Redox-Activation of Neutrophils Induced by Pericardium Scaffolds
Abstract
:1. Introduction
2. Results
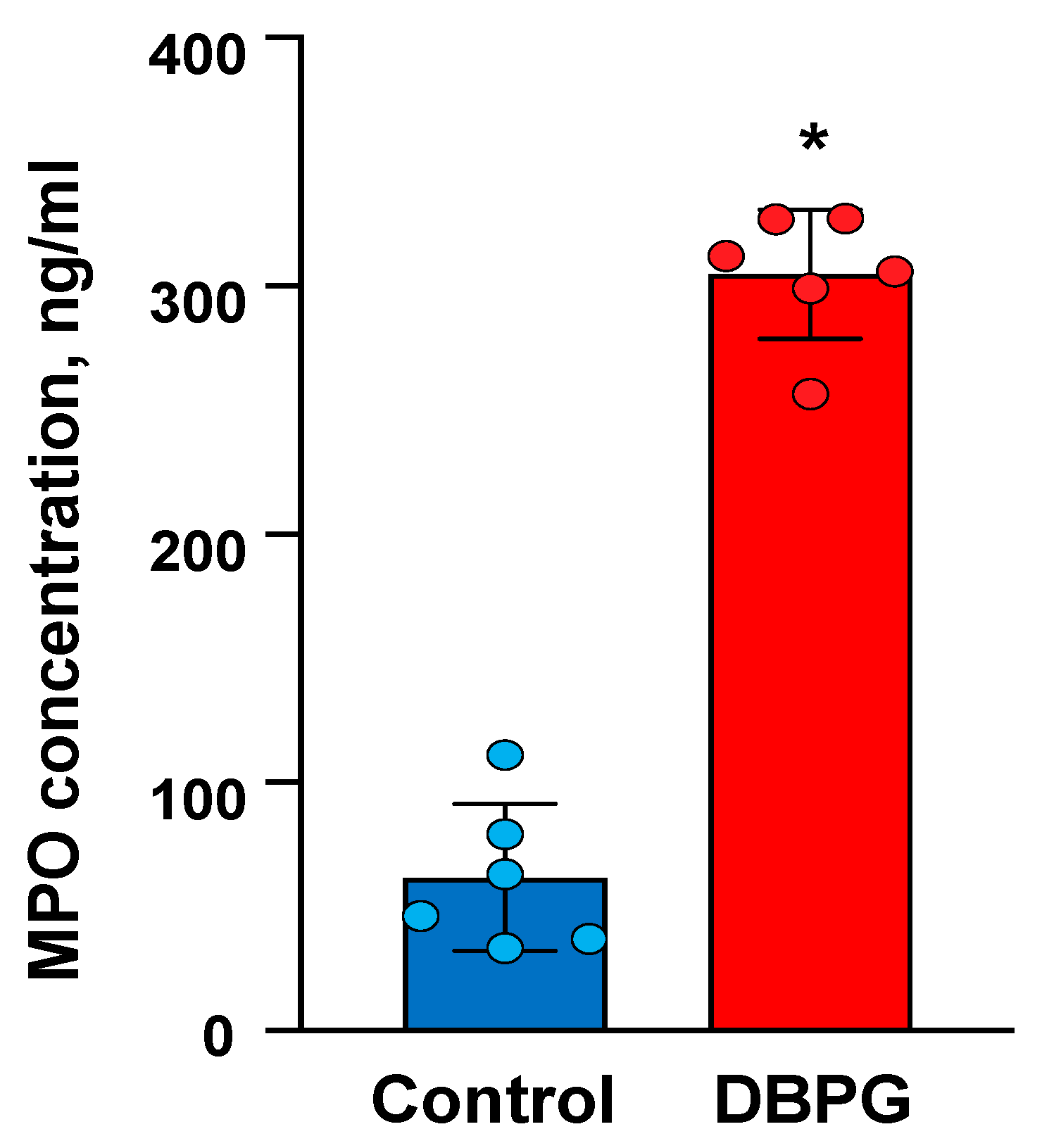
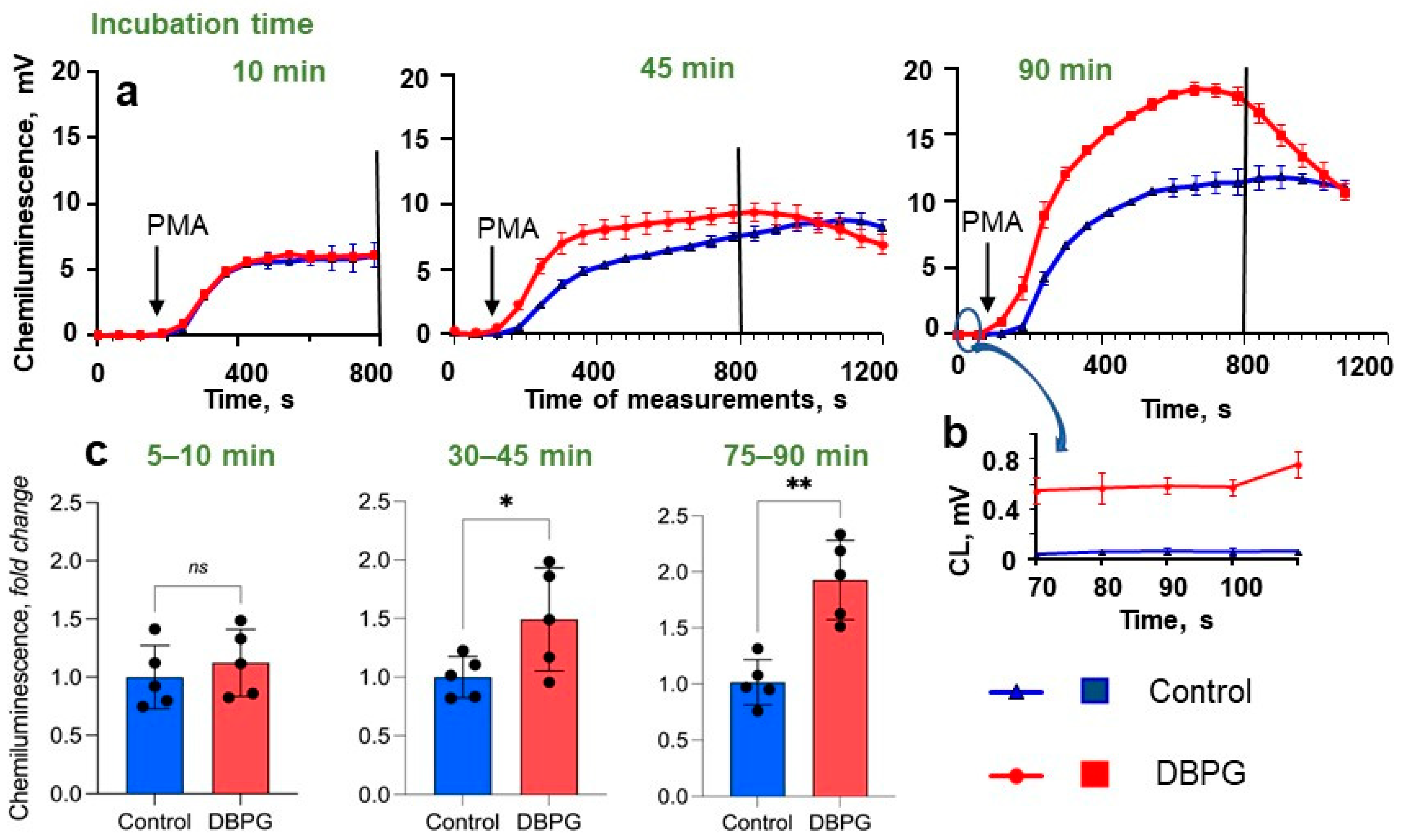
2.1. Activation of Neutrophils by Pericardium Scaffolds in Whole Blood
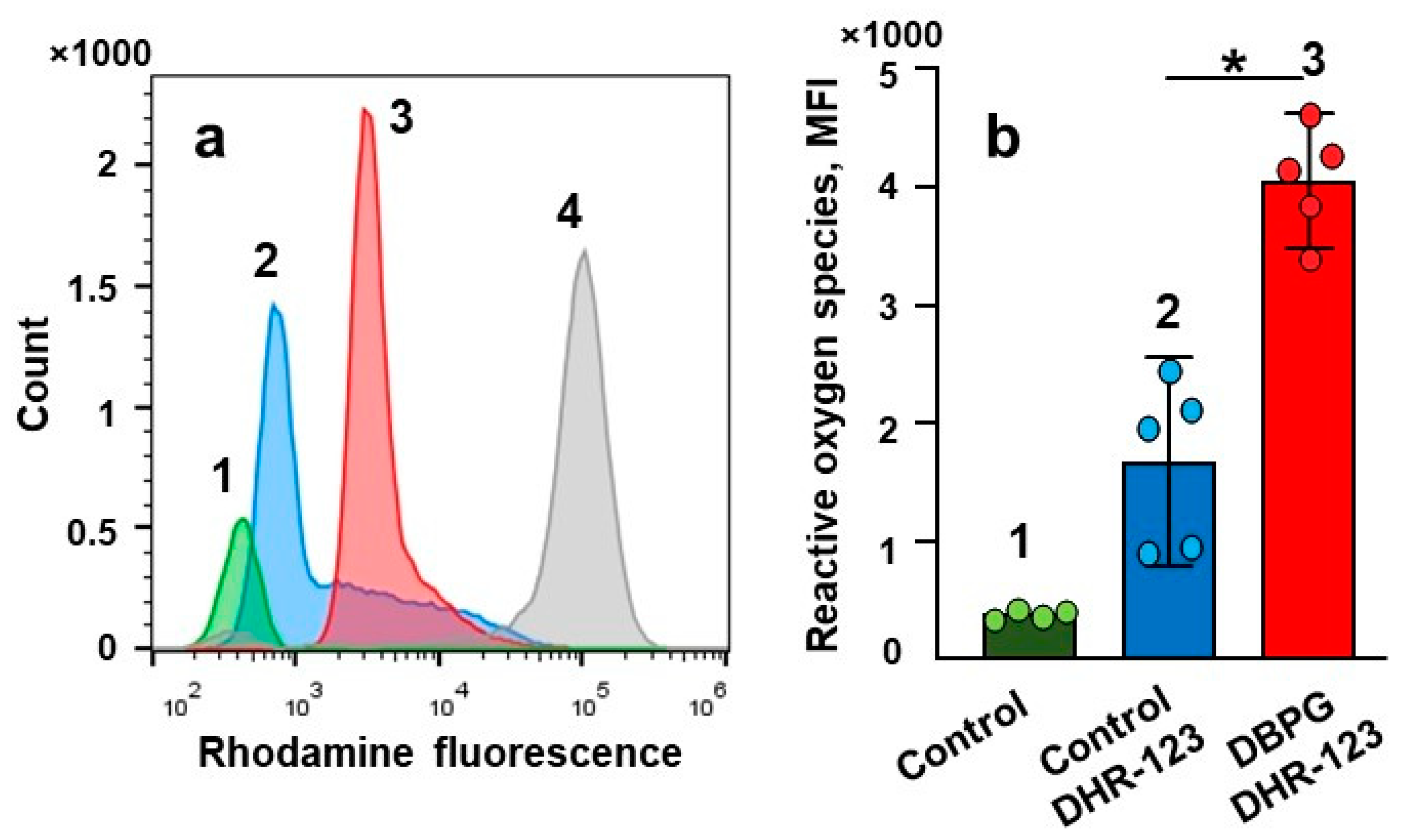
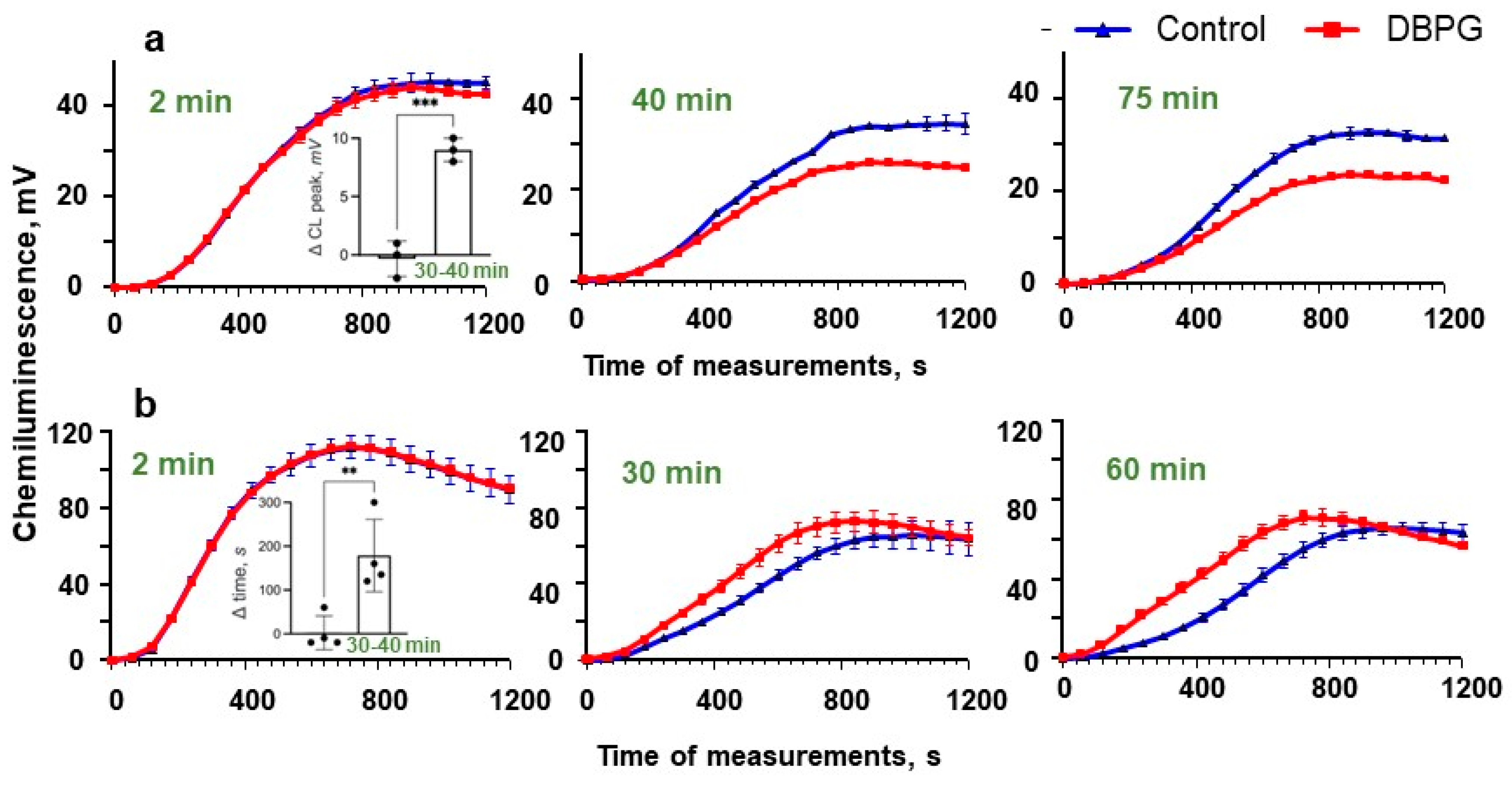
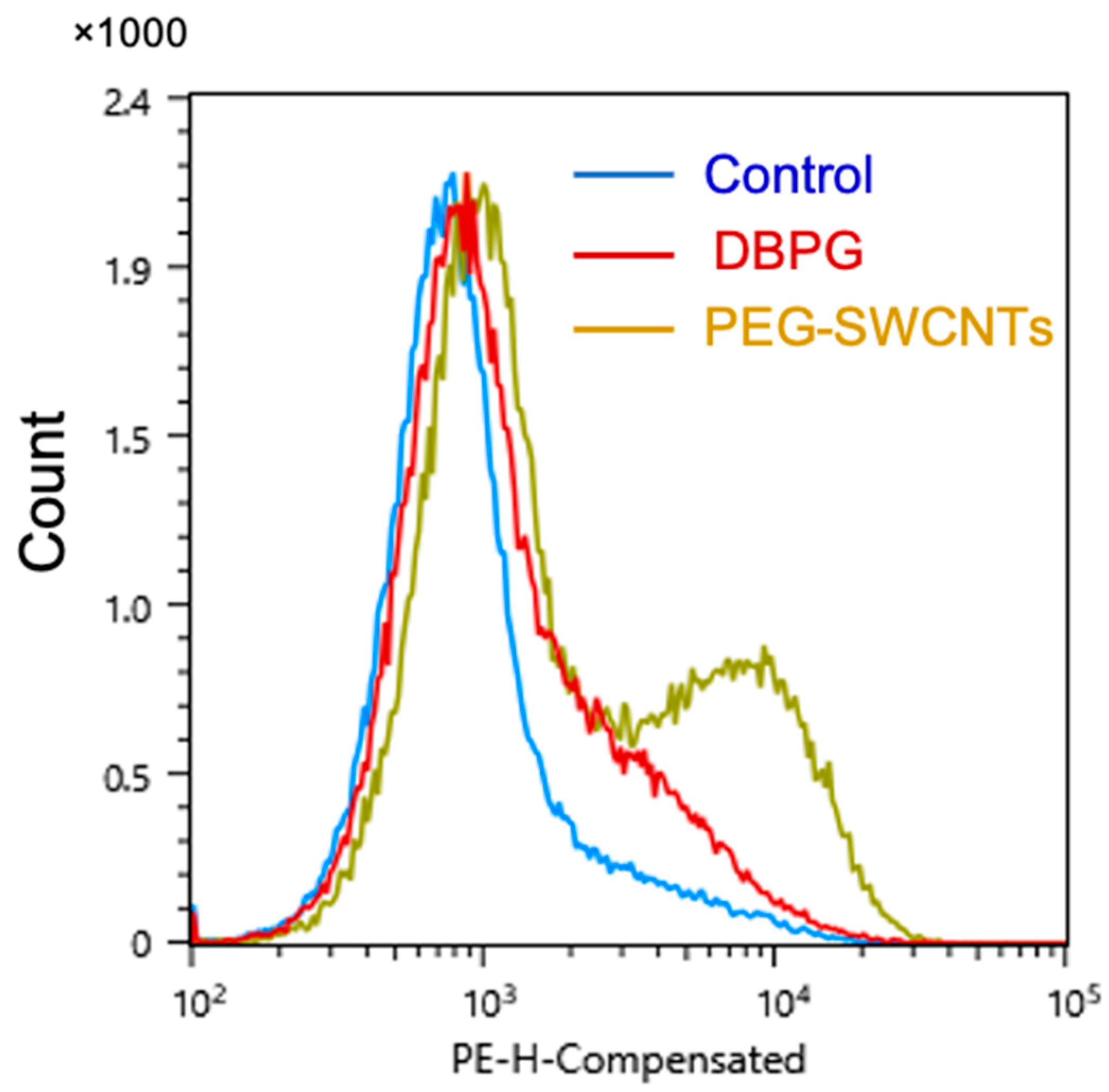
2.2. Activation of Isolated Neutrophils by Pericardium Scaffold
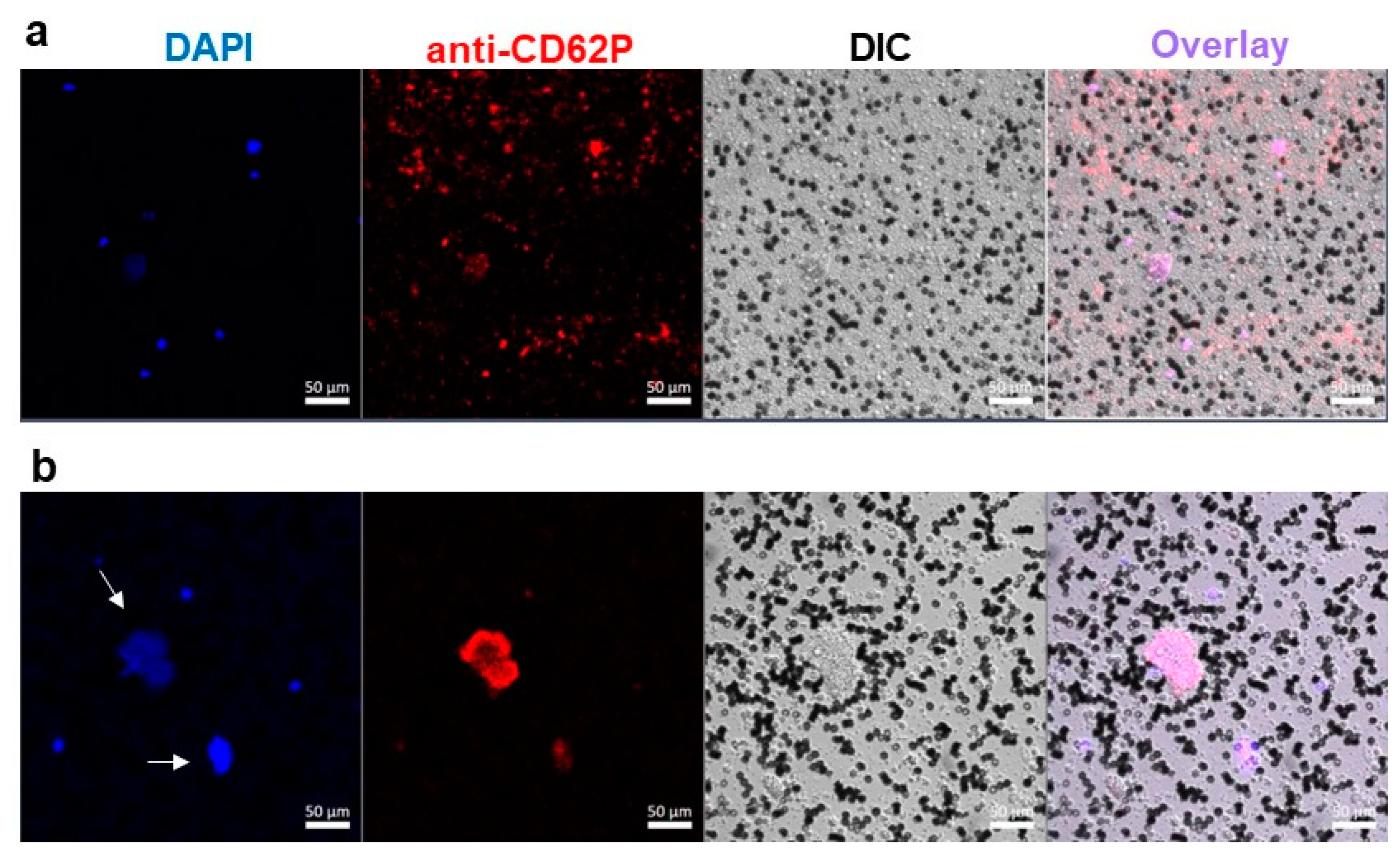
2.3. Platelet Activation in Blood Exposed to DBPG
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Myeloperoxidase Measurements
4.3. Flow Cytometry
4.4. Luminol-Dependent Chemiluminescence
4.5. Confocal Microscopy
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | luminol-dependent chemiluminescence |
| DP | decellularized pericardium |
| DBP | decellularized bovine pericardium (collagen type I) |
| DBPG DBP | crosslinked with genipin |
| DBP-EGDE DBPG | crosslinked with ethylene glycol diglycidyl ether |
| DHR123 | dihydrorhodamine 123 |
| DIC | differential interference contrast |
| ErPB | erythrocyte-poor blood |
| FLIM | fluorescence lifetime imaging |
| MFI | median fluorescence intensity |
| MPO | myeloperoxidase |
| NET | neutrophil extracellular traps |
| PE | phycoerythrin |
| PEG-SWCNTs | pegylated single-walled carbon nanotubes |
| PMA | phorbol 12-myristate 13-acetate |
| PRP | platelet rich plasma |
