermatozoa. J. Androl. 1991, 12, 323–330. [Google Scholar] [CrossRef]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular PH in Sperm Physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef]
- Tajima, Y.; Okamura, N.; Sugita, Y. The Activating Effects of Bicarbonate on Sperm Motility and Respiration at Ejaculation. Biochim. Biophys. Acta BBA Gen. Subj. 1987, 924, 519–529. [Google Scholar] [CrossRef]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; de la Vega-Beltran, J.L.; da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The Tyrosine Kinase FER Is Responsible for the Capacitationassociated Increase in Tyrosine Phosphorylation in Murine Sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef]
- Battistone, M.A.; da Ros, V.G.; Salicioni, A.M.; Navarrete, F.A.; Krapf, D.; Visconti, P.E.; Cuasnicú, P.S. Functional Human Sperm Capacitation Requires Both Bicarbonate-Dependent PKA Activation and down-Regulation of Ser/Thr Phosphatases by Src Family Kinases. Mol. Hum. Reprod. 2013, 19, 570. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Suarez, S.S. Different Signaling Pathways in Bovine Sperm Regulate Capacitation and Hyperactivation. Biol. Reprod. 2004, 70, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.; Santos, M.; Martins, F.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.B. Protein Phosphatase 1 Is a Key Player in Nuclear Events. Cell Signal. 2015, 27, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The Origins of Protein Phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Dey, S.; Eisa, A.; Kline, D.; Wagner, F.F.; Abeysirigunawardena, S.; Vijayaraghavan, S. Roles of Glycogen Synthase Kinase 3 Alpha and Calcineurin in Regulating the Ability of Sperm to Fertilize Eggs. FASEB J. 2019, 34, 1247–1269. [Google Scholar] [CrossRef]
- Signorelli, J.R.; Díaz, E.S.; Fara, K.; Barón, L.; Morales, P. Protein Phosphatases Decrease Their Activity during Capacitation: A New Requirement for This Event. PLoS ONE 2013, 8, e81286. [Google Scholar] [CrossRef]
- Goldberg, J.; Huang, H.B.; Kwon, Y.G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three-Dimensional Structure of the Catalytic Subunit of Protein Serine/Threonine Phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar] [CrossRef]
- Barford, D.; Das, A.K.; Egloff, M.P. The Structure and Mechanism of Protein Phosphatases: Insights into Catalysis and Regulation. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 133–164. [Google Scholar] [CrossRef]
- Da Cruz e Silva, E.; Fox, C.A.; Ouimet, C.C.; Gustafson, E.; Watson, S.J.; Greengard, P. Differential Expression of Protein Phosphatase 1 Isoforms in Mammalian Brain. J. Neurosci. 1995, 15, 3375–3389. [Google Scholar] [CrossRef]
- Berndt, N.; Campbell, D.G.; Caudwell, F.B.; Cohen, P.; da Cruz e Silva, E.F.; da Cruz e Silva, O.B.; Cohen, P.T.W. Isolation and Sequence Analysis of a CDNA Clone Encoding a Type-1 Protein Phosphatase Catalytic Subunit: Homology with Protein Phosphatase 2A. FEBS Lett. 1987, 223, 340–346. [Google Scholar] [CrossRef]
- Lee, J.H.; You, J.; Dobrota, E.; Skalnik, D.G. Identification and Characterization of a Novel Human PP1 Phosphatase Complex. J. Biol. Chem. 2010, 285, 24466. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; De Wever, V.; Derua, R.; Winkler, C.; Beullens, M.; Van Eynde, A.; Bollen, M. A Substrate-Trapping Strategy for Protein Phosphatase PP1 Holoenzymes Using Hypoactive Subunit Fusions. J. Biol. Chem. 2018, 293, 15152–15162. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, I.; Ferreira, M.; Bollen, M. Biogenesis and Activity Regulation of Protein Phosphatase 1. Biochem. Soc. Trans. 2017, 45, 89–99. [Google Scholar] [CrossRef]
- Alanis-Lobato, G.; Andrade-Navarro, M.A.; Schaefer, M.H. HIPPIE v2.0: Enhancing Meaningfulness and Reliability of Protein-Protein Interaction Networks. Nucleic Acids Res. 2017, 45, gkw985. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Cheng, L.; Puri, P.; Soler, D.; Vijayaraghavan, S. Protein Phosphatase PP1 Gamma 2 in Sperm Morphogenesis and Epididymal Initiation of Sperm Motility. Asian J. Androl. 2007, 9, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Varmuza, S.; Jurisicova, A.; Okano, K.; Hudson, J.; Boekelheide, K.; Shipp, E.B. Spermiogenesis is Impaired in Mice Bearing a Targeted Mutation in the Protein Phosphatase 1cγ Gene. Dev. Biol. 1999, 205, 98–110. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Kline, D.; Lu, J.; Orth, J.; Pilder, S.; Vijayaraghavan, S. Analysis of Ppp1cc-Null Mice Suggests a Role for PP1gamma2 in Sperm Morphogenesis. Biol. Reprod. 2007, 76, 992–1001. [Google Scholar] [CrossRef]
- Sinha, N.; Puri, P.; Nairn, A.C.; Vijayaraghavan, S. Selective Ablation of Ppp1cc Gene in Testicular Germ Cells Causes Oligo-Teratozoospermia and Infertility in Mice. Biol. Reprod. 2013, 89, 1–15. [Google Scholar] [CrossRef]
- Sinha, N.; Pilder, S.; Vijayaraghavan, S. Significant Expression Levels of Transgenic PPP1CC2 in Testis and Sperm Are Required to Overcome the Male Infertility Phenotype of Ppp1cc Null Mice. PLoS ONE 2012, 7, e47623. [Google Scholar] [CrossRef]
- Goswami, S.; Korrodi-Gregório, L.; Sinha, N.; Bhutada, S.; Bhattacharjee, R.; Kline, D.; Vijayaraghavan, S. Regulators of the Protein Phosphatase PP1γ2, PPP1R2, PPP1R7, and PPP1R11 Are Involved in Epididymal Sperm Maturation. J. Cell. Physiol. 2019, 234, 3105–3118. [Google Scholar] [CrossRef]
- Mishra, S.; Somanath, P.R.; Huang, Z.; Vijayaraghavan, S. Binding and Inactivation of the Germ Cell-Specific Protein Phosphatase PP1gamma2 by Sds22 during Epididymal Sperm Maturation. Biol. Reprod. 2003, 69, 1572–1579. [Google Scholar] [CrossRef]
- Korrodi-Gregório, L.; Ferreira, M.; Vintém, A.P.; Wu, W.; Muller, T.; Marcus, K.; Vijayaraghavan, S.; Brautigan, D.L.; da Cruz e Silva, O.A.B.; Fardilha, M.; et al. Identification and Characterization of Two Distinct PPP1R2 Isoforms in Human Spermatozoa. BMC Cell Biol. 2013, 14, 15. [Google Scholar] [CrossRef]
- Schwarz, T.; Prieler, B.; Schmid, J.A.; Grzmil, P.; Neesen, J. Ccdc181 Is a Microtubule-Binding Protein That Interacts with Hook1 in Haploid Male Germ Cells and Localizes to the Sperm Tail and Motile Cilia. Eur. J. Cell Biol. 2017, 96, 276–288. [Google Scholar] [CrossRef]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in Sperm Glycogen Synthase Kinase-3 Serine Phosphorylation and Activity Accompany Motility Initiation and Stimulation. J. Androl. 2004, 25, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Mohan, J.; Gray, H.; Khatra, B.; Carr, D.W. A Role for Phosphorylation of Glycogen Synthase Kinase-3alpha in Bovine Sperm Motility Regulation. Biol. Reprod. 2000, 62, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Janssens, V.; Goris, J. Protein Phosphatase 2A: A Highly Regulated Family of Serine/Threonine Phosphatases Implicated in Cell Growth and Signalling. Biochem. J. 2001, 353, 417–439. [Google Scholar] [CrossRef]
- Dudiki, T.; Kadunganattil, S.; Ferrara, J.K.; Kline, D.W.; Vijayaraghavan, S. Changes in Carboxy Methylation and Tyrosine Phosphorylation of Protein Phosphatase PP2A Are Associated with Epididymal Sperm Maturation and Motility. PLoS ONE 2015, 10, e0141961. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Li, Z.; Strack, S.; Stock, J.B.; Shi, Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell 2006, 127, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E. Protein Phosphatase 2A: The Trojan Horse of Cellular Signaling. Cell Signal. 2001, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Signorelli, J.R.; Diaz, E.S. Protein Phosphatase-Type 2A (PP2A) Is Involved in the Initial Events of Human Sperm Capacitation. Biol. Reprod. 2010, 83, 172. [Google Scholar] [CrossRef]
- Ahmad, K.; Bracho, G.E.; Wolf, D.P.; Tash, J.S. Regulation of Human Sperm Motility and Hyperactivation Components by Calcium, Calmodulin, and Protein Phosphatases. Arch. Androl. 1995, 35, 187–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, H.; Satouh, Y.; Mashiko, D.; Muto, M.; Nozawa, K.; Shiba, K.; Fujihara, Y.; Isotani, A.; Inaba, K.; Ikawa, M. Sperm Calcineurin Inhibition Prevents Mouse Fertility with Implications for Male Contraceptive. Science 2015, 350, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, C.R.; Parge, H.E.; Knighton, D.R.; Lewis, C.T.; Pelletier, L.A.; Tempczyk, A.; Kalish, V.J.; Tucker, K.D.; Showalter, R.E.; Moomaw, E.W.; et al. Crystal Structures of Human Calcineurin and the Human FKBP12–FK506–Calcineurin Complex. Nature 1995, 378, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Harrison, S.C. Crystal Structure of Human Calcineurin Complexed with Cyclosporin A and Human Cyclophilin. Proc. Natl. Acad. Sci. USA 2002, 99, 13522–13526. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Hoskins, D.D. Changes in the Mitochondrial Calcium Influx and Efflux Properties Are Responsible for the Decline in Sperm Calcium during Epididymal Maturation. Mol. Reprod. Dev. 1990, 25, 186–194. [Google Scholar] [CrossRef]
- Buffone, M.G.; Wertheimer, E.V.; Visconti, P.E.; Krapf, D. Central Role of Soluble Adenylyl Cyclase and CAMP in Sperm Physiology. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 2610–2620. [Google Scholar] [CrossRef]
- Salvi, F.; Hoermann, B.; del Pino García, J.; Fontanillo, M.; Derua, R.; Beullens, M.; Bollen, M.; Barabas, O.; Köhn, M. Towards Dissecting the Mechanism of Protein Phosphatase-1 Inhibition by Its C-Terminal Phosphorylation. ChemBioChem 2021, 22, 834–838. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble Adenylyl Cyclase as an Evolutionarily Conserved Bicarbonate Sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble Adenylyl Cyclase (SAC) Is Indispensable for Sperm Function and Fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef]
- Freitas, M.J.; Silva, J.V.; Brothag, C.; Regadas-Correia, B.; Fardilha, M.; Vijayaraghavan, S. Isoform-Specific GSK3A Activity Is Negatively Correlated with Human Sperm Motility. Mol. Hum. Reprod. 2019, 25, 171–183. [Google Scholar] [CrossRef]
- Swingle, M.R.; Honkanen, R.E. Inhibitors of Serine/Threonine Protein Phosphatases: Biochemical and Structural Studies Provide Insight for Further Development. Curr. Med. Chem. 2019, 26, 2634–2660. [Google Scholar] [CrossRef]
- Woydziak, Z.; Yucel, A.; Chamberlin, A. Tautomycetin Synthetic Analogues: Selective Inhibitors of Protein Phosphatase I. ChemMedChem 2021, 16, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yogesha, S.D.; Mayfield, J.E.; Gill, G.N.; Zhang, Y. Viewing Serine/Threonine Protein Phosphatases through the Eyes of Drug Designers. FEBS J. 2013, 280, 4739–4760. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Tumor Promotion by Inhibitors of ProteinZ Phosphatases 1 and 2A: The Okadaic Acid Class of Compounds. Adv. Cancer Res. 1993, 61, 143–194. [Google Scholar] [CrossRef]
- Roberge, M.; Tudan, C.; Hung, S.M.; Harder, K.W.; Jirik, F.R.; Anderson, H. Antitumor Drug Fostriecin Inhibits the Mitotic Entry Checkpoint and Protein Phosphatases 1 and 2A. Cancer Res. 1994, 54, 6115–6121. [Google Scholar] [PubMed]
- Walsh, A.H.; Cheng, A.; Honkanen, R.E. Fostriecin, an Antitumor Antibiotic with Inhibitory Activity against Serine/Threonine Protein Phosphatases Types 1 (PP1) and 2A (PP2A), Is Highly Selective for PP2A. FEBS Lett. 1997, 416, 230–234. [Google Scholar] [CrossRef]
- Ishihara, H.; Martin, B.L.; Brautigan, D.L.; Karaki, H.; Ozaki, H.; Kato, Y.; Fusetani, N.; Watabe, S.; Hashimoto, K.; Uemura, D.; et al. Calyculin A and Okadaic Acid: Inhibitors of Protein Phosphatase Activity. Biochem. Biophys. Res. Commun. 1989, 159, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Klee, C.B.; Bierer, B.E.; Burakoff, S.J. Calcineurin Phosphatase Activity in T Lymphocytes Is Inhibited by FK 506 and Cyclosporin A. Proc. Natl. Acad. Sci. USA 1992, 89, 3686. [Google Scholar] [CrossRef]
- Laidley, C.W.; Cohen, E.; Casida, J.E. Protein Phosphatase in Neuroblastoma Cells: [3H]Cantharidin Binding Site in Relation to Cytotoxicity. J. Pharmacol. Exp. Ther. 1997, 280, 1152–1158. [Google Scholar]
- Enan, E.; Matsumura, F. Specific Inhibition of Calcineurin by Type II Synthetic Pyrethroid Insecticides. Biochem. Pharm. 1992, 43, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Klumpp, S. Tautomycin from the Bacterium Streptomyces Verticillatus. Another Potent and Specific Inhibitor of Protein Phosphatases 1 and 2A. FEBS Lett. 1990, 277, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.A.; Stewart, S.G.; Gordon, C.P.; Ackland, S.P.; Gilbert, J.; Sauer, B.; Sakoff, J.A.; McCluskey, A. Norcantharidin Analogues: Synthesis, Anticancer Activity and Protein Phosphatase 1 and 2A Inhibition. ChemMedChem 2008, 3, 1878–1892. [Google Scholar] [CrossRef]
- Sakoff, J.A.; Ackland, S.P.; Baldwin, M.L.; Keane, M.A.; McCluskey, A. Anticancer Activity and Protein Phosphatase 1 and 2A Inhibition of a New Generation of Cantharidin Analogues. Investig. New Drugs 2002, 20, 1–11. [Google Scholar] [CrossRef]
- Cohen, P.T.W. Protein Phosphatase 1—Targeted in Many Directions. J. Cell Sci. 2002, 115, 241–256. [Google Scholar] [CrossRef]
- Ammosova, T.; Platonov, M.; Yedavalli, V.R.K.; Obukhov, Y.; Gordeuk, V.R.; Jeang, K.T.; Kovalskyy, D.; Nekhai, S. Small Molecules Targeted to a Non-Catalytic “RVxF” Binding Site of Protein Phosphatase-1 Inhibit HIV-1. PLoS ONE 2012, 7, e39481. [Google Scholar] [CrossRef]
- Ammosova, T.; Platonov, M.; Ivanov, A.; Kont, Y.S.; Kumari, N.; Kehn-Hall, K.; Jerebtsova, M.; Kulkarni, A.A.; Üren, A.; Kovalskyy, D.; et al. 1E7-03, a Low MW Compound Targeting Host Protein Phosphatase-1, Inhibits HIV-1 Transcription. Br. J. Pharmacol. 2014, 171, 5059–5075. [Google Scholar] [CrossRef]
- Ilinykh, P.A.; Tigabu, B.; Ivanov, A.; Ammosova, T.; Obukhov, Y.; Garron, T.; Kumari, N.; Kovalskyy, D.; Platonov, M.O.; Naumchik, V.S.; et al. Role of Protein Phosphatase 1 in Dephosphorylation of Ebola Virus VP30 Protein and Its Targeting for the Inhibition of Viral Transcription. J. Biol. Chem. 2014, 289, 22723–22738. [Google Scholar] [CrossRef]
- Ashizawa, K.; Wishart, G.J.; Tomonaga, H.; Nishinakama, K.; Tsuzuki, Y. Presence of Protein Phosphatase Type 1 and Its Involvement in Temperature-Dependent Flagellar Movement of Fowl Spermatozoa. FEBS Lett. 1994, 350, 130–134. [Google Scholar] [CrossRef][Green Version]
- Ashizawa, K.; Magome, A.; Tsuzuki, Y. Stimulation of Motility and Respiration of Intact Fowl Spermatozoa by Calyculin A, a Specific Inhibitor of Protein Phosphatase-1 and -2A, via a Ca2+-Dependent Mechanism. Reproduction 1995, 105, 109–114. [Google Scholar] [CrossRef][Green Version]
- Ashizawa, K.; Wishart, G.J.; Katayama, S.; Takano, D.; Ranasinghe, A.R.A.H.; Narumi, K.; Tsuzuki, Y. Regulation of Acrosome Reaction of Fowl Spermatozoa: Evidence for the Involvement of Protein Kinase C and Protein Phosphatase-Type 1 and/or -Type 2A. Reproduction 2006, 131, 1017–1024. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goto, N.; Harayama, H. Calyculin A-Sensitive Protein Phosphatases Are Involved in Maintenance of Progressive Movement in Mouse Spermatozoa in Vitro by Suppression of Autophosphorylation of Protein Kinase A. J. Reprod. Dev. 2009, 55, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D.; Arcelay, E.; Wertheimer, E.V.; Sanjay, A.; Pilder, S.H.; Salicioni, A.M.; Visconti, P.E. Inhibition of Ser/Thr Phosphatases Induces Capacitation-Associated Signaling in the Presence of Src Kinase Inhibitors. J. Biol. Chem. 2010, 285, 7977–7985. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Harrison, R.A.P.; Tsolova, M.; Jebe, E.; Töpfer-Petersen, E. Signalling Pathways Involved in the Control of Sperm Cell Volume. Reproduction 2007, 133, 61–73. [Google Scholar] [CrossRef][Green Version]
- Harayama, H.; Noda, T.; Ishikawa, S.; Shidara, O. Relationship between Cyclic AMP-Dependent Protein Tyrosine Phosphorylation and Extracellular Calcium during Hyperactivation of Boar Spermatozoa. Mol. Reprod. Dev. 2012, 79, 727–739. [Google Scholar] [CrossRef]
- Huang, Z.; Vijayaraghavan, S. Increased Phosphorylation of a Distinct Subcellular Pool of Protein Phosphatase, PP1γ2, During Epididymal Sperm Maturation. Biol. Reprod. 2004, 70, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Wolf, D.P.; Trautman, K.C.; Vijayaraghavan, S. Motility Potential of Macaque Epididymal Sperm: The Role of Protein Phosphatase and Glycogen Synthase Kinase-3 Activities. J. Androl. 1999, 20, 47–53. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic Adenosine 3′,5′monophosphate-Dependent Regulation of Protein Tyrosine Phosphorylation in Relation to Human Sperm Capacitation and Motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef]
- Carrera, A.; Moos, J.; Ning, X.P.; Gerton, G.L.; Tesarik, J.; Kopf, G.S.; Moss, S.B. Regulation of Protein Tyrosine Phosphorylation in Human Sperm by a Calcium/Calmodulin-Dependent Mechanism: Identification of A Kinase Anchor Proteins as Major Substrates for Tyrosine Phosphorylation. Dev. Biol. 1996, 180, 284–296. [Google Scholar] [CrossRef]
- Bennett, J.C.; Roggero, C.M.; Mancifesta, F.E.; Mayorga, L.S. Calcineurin-Mediated Dephosphorylation of Synaptotagmin VI Is Necessary for Acrosomal Exocytosis. J. Biol. Chem. 2010, 285, 26269–26278. [Google Scholar] [CrossRef]
- Ashizawa, K.; Wishart, G.J.; Ranasinghe, A.R.A.H.; Katayama, S.; Tsuzuki, Y. Protein Phosphatase-Type 2B Is Involved in the Regulation of the Acrosome Reaction but Not in the Temperature-Dependent Flagellar Movement of Fowl Spermatozoa. Reproduction 2004, 128, 783–787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takai, A.; Sasaki, K.; Nagai, H.; Mieskes, G.; Isobe, M.; Isono, K.; Yasumoto, T. Inhibition of Specific Binding of Okadaic Acid to Protein Phosphatase 2A by Microcystin-LR, Calyculin-A and Tautomycin: Method of Analysis of Interactions of Tight-Binding Ligands with Target Protein. Biochem. J. 1995, 306, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Brew, K.; Lee, E.Y.C. Mutational Analysis of the Catalytic Subunit of Muscle Protein Phosphatase-1. Biochemistry 1996, 35, 6276–6282. [Google Scholar] [CrossRef]
- Bialojan, C.; Takai, A. Inhibitory Effect of a Marine-Sponge Toxin, Okadaic Acid, on Protein Phosphatases. Specificity and Kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef]
- Clipstone, N.A.; Fiorentino, D.F.; Crabtree, G.R. Molecular Analysis of the Interaction of Calcineurin with Drug-Immunophilin Complexes. J. Biol. Chem. 1994, 269, 26431–26437. [Google Scholar] [CrossRef]
- Milan, D.; Griffith, J.; Su, M.; Price, E.R.; McKeon, F. The Latch Region of Calcineurin B Is Involved in Both Immunosuppressant-Immunophilin Complex Docking and Phosphatase Activation. Cell 1994, 79, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Yenugu, S. Pyrethroid Based Pesticides–Chemical and Biological Aspects. Crit. Rev. Toxicol. 2021, 51, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sun, Y.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.R.; Yuan, Z.; Wang, X.; Martínez, M.A. Deltamethrin Toxicity: A Review of Oxidative Stress and Metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar] [CrossRef]
- Cancel, A.M.; Lobdell, D.; Mendola, P.; Perreault, S.D. Objective Evaluation of Hyperactivated Motility in Rat Spermatozoa Using Computer-Assisted Sperm Analysis. Hum. Reprod. 2000, 15, 1322–1328. [Google Scholar] [CrossRef]
- Mortimer, D.; Mortimer, S.T. Computer-Aided Sperm Analysis (CASA) of Sperm Motility and Hyperactivation. Methods Mol. Biol. 2013, 927, 77–87. [Google Scholar] [CrossRef]
- Li, Y.M.; Mackintosh, C.; Casida, J.E. Protein Phosphatase 2A and Its [3H]Cantharidin/[3H]Endothall Thioanhydride Binding Site. Inhibitor Specificity of Cantharidin and ATP Analogues. Biochem. Pharm. 1993, 46, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.E. Cantharidin, Another Natural Toxin That Inhibits the Activity of Serine/Threonine Protein Phosphatases Types 1 and 2A. FEBS Lett. 1993, 330, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Fujinoki, M.; Shibahara, H.; Suzuki, M. Regulation of Hyperactivation by PPP2 in Hamster Spermatozoa. Reproduction 2010, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A.; Elhanbly, S.; Abdalla, H.; Serria, M.S.; Aziz, A.; El-Dakrooy, S.A.; El-Bakary, A.A.; Mostafa, T. In Vitro Study of Cypermethrin on Human Spermatozoa and the Possible Protective Role of Vitamins C and E. Andrologia 2014, 46, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, C.; Gao, S.Q.; Kong, T.T.; Chen, L.; Li, X.F.; Song, L.; Wang, Y.B. Effects of Permethrin, Cypermethrin and 3-Phenoxybenzoic Acid on Rat Sperm Motility In vitro Evaluated with Computer-Assisted Sperm Analysis. Toxicol. In Vitro 2010, 24, 382–386. [Google Scholar] [CrossRef] [PubMed]
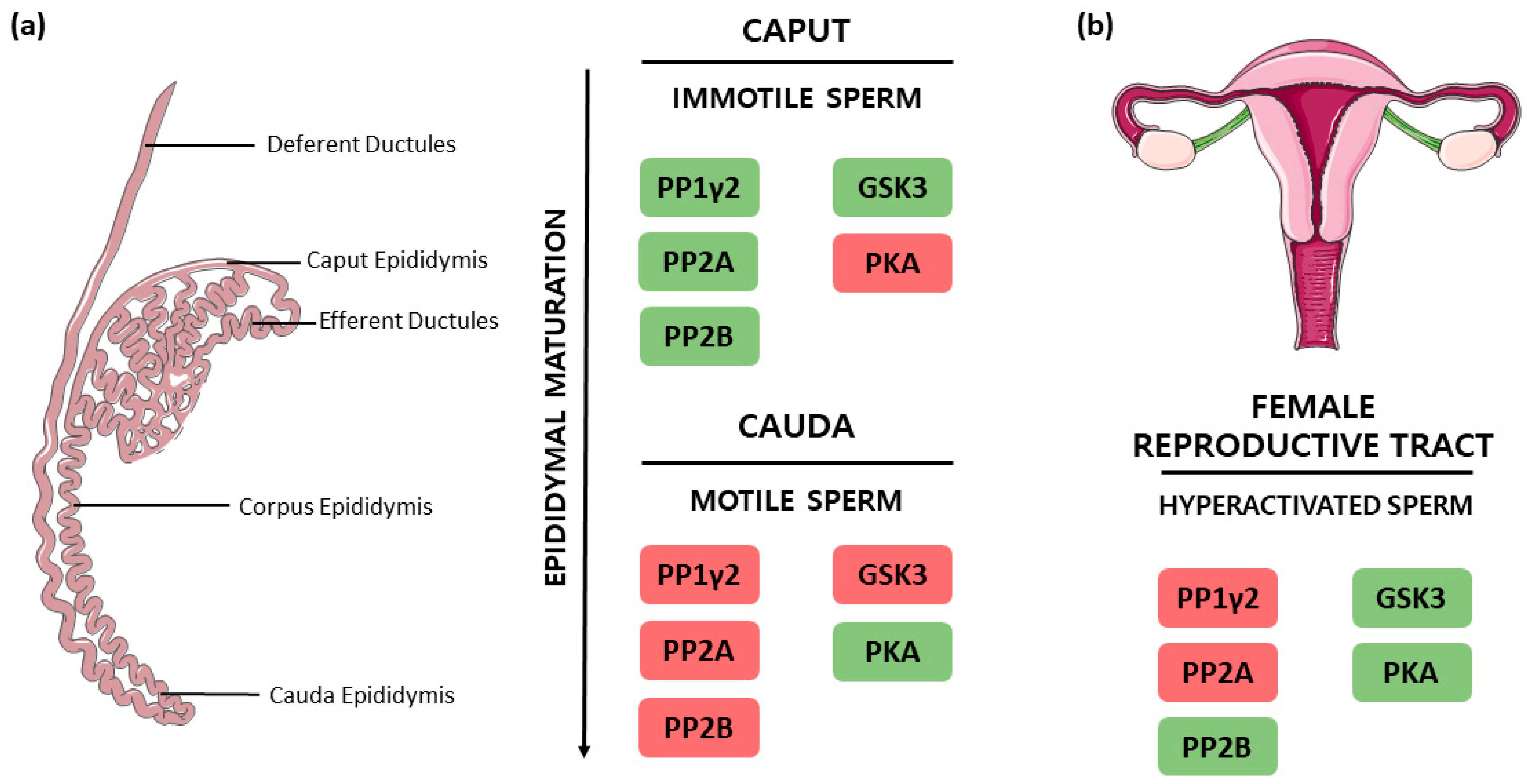
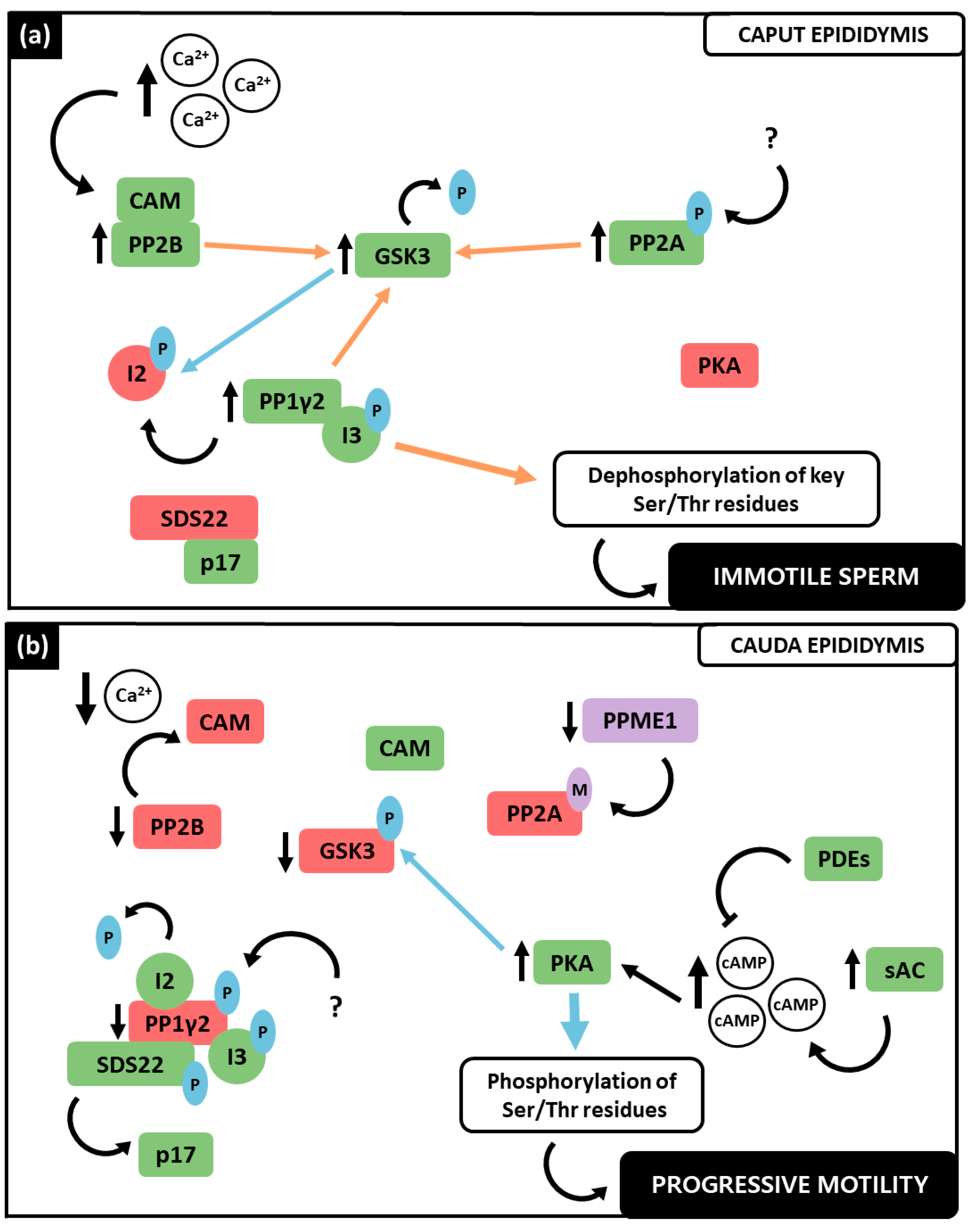
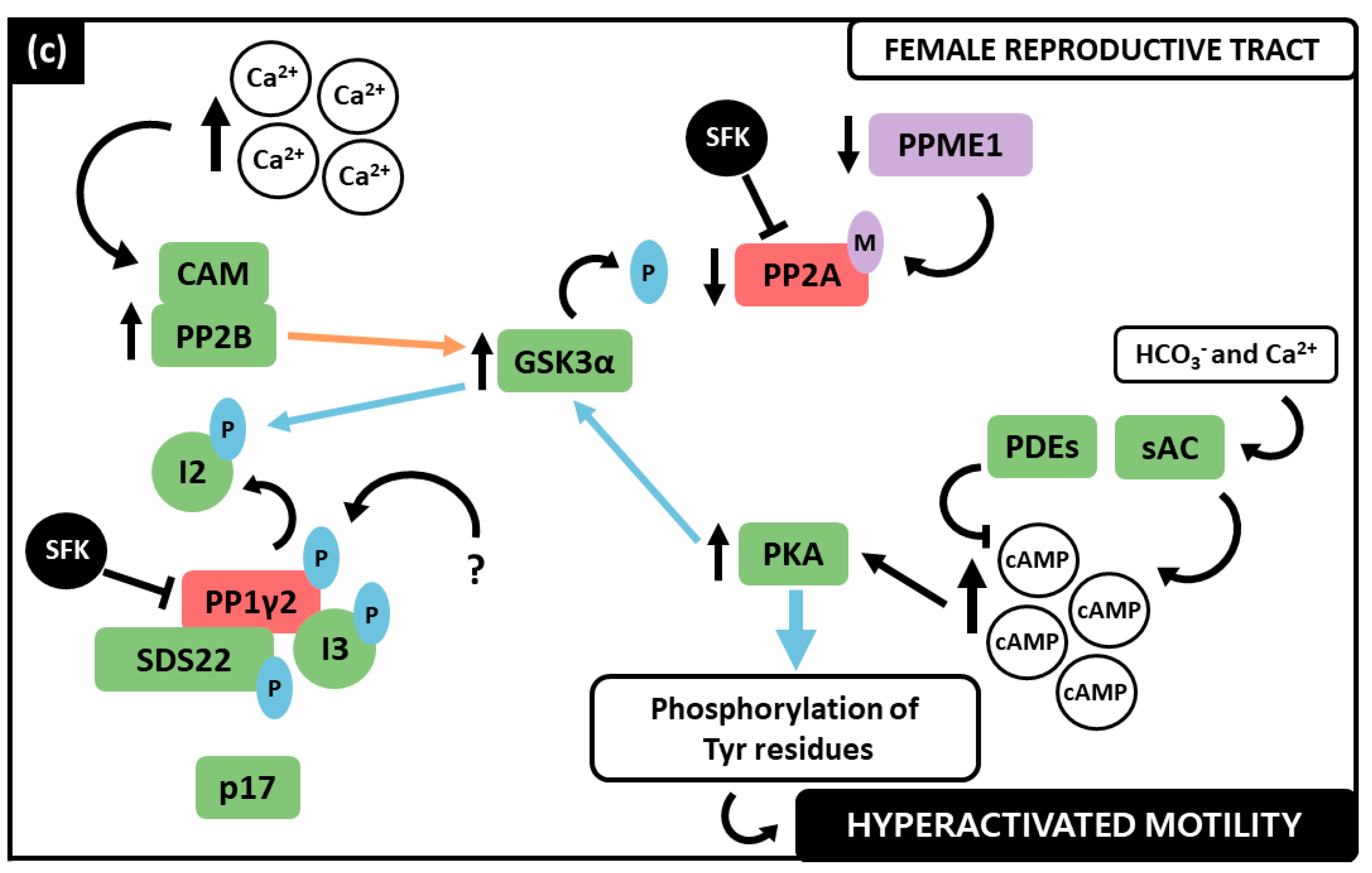
| PP Inhibitor | Model | Concentration | Outcome | Reference |
|---|---|---|---|---|
| Calyculin A | Fowl sperm | 0.1μM | Loss of motility following the addition of CaCl2 to demembranated spermatozoa, which was gradually restored by addition of EGTA. | Ashizawa et al. [90] 1994 |
| 1.0 μM | Activation of intact sperm motility and stimulation of metabolic activity at 40 degrees. | Ashizawa et al. [91] 1995 | ||
| (1) Maximal effect: 1000 nM (2) 100 nM | (1) Induction of vigorous motility, stimulation of acrosome reaction in the presence of IPVL; (2) Significantly decreased ATP concentrations of spermatozoa. | Ashizawa et al. [92] 2006 | ||
| Mouse sperm | Maximal effects: 125 nM | Induced phosphorylation of several flagellar proteins, as well as PKA, inactivating it; Reduced progressive flagellar movement, inducing the hyperactivation-like motility pattern type. | Goto et al. [93] 2009 | |
| 0.1, 1, 3, 10, 100, 1000 nM | Overcome the block of capacitation-associated parameters by SKI606 and SU6656, such as PKA inhibition and tyrosine phosphorylation in a dose-dependent manner. | Krapf et al. [94] 2010 | ||
| Boar sperm | Maximal effect: 10 nM | Increased hypotonic volume, blocked the regulatory volume decrease (RVD) process, and increased relative cell volume. | Petrunkina et al. [95] 2007 | |
| 50 and 100 nM | Promotion of hyperactivation and cAMP-induced protein tyrosine phosphorylation identically at both concentrations. | Harayama et al. [96] 2012 | ||
| Bovine sperm | PP inhibition: 1.0 nM; Maximal effect: 3.4 nM | Activation of motility on caput and caudal spermatozoa; Demonstration of GSK3’s presence in bovine sperm. | Vijayaraghavan et al. [9] 1996 | |
| 50 nM | Increase of phosphorylated PP1γ2 in both caput and caudal epididymal spermatozoa. | Huang et al. [97] 2004 | ||
| Monkey sperm | 0.59 nM | Increase in %motility and an acceleration in mean path velocity; | Smith et al. [8] 1996 | |
| 100 nM | Increase in motile cells of the caput sperm, without any effect on their path velocity. | Smith et al. [98] 1999 | ||
| Human sperm | IC50: 0.75 nM | Increase in %motility and an acceleration in mean path velocity; Demonstration that sperm contains PP1 and its regulators. | Smith et al. [8] 1996 | |
| 100 nM | Increase in p105/81 phosphotyrosine levels and stimulation of sperm capacitation. | Leclerc et al. [99] 1996 | ||
| Okadaic acid | Fowl sperm | 1.0 μM | Loss of motility following the addition of CaCl2 to demembranated sperm, which was gradually restored by addition of EGTA. | Ashizawa et al. [90] 1994 |
| Maximal effect: 1000 nM | Less vigorous motility stimulation, induction of acrosome reaction in the presence of IPVL. | Ashizawa et al. [92] 2006 | ||
| Mouse sperm | 0.1, 1, 3, 10, 100, 1000 nM | Overcome the block of capacitation-associated parameters by SKI606 and SU6656, such as PKA inhibition and tyrosine phosphorylation in a dose-dependent manner. | Krapf et al. [94] 2010 | |
| Boar sperm | Maximal effect: 10 nM | Increased hypotonic volume, blocked the regulatory volume decrease (RVD) process, and increased relative cell volume. | Petrunkina et al. [95] 2007 | |
| Bovine sperm | PP inhibition: 1 μM; Maximal effect: 5 μM | Activation of motility on caput and caudal sperm; Demonstration of GSK3’s presence in bovine sperm. | Vijayaraghavan et al. [9] 1996 | |
| 5 nM | Increase of sperm motility parameters (%motility, velocity, and lateral head amplitude), as well as elevation of dimethyl PP2A and tyrosine phosphorylated PP2A. | Dudiki et al. [57] 2015 | ||
| Monkey sperm | 37.2 nM | Increase in %motility and an acceleration in mean path velocity. | Smith et al. [8] 1996 | |
| Human sperm | 1.0 μM | Alteration of velocity along the curvilinear path and amplitude of the lateral displacement of the head; Inhibition of Ca2+-dependent dephosphorylation of cAMP-dependent phosphoproteins in capacitating sperm. | Ahmad et al. [61] 1995 | |
| 38.8 nM | Increase in %motility and an acceleration in mean path velocity; Demonstration that sperm contains PP1 and its regulators. | Smith et al. [8] 1996 | ||
| (1) 1 μM (2) 100 nM | (1) Increase in p105/81 phosphotyrosine levels; (2) Stimulation of sperm capacitation. | Leclerc et al. [99] 1996 | ||
| 100 nM | Inhibited the Ca2+-stimulated dephosphorylation of human sperm phosphotyrosine-containing proteins. | Carrera et al. [100] 1996 | ||
| (IC50) PP1: 10 nM PP2A: 0.1 nM | Increased phosphorylation on threonine residues; Demonstration that the activity of this PP decreases during the capacitation process. | Signorelli et al. [36] 2013 | ||
| Cyclosporin A | Human sperm | 2 μM | Blocked acrosomal exocytosis, suggesting PP2B is required in the early steps of the secretory process of the acrosome reaction. | Bennet et al. [101] 2010 |
| Deltamethrin | Fowl sperm | 1–100 nM Maximal effect: 10 nM. | Did not permit the restoration of motility at 40 °C but stimulated the acrosome reaction in the presence of IPVL. | Ashizawa et al. [102] 2004 |
| Human sperm | 10 nM; | Inhibited the Ca2+-stimulated dephosphorylation of human sperm phosphotyrosine-containing proteins. | Carrera et al. [100] 1996 | |
| (IC50) PP2B: 0.1 nM | Increased phosphorylation on threonine residues; Demonstration that the activity of this PP decreases during the capacitation process. | Signorelli et al. [36] 2013 | ||
| Endothall | Human sperm | (IC50) PP2A: 90 nM | Increased phosphorylation on threonine residues; Demonstration that the activity of this PP decreases during the capacitation process. | Signorelli et al. [36] 2013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.F.; Santiago, J.; Silva, J.V.; Oliveira, P.F.; Fardilha, M. PP1, PP2A and PP2B Interplay in the Regulation of Sperm Motility: Lessons from Protein Phosphatase Inhibitors. Int. J. Mol. Sci. 2022, 23, 15235. https://doi.org/10.3390/ijms232315235
Ferreira AF, Santiago J, Silva JV, Oliveira PF, Fardilha M. PP1, PP2A and PP2B Interplay in the Regulation of Sperm Motility: Lessons from Protein Phosphatase Inhibitors. International Journal of Molecular Sciences. 2022; 23(23):15235. https://doi.org/10.3390/ijms232315235
Chicago/Turabian StyleFerreira, Ana F., Joana Santiago, Joana V. Silva, Pedro F. Oliveira, and Margarida Fardilha. 2022. "PP1, PP2A and PP2B Interplay in the Regulation of Sperm Motility: Lessons from Protein Phosphatase Inhibitors" International Journal of Molecular Sciences 23, no. 23: 15235. https://doi.org/10.3390/ijms232315235
APA StyleFerreira, A. F., Santiago, J., Silva, J. V., Oliveira, P. F., & Fardilha, M. (2022). PP1, PP2A and PP2B Interplay in the Regulation of Sperm Motility: Lessons from Protein Phosphatase Inhibitors. International Journal of Molecular Sciences, 23(23), 15235. https://doi.org/10.3390/ijms232315235










