Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
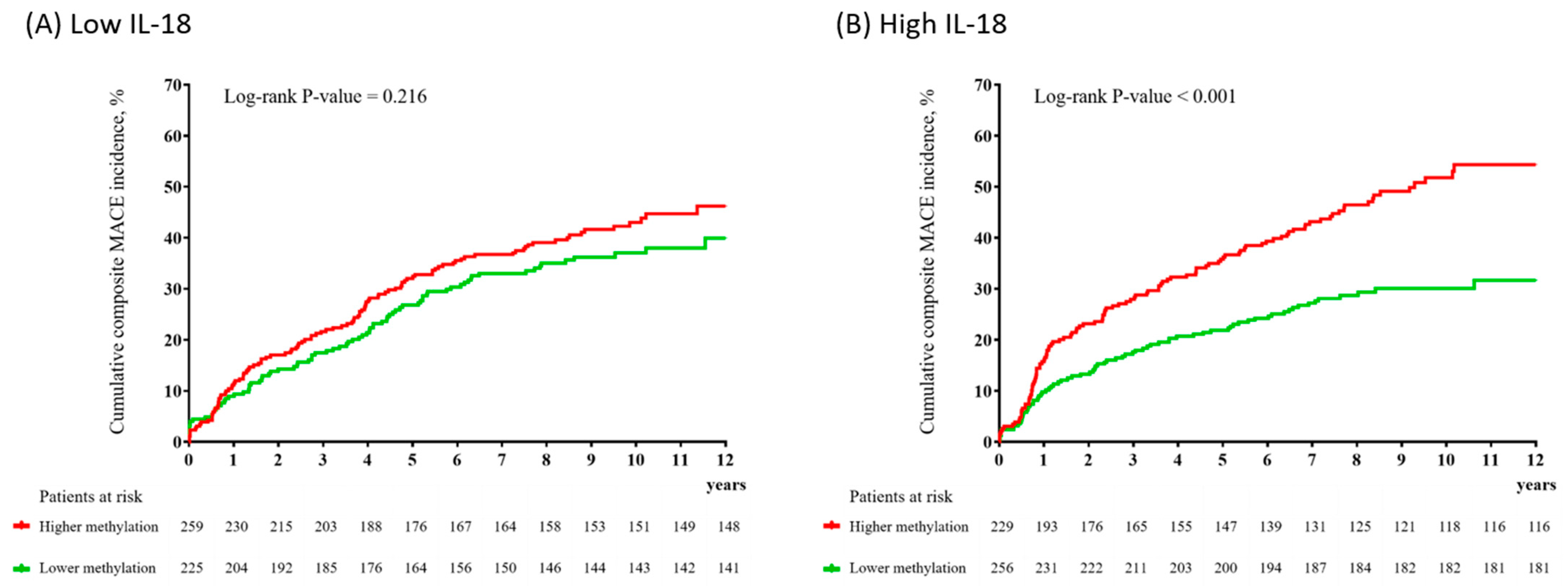
2. Results
3. Discussion
4. Methods
4.1. Study Overview and Participants
4.2. Primary Measures
4.2.1. Interleukin-18 Level
4.2.2. BDNF Methylation Status
4.3. Baseline Covariates
4.4. Outcomes
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donovan, M.J.; Hahn, R.; Tessarollo, L.; Hempstead, B.L. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat. Genet. 1996, 14, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Lin, M.I.; Wiegn, P.; Ringstedt, T.; Kraemer, R.; Hahn, R.; Wang, S.; Ibanez, C.F.; Rafii, S.; Hempstead, B.L. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000, 127, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Tessarollo, L.; Tsoulfas, P.; Donovan, M.J.; Palko, M.E.; Blair-Flynn, J.; Hempstead, B.L.; Parada, L.F. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 14776–14781. [Google Scholar] [CrossRef]
- Hang, P.Z.; Zhu, H.; Li, P.F.; Liu, J.; Ge, F.Q.; Zhao, J.; Du, Z.M. The Emerging Role of BDNF/TrkB Signaling in Cardiovascular Diseases. Life 2021, 11, 70. [Google Scholar] [CrossRef]
- Martinowich, K.; Hattori, D.; Wu, H.; Fouse, S.; He, F.; Hu, Y.; Fan, G.; Sun, Y.E. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003, 302, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stewart, R.; Kim, J.W.; Kang, H.J.; Lee, J.Y.; Kim, S.Y.; Kim, S.W.; Shin, I.S.; Hong, Y.J.; Ahn, Y.; et al. Modifying effects of depression on the association between BDNF methylation and prognosis of acute coronary syndrome. Brain Behav. Immun. 2019, 81, 422–429. [Google Scholar] [CrossRef]
- Hansson, G.K.; Robertson, A.K.; Soderberg-Naucler, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006, 1, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Nanchen, D.; Klingenberg, R.; Gencer, B.; Raber, L.; Carballo, D.; von Eckardstein, A.; Windecker, S.; Rodondi, N.; Luscher, T.F.; Mach, F.; et al. Inflammation during acute coronary syndromes—Risk of cardiovascular events and bleeding. Int. J. Cardiol. 2019, 287, 13–18. [Google Scholar] [CrossRef]
- Mallat, Z.; Henry, P.; Fressonnet, R.; Alouani, S.; Scoazec, A.; Beaufils, P.; Chvatchko, Y.; Tedgui, A. Increased plasma concentrations of interleukin-18 in acute coronary syndromes. Heart 2002, 88, 467–469. [Google Scholar] [CrossRef]
- Furtado, M.V.; Rossini, A.P.; Campani, R.B.; Meotti, C.; Segatto, M.; Vietta, G.; Polanczyk, C.A. Interleukin-18: An independent predictor of cardiovascular events in patients with acute coronary syndrome after 6 months of follow-up. Coron. Artery Dis. 2009, 20, 327–331. [Google Scholar] [CrossRef]