| ROS | reactive oxygen species |
| RT | room temperature |
References
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Sun, Y.; Pedersen, T.O.; Xue, Y.; Nickel, J.; Waag, T.; Finne-Wistrand, A.; Steinmüller-Nethl, D.; Krueger, A.; Costea, D.E.; et al. In Vivo Host Response and Degradation of Copolymer Scaffolds Functionalized with Nanodiamonds and Bone Morphogenetic Protein 2. Adv. Heal. Mater. 2016, 5, 730–742. [Google Scholar] [CrossRef] [Green Version]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2018, 192, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, A.; Gafarova, E.R.; Istranov, L.P.; Istranova, V.; Ma, X.; Xu, J.; Guo, W.; Atala, A.; Timashev, P.S. Mammalian Pericardium-Based Bioprosthetic Materials in Xenotransplantation and Tissue Engineering. Biotechnol. J. 2020, 15, e1900334. [Google Scholar] [CrossRef]
- Gauvin, R.; Marinov, G.; Mehri, Y.; Klein, J.; Li, B.; Larouche, D.; Guzman, R.; Zhang, Z.; Germain, L.; Guidoin, R. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J. Biomater. Appl. 2012, 28, 552–565. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Elagin, V.; Kuznetsova, D.; Grebenik, E.; Zolotov, D.A.; Istranov, L.; Zharikova, T.; Istranova, E.; Polozova, A.; Reunov, D.; Kurkov, A.; et al. Multiparametric Optical Bioimaging Reveals the Fate of Epoxy Crosslinked Biomeshes in the Mouse Subcutaneous Implantation Model. Front. Bioeng. Biotechnol. 