Light Emission from M-Type Enantiomer of 2,13-bis(hydroxymethyl)[7]-thiaheterohelicene Molecules Adsorbed on Au(111) and C60/Au(111) Surfaces Investigated by STM-LE
Abstract
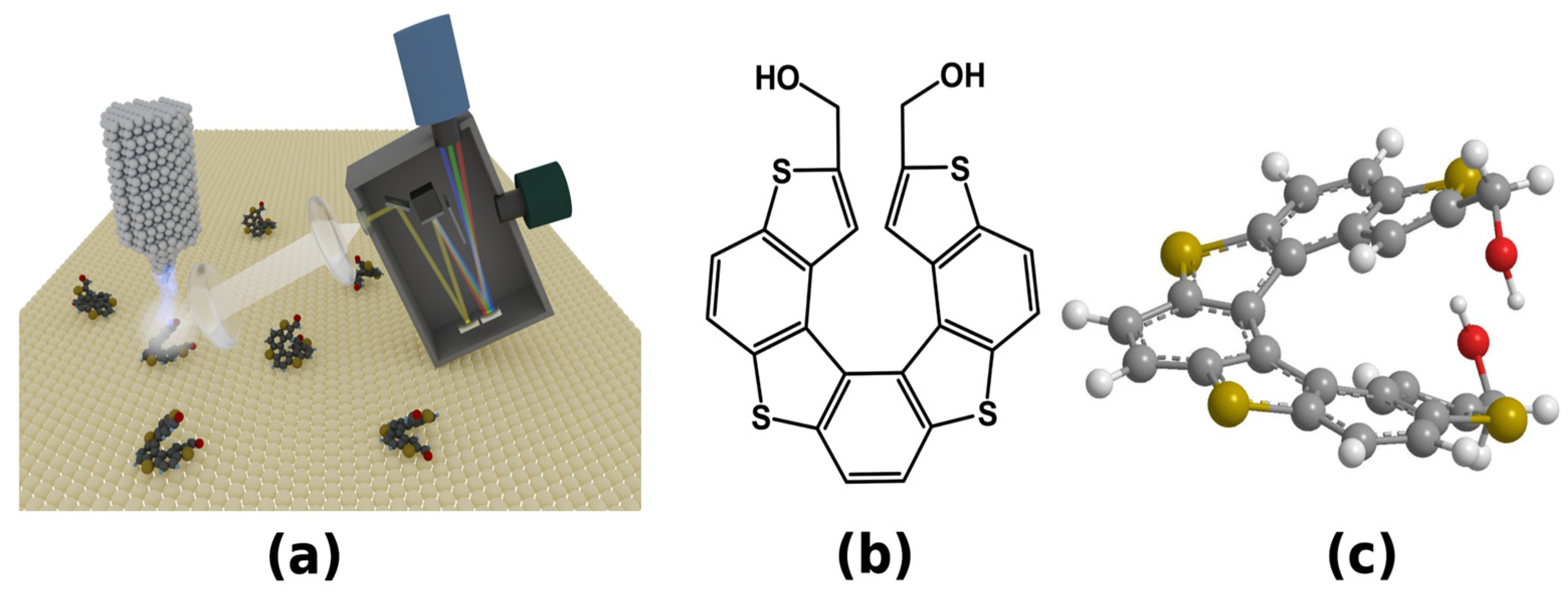
:1. Introduction
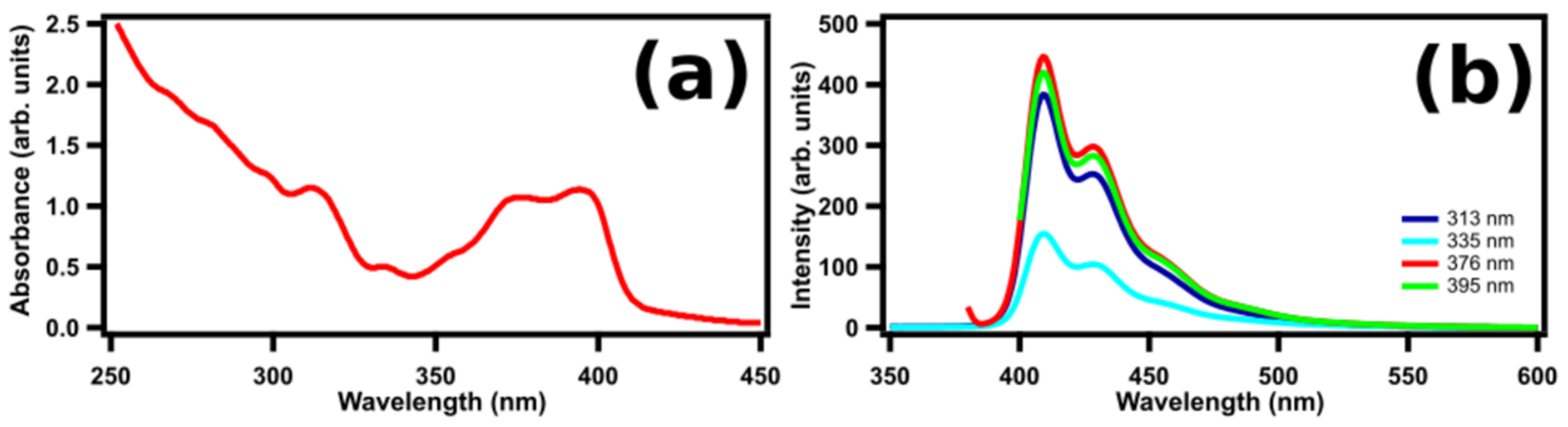
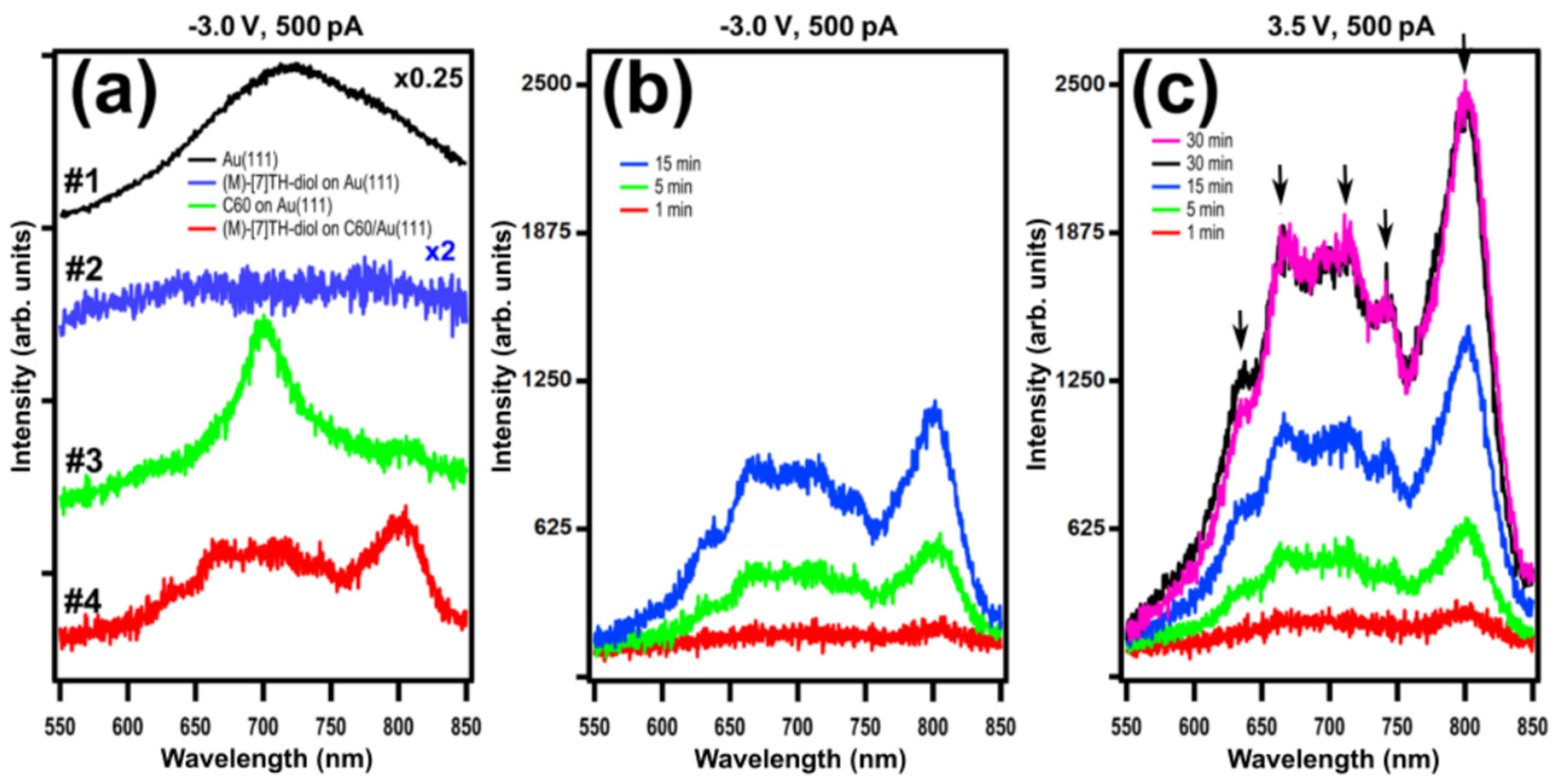
2. Results and Discussion
2.1. STM-LE Investigation of (M)-[7]TH-diol Molecules Adsorbed on Au(111)
2.2. C60 Buffer Layer Grown on Au(111)
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, X.H.; Nazin, G.V.; Hot, W. Vibrationally Resolved Fluorescence Excited with Submolecular Precision. Science 2003, 299, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Ćavar, E.; Blüm, M.C.; Pivetta, M.; Patthey, F.; Chergui, M.; Schneider, W.D. Fluorescence and Phosphorescence from Individual C60 Molecules Excited by Local Electron Tunneling. Phys. Rev. Lett. 2005, 95, 196102. [Google Scholar] [CrossRef] [Green Version]
- Lutz, T.; Große, C.; Dette, C.; Kabakchiev, A.; Schramm, F.; Ruben, M.; Gutzler, R.; Kuhnke, K.; Schlickum, U.; Kern, K. Molecular Orbital Gates for Plasmon Excitation. Nano Lett. 2013, 13, 2846–2850. [Google Scholar] [CrossRef]
- Krukowski, P.; Tsuzuki, T.; Minagawa, Y.; Yajima, N.; Chaunchaiyakul, S.; Akai-Kasaya, M.; Saito, A.; Miyake, Y.; Katayama, M.; Kuwahara, Y. Detection of Light Emission from (S)-PTCDI Molecules Adsorbed on Au(111) and NiAl(110) Surfaces Induced by a Scanning Tunneling Microscope. J. Phys. Chem. C 2016, 120, 3964–3977. [Google Scholar] [CrossRef]
- Krukowski, P.; Chaunchaiyakul, S.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Adsorption and Light Emission of a Racemic Mixture of [7]Thiaheterohelicene-2,13-Carboxaldehyde on Au(111), Cu(001), and Nial(110) Surfaces Investigated Using a Scanning Tunneling Microscope. J. Phys. Chem. C 2021, 125, 9419–9427. [Google Scholar] [CrossRef]
- Geng, F.; Kuang, Y.; Yu, Y.; Liao, Y.; Zhang, Y.; Zhang, Y.; Dong, Z. Tunneling Electron Induced Luminescence from Porphyrin Molecules on Monolayer Graphene. J. Lumin. 2015, 157, 39–45. [Google Scholar] [CrossRef]
- Kaiser, K.; Gross, L.; Schulz, F. A Single-Molecule Chemical Reaction Studied by High-Resolution Atomic Force Microscopy and Scanning Tunneling Microscopy Induced Light Emission. ACS Nano 2019, 13, 6947–6954. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.C.; Kiraly, B.; Strik, J.H.; Khajetoorians, A.A.; Wegner, D. Plasmon-Driven Motion of an Individual Molecule. Nano Lett. 2021, 21, 5006–5012. [Google Scholar] [CrossRef]
- Chen, G.; Luo, Y.; Gao, H.; Jiang, J.; Yu, Y.; Zhang, L.; Zhang, Y.; Li, X.; Zhang, Z.; Dong, Z. Spin-Triplet-Mediated Up-Conversion and Crossover Behavior in Single-Molecule Electroluminescence. Phys. Rev. Lett. 2019, 122, 177401. [Google Scholar] [CrossRef]
- Rai, V.; Gerhard, L.; Sun, Q.; Holzer, C.; Repän, T.; Krstić, M.; Yang, L.; Wegener, M.; Rockstuhl, C.; Wulfhekel, W. Boosting Light Emission from Single Hydrogen Phthalocyanine Molecules by Charging. Nano Lett. 2020, 20, 7600–7605. [Google Scholar] [CrossRef]
- Liu, H.; Ie, Y.; Yoshinobu, T.; Aso, Y.; Iwasaki, H.; Nishitani, R. Plasmon-Enhanced Molecular Fluorescence from an Organic Film in a Tunnel Junction. Appl. Phys. Lett. 2006, 88, 61901. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.W.; Nazin, G.V.; Ho, W. Intramolecular Photon Emission from a Single Molecule in a Scanning Tunneling Microscope. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77, 205430. [Google Scholar] [CrossRef]
- Liu, H.W.; Nishitani, R.; Han, T.Z.; Ie, Y.; Aso, Y.; Iwasaki, H. STM Fluorescence of Porphyrin Enhanced by a Strong Plasmonic Field and Its Nanoscale Confinement in an STM Cavity. Phys. Rev. B-Condens. Matter Mater. Phys. 2009, 79, 125415. [Google Scholar] [CrossRef] [Green Version]
- Rossel, F.; Pivetta, M.; Schneider, W.D. Luminescence Experiments on Supported Molecules with the Scanning Tunneling Microscope. Surf. Sci. Rep. 2010, 65, 129–144. [Google Scholar] [CrossRef]
- Schneider, N.L.; Matino, F.; Schull, G.; Gabutti, S.; Mayor, M.; Berndt, R. Light Emission from a Double-Decker Molecule on a Metal Surface. Phys. Rev. B-Condens. Matter Mater. Phys. 2011, 84, 153403. [Google Scholar] [CrossRef]
- Rzeźnicka, I.I.; Yamada, T.; Kawai, M. Light Emission from PTCDA Bilayer on Au(111) Induced in Scanning Tunneling Microscopy. Surf. Sci. 2011, 605, 2032–2037. [Google Scholar] [CrossRef]
- Fujiki, A.; Miyake, Y.; Oshikane, Y.; Akai-Kasaya, M.; Saito, A.; Kuwahara, Y. STM-Induced Light Emission from Thin Films of Perylene Derivatives on the HOPG and Au Substrates. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.L.; Berndt, R. Plasmonic Exciation of Light Emission and Absorption by Porphyrine Molecules in a Scanning Tunneling Microscope. Phys. Rev. B-Condens. Matter Mater. Phys. 2012, 86, 35445. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- Gingras, G. One Hundred Years of Helicene Chemistry. Part 3: Applications and Properties of Carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef]
- Zhao, W.L.; Li, M.; Lu, H.Y.; Chen, C.F. Advances in Helicene Derivatives with Circularly Polarized Luminescence. Chem. Commun. 2019, 55, 13793–13803. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, N.; Miao, J.; Zhan, L.; Gong, S.; Huang, Z.; Yang, C. Simple Double Hetero[5]Helicenes Realize Highly Efficient and Narrowband Circularly Polarized Organic Light-Emitting Diodes. CCS Chem. 2022, 4, 3463–3471. [Google Scholar] [CrossRef]
- Bracciale, M.P.; Kwon, G.; Ho, D.; Kim, C.; Santarelli, M.L.; Marrocchi, A. Synthesis, Characterization, and Thin-Film Transistor Response of Benzo[i]Pentahelicene-3,6-Dione. Molecules 2022, 27, 863. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.H. Stereochemical Recognition of Helicenes on Metal Surfaces. Acc. Chem. Res. 2016, 49, 1182–1190. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Krukowski, P.; Tsuzuki, T.; Minagawa, Y.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Self-Assembly Formation of M-Type Enantiomer of 2,13-Bis(Hydroxymethyl)[7]-Thiaheterohelicene Molecules on Au(111) Surface Investigated by STM/CITS. J. Phys. Chem. C 2015, 119, 21434–21442. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Setiadi, A.; Krukowski, P.; Catalan, F.C.I.; Akai-Kasaya, M.; Saito, A.; Hayazawa, N.; Kim, Y.; Osuga, H.; Kuwahara, Y. Nanoscale Dehydrogenation Observed by Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2017, 121, 18162–18168. [Google Scholar] [CrossRef]
- Krukowski, P.; Hattori, T.; Okada, M.; Piskorski, M.; Lutsyk, I.; Saito, A.; Osuga, H.; Kuwahara, Y. Study of Stereochemical Crystallization of Racemic Mixtures of [5] and [7]Thiaheterohelicene Molecules on Ag(111) Surface by Scanning Tunneling Microscopy and Raman Scattering Spectroscopy. Appl. Surf. Sci. 2022, 589, 152860. [Google Scholar] [CrossRef]
- Krukowski, P.; Kozlowski, W.; Olejniczak, W.; Klusek, Z.; Puchalski, M.; Dabrowski, P.; Kowalczyk, P.J.; Gwozdzinski, K.; Grabowski, G. Formation of Dense Nitroxide Radical Layers on the Au(1 1 1) Substrate for ESN-STM Measurement. Appl. Surf. Sci. 2008, 255, 1921–1928. [Google Scholar] [CrossRef]
- Krukowski, P.; Kowalczyk, P.J.; Krzyczmonik, P.; Olejniczak, W.; Klusek, Z.; Puchalski, M.; Gwozdzinski, K. Electrochemical Behaviour of Gold Modified with Contaminated TMP Amine Adlayers Studied by STM, CV, EPR. Appl. Surf. Sci. 2009, 255, 3946–3952. [Google Scholar] [CrossRef]
- Zhang, T.; Svensson, P.H.W.; Brumboiu, I.E.; Lanzilotto, V.; Grazioli, C.; Guarnaccio, A.; Johansson, F.O.L.; Beranová, K.; Coreno, M.; de Simone, M.; et al. Clarifying the Adsorption of Triphenylamine on Au(111): Filling the HOMO–LUMO Gap. J. Phys. Chem. C 2022, 126, 1635–1643. [Google Scholar] [CrossRef]
- Tao, X.; Dong, Z.C.; Yang, J.L.; Luo, Y.; Hou, J.G.; Aizpurua, J. Influence of a Dielectric Layer on Photon Emission Induced by a Scanning Tunneling Microscope. J. Chem. Phys. 2009, 130, 84706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitani, R.; Tateishi, Y.; Arakawa, H.; Kasuya, A.; Sumiyama, K. STM-Induced Luminescence for Alkanethiol Films on Au(1 1 1) Surface. Acta Phys. Pol. A 2003, 104, 269–279. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, Y.; Yu, Y.; Kuang, Y.; Liao, Y.; Dong, Z.; Hou, J. Modulation of Nanocavity Plasmonic Emission by Local Molecular States of C_60 on Au(111). Opt. Express 2012, 20, 26725. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.C.; Trifonov, A.S.; Guo, X.L.; Amemiya, K.; Yokoyama, S.; Kamikado, T.; Yamada, T.; Mashiko, S.; Okamoto, T. Tunneling Electron Induced Photon Emission from Monolayered H2TBP Porphyrin Molecules on Cu( 1 0 0 ). Surf. Sci. 2003, 532–535, 237–243. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krukowski, P.; Hattori, T.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Light Emission from M-Type Enantiomer of 2,13-bis(hydroxymethyl)[7]-thiaheterohelicene Molecules Adsorbed on Au(111) and C60/Au(111) Surfaces Investigated by STM-LE. Int. J. Mol. Sci. 2022, 23, 15399. https://doi.org/10.3390/ijms232315399
Krukowski P, Hattori T, Akai-Kasaya M, Saito A, Osuga H, Kuwahara Y. Light Emission from M-Type Enantiomer of 2,13-bis(hydroxymethyl)[7]-thiaheterohelicene Molecules Adsorbed on Au(111) and C60/Au(111) Surfaces Investigated by STM-LE. International Journal of Molecular Sciences. 2022; 23(23):15399. https://doi.org/10.3390/ijms232315399
Chicago/Turabian StyleKrukowski, Paweł, Takuma Hattori, Megumi Akai-Kasaya, Akira Saito, Hideji Osuga, and Yuji Kuwahara. 2022. "Light Emission from M-Type Enantiomer of 2,13-bis(hydroxymethyl)[7]-thiaheterohelicene Molecules Adsorbed on Au(111) and C60/Au(111) Surfaces Investigated by STM-LE" International Journal of Molecular Sciences 23, no. 23: 15399. https://doi.org/10.3390/ijms232315399