- Hartford, M.; Wiklund, O.; Hulten, L.M.; Persson, A.; Karlsson, T.; Herlitz, J.; Hulthe, J.; Caidahl, K. Interleukin-18 as a predictor of future events in patients with acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Akerblom, A.; James, S.K.; Lakic, T.G.; Becker, R.C.; Cannon, C.P.; Steg, P.G.; Himmelmann, A.; Katus, H.A.; Storey, R.F.; Wallentin, L.; et al. Interleukin-18 in patients with acute coronary syndromes. Clin. Cardiol. 2019, 42, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Krumm, B.; Xiang, Y.; Deng, J. Structural biology of the IL-1 superfamily: Key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014, 23, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Seljeflot, I.; Hjerkinn, E.M.; Arnesen, H. Interleukin-18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: Synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009, 32, 486–492. [Google Scholar] [CrossRef]
- Formanowicz, D.; Wanic-Kossowska, M.; Pawliczak, E.; Radom, M.; Formanowicz, P. Usefulness of serum interleukin-18 in predicting cardiovascular mortality in patients with chronic kidney disease-systems and clinical approach. Sci. Rep. 2015, 5, 18332. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Kim, J.M.; Bae, K.Y.; Kang, H.J.; Kim, S.W.; Shin, I.S.; Hong, Y.J.; Kim, J.H.; Shin, H.Y.; Ahn, Y.; Kim, J.K.; et al. Design and Methodology for the Korean Observational and Escitalopram Treatment Studies of Depression in Acute Coronary Syndrome: K-DEPACS and EsDEPACS. Psychiatry Investig. 2014, 11, 89–94. [Google Scholar] [CrossRef]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E., Jr.; Chavey, W.E., 2nd; Fesmire, F.M.; Hochman, J.S.; Levin, T.N.; et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e179–e347. [Google Scholar] [CrossRef]
- Breitling, L.P.; Salzmann, K.; Rothenbacher, D.; Burwinkel, B.; Brenner, H. Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur. Heart J. 2012, 33, 2841–2848. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Killip, T., 3rd; Kimball, J.T. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am. J. Cardiol. 1967, 20, 457–464. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, Y.; Zhang, Y.; Chen, Z.Y. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem. Biophys. Res. Commun. 2011, 415, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kinugawa, S.; Homma, T.; Masaki, Y.; Furihata, T.; Yokota, T.; Matsushima, S.; Takada, S.; Kadoguchi, T.; Oba, K.; et al. Serum brain-derived neurotropic factor level predicts adverse clinical outcomes in patients with heart failure. J. Card. Fail. 2015, 21, 300–306. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef]
- Lubin, F.D.; Roth, T.L.; Sweatt, J.D. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 2008, 28, 10576–10586. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690. [Google Scholar] [CrossRef]
- Heinisch, R.H.; Zanetti, C.R.; Comin, F.; Fernandes, J.L.; Ramires, J.A.; Serrano, C.V., Jr. Serial changes in plasma levels of cytokines in patients with coronary artery disease. Vasc. Health Risk Manag. 2005, 1, 245–250. [Google Scholar] [PubMed]
- Kang, H.J.; Kim, J.M.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Kim, H.R.; Shin, M.G.; Yoon, J.S. Longitudinal associations between BDNF promoter methylation and late-life depression. Neurobiol. Aging 2015, 36, 1764.e1–1764.e7. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; O’Connor, C.M.; Califf, R.M.; Swedberg, K.; Schwartz, P.; Bigger, J.T., Jr.; Krishnan, K.R.; van Zyl, L.T.; Swenson, J.R.; Finkel, M.S.; et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002, 288, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.M.; Brain, U.; Austin, J.; Oberlander, T.F. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS ONE 2010, 5, e12201. [Google Scholar] [CrossRef]
| Low IL-18 (n = 484) | High IL-18 (n = 485) | p-Value for Interaction | |
|---|---|---|---|
| Major adverse cardiac events | 1.25 (0.83–1.88) | 2.15 (1.42–3.26) ‡ | 0.019 |
| All-cause mortality | 1.26 (0.76–2.09) | 1.89 (1.11–3.22) * | 0.192 |
| Cardiac death | 1.11 (0.60–2.05) | 1.60 (0.81–3.17) | 0.250 |
| Myocardial infarction | 0.89 (0.47–1.69) | 1.98 (1.07–3.67) * | 0.027 |
| Percutaneous coronary intervention | 1.09 (0.65–1.82) | 1.81 (1.01–3.23) * | 0.147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Kang, H.-J.; Kim, J.-W.; Kim, H.K.; Kang, H.-C.; Kim, S.-W.; Kim, J.-C.; Ahn, Y.; Jeong, M.H.; Kim, J.-M. Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. Int. J. Mol. Sci. 2022, 23, 15270. https://doi.org/10.3390/ijms232315270
Choi W, Kang H-J, Kim J-W, Kim HK, Kang H-C, Kim S-W, Kim J-C, Ahn Y, Jeong MH, Kim J-M. Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. International Journal of Molecular Sciences. 2022; 23(23):15270. https://doi.org/10.3390/ijms232315270
Chicago/Turabian StyleChoi, Wonsuk, Hee-Ju Kang, Ju-Wan Kim, Hee Kyung Kim, Ho-Cheol Kang, Sung-Wan Kim, Jung-Chul Kim, Youngkeun Ahn, Myung Ho Jeong, and Jae-Min Kim. 2022. "Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome" International Journal of Molecular Sciences 23, no. 23: 15270. https://doi.org/10.3390/ijms232315270
APA StyleChoi, W., Kang, H.-J., Kim, J.-W., Kim, H. K., Kang, H.-C., Kim, S.-W., Kim, J.-C., Ahn, Y., Jeong, M. H., & Kim, J.-M. (2022). Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. International Journal of Molecular Sciences, 23(23), 15270. https://doi.org/10.3390/ijms232315270