2020, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Grebenik, E.A.; Istranov, L.P.; Istranova, E.V.; Churbanov, S.N.; Shavkuta, B.S.; Dmitriev, R.I.; Veryasova, N.N.; Kotova, S.L.; Kurkov, A.V.; Shekhter, A.B.; et al. Chemical cross-linking of xenopericardial biomeshes: A bottom-up study of structural and functional correlations. Xenotransplantation 2019, 26, e12506. [Google Scholar] [CrossRef]
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2019, 48, 2132–2153. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, X.; Wu, Q.; Li, Y.; Zhou, Y.; Li, L.; Bu, H. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci. Rep. 2016, 6, 24779. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.-C.; Liang, H.-C.; Sung, H.-W. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 2001, 23, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Klut, M.E.; Walker, B.A.; Hogg, J.C. The use of flow cytometry to measure neutrophil function. J. Immunol. Methods 1999, 232, 23–43. [Google Scholar] [CrossRef]
- Nauseef, W.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Mikhalchik, E.; Basyreva, L.Y.; Gusev, S.A.; Panasenko, O.M.; Klinov, D.V.; Barinov, N.A.; Morozova, O.V.; Moscalets, A.P.; Maltseva, L.N.; Filatova, L.Y.; et al. Activation of Neutrophils by Mucin–Vaterite Microparticles. Int. J. Mol. Sci. 2022, 23, 10579. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef] [Green Version]
- Page, C.; Pitchford, S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int. Immunopharmacol. 2013, 17, 1176–1184. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef]
- Jannasch, M.; Gaetzner, S.; Groeber, F.; Weigel, T.; Walles, H.; Schmitz, T.; Hansmann, J. An in vitro model mimics the contact of biomaterials to blood components and the reaction of surrounding soft tissue. Acta Biomater. 2019, 89, 227–241. [Google Scholar] [CrossRef]
- Yakimov, B.P.; Vlasova, I.I.; Efremov, Y.M.; Maksimov, E.G.; Shirshin, E.A.; Kagan, V.E.; Timashev, P.S. Detection of HOCl-driven degradation of the pericardium scaffolds by label-free multiphoton fluorescence lifetime imaging. Sci. Rep. 2022, 12, 10329. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Staňková, L.; Eckhardt, A.; Svobodová, J.; Musilkova, J.; Pala, J.; Hadraba, D.; Brynda, E.; Koňařík, M.; Pirk, J.; et al. Modification of Human Pericardium by Chemical Crosslinking. Physiol. Res. 2020, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef] [PubMed]
- Mol, S.; Hafkamp, F.M.J.; Varela, L.; Simkhada, N.; Taanman-Kueter, E.W.; Tas, S.W.; Wauben, M.H.M.; Kormelink, T.G.; de Jong, E.C. Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli. Int. J. Mol. Sci. 2021, 22, 10106. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Mikhalchik, E.V.; Gusev, A.A.; Balabushevich, N.; Gusev, S.A.; Kazarinov, K.D. Extremely high-frequency electromagnetic radiation enhances neutrophil response to particulate agonists. Bioelectromagnetics 2017, 39, 144–155. [Google Scholar] [CrossRef]
- Nirala, N.R.; Harel, Y.; Lellouche, J.-P.; Shtenberg, G. Ultrasensitive haptoglobin biomarker detection based on amplified chemiluminescence of magnetite nanoparticles. J. Nanobiotechnol. 2020, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [Green Version]
- Vakhrusheva, T.V.; Gusev, A.A.; Gusev, S.A.; Vlasova, I.I. Albumin reduces thrombogenic potential of single-walled carbon nanotubes. Toxicol. Lett. 2013, 221, 137–145. [Google Scholar] [CrossRef]
- Lisman, T. Platelet–neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2017, 371, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Hachim, D.; Lopresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef]
- Alhamdi, J.R.; Peng, T.; Al-Naggar, I.M.; Hawley, K.L.; Spiller, K.L.; Kuhn, L.T. Controlled M1-to-M2 transition of aged macrophages by calcium phosphate coatings. Biomaterials 2019, 196, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Antmen, E.; Vrana, N.E.; Hasirci, V. The role of biomaterials and scaffolds in immune responses in regenerative medicine: Macrophage phenotype modulation by biomaterial properties and scaffold architectures. Biomater. Sci. 2021, 9, 8090–8110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shklover, J.; Parvizi, M.; Sherlock, B.E.; Garcia, A.A.; Haudenschild, A.K.; Griffiths, L.G.; Marcu, L. Label-Free Assessment of Collagenase Digestion on Bovine Pericardium Properties by Fluorescence Lifetime Imaging. Ann. Biomed. Eng. 2018, 46, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Jančinová, V.; Drábiková, K.; Nosál, R.; Račková, L.; Majekova, M.; Holomanova, D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox Rep. 2006, 11, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bedouhène, S.; Moulti-Mati, F.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. Am. J. blood Res. 2017, 7, 41–48. [Google Scholar]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Ledo, A.; Fernandes, E.; Salvador, A.; Laranjinha, J.; Barbosa, R. In vivo hydrogen peroxide diffusivity in brain tissue supports volume signaling activity. Redox Biol. 2022, 50. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Jaken, S. Protein kinase C signaling and oxidative stress. Free. Radic. Biol. Med. 2000, 28, 1349–1361. [Google Scholar] [CrossRef]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous Acid Oxygenates the Cysteine Switch Domain of Pro-matrilysin (MMP-7): A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rapture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Masyutin, A.G.; Bagrov, D.V.; Vlasova, I.I.; Nikishin, I.I.; Klinov, D.V.; Sychevskaya, K.A.; Onishchenko, G.E.; Erokhina, M.V. Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite. Nanomaterials 2018, 8, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, K.; Mahoney, J.R.; Coury, A.J.; Eaton, J.W. Degradation of biomaterials by phagocyte-derived oxidants. J. Clin. Investig. 1993, 92, 2360–2367. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Harmsen, M.; van Luyn, M.J.; Bank, R.A. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 2010, 31, 9192–9201. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Shortridge, C.; Fakhrabadi, E.A.; Wuescher, L.M.; Worth, R.G.; Liberatore, M.W.; Yildirim-Ayan, E. Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold. Int. J. Mol. Sci. 2021, 22, 1134. [Google Scholar] [CrossRef]
- Karkanitsa, M.; Fathi, P.; Ngo, T.; Sadtler, K. Mobilizing Endogenous Repair Through Understanding Immune Reaction With Biomaterials. Front. Bioeng. Biotechnol. 2021, 9, 730938. [Google Scholar] [CrossRef]
- Vlasova, I.I. The effect of oxidatively modified low-density lipoproteins on platelet aggregability and membrane fluidity. Platelets 2000, 11, 406–414. [Google Scholar] [CrossRef]
- Giaglis, S.; Chowdhury, C.S.; van Breda, S.V.; Stoikou, M.; Tiaden, A.N.; Daoudlarian, D.; Schaefer, G.; Buser, A.; Walker, U.A.; Lapaire, O.; et al. Circulatory Neutrophils Exhibit Enhanced Neutrophil Extracellular Trap Formation in Early Puerperium: NETs at the Nexus of Thrombosis and Immunity. Int. J. Mol. Sci. 2021, 22, 13646. [Google Scholar] [CrossRef]
- Ollivier, V.; Roques, C.; Receveur, N.; Gratz, M.; Feldman, L.; Letourneur, D.; Gachet, C.; Mangin, P.H.; Jandrot-Perrus, M. Bioreactivity of stent material: Activation of platelets, coagulation, leukocytes and endothelial cell dysfunction in vitro. Platelets 2016, 28, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.W.; Chen, C.N.; Huang, R.N.; Hsu, J.C.; Chang, W.H. In vitro surface characterization of a biological patch fixed with a naturally occurring crosslinking agent. Biomaterials 2000, 21, 1353–1362. [Google Scholar] [CrossRef]
- Moniot, A.; Braux, J.; Siboni, R.; Guillaume, C.; Audonnet, S.; Allart-Simon, I.; Sapi, J.; Tirouvanziam, R.; Gérard, S.; Gangloff, S.C.; et al. Inhibition of Recruitment and Activation of Neutrophils by Pyridazinone-Scaffold-Based Compounds. Int. J. Mol. Sci. 2022, 23, 7226. [Google Scholar] [CrossRef] [PubMed]
- Pliyev, B.K.; Dimitrieva, T.V.; Savchenko, V.G. Cytokine-mediated induction of MHC class II in human neutrophils is dependent on NADPH oxidase activity. Eur. J. Cell Biol. 2015, 94, 67–70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasova, I.I.; Suleimanov, S.K.; Mikhalchik, E.V.; Urmantaeva, N.T.; Salimov, E.L.; Ragimov, A.A.; Khlebnikova, T.M.; Timashev, P.S. Redox-Activation of Neutrophils Induced by Pericardium Scaffolds. Int. J. Mol. Sci. 2022, 23, 15468. https://doi.org/10.3390/ijms232415468
Vlasova II, Suleimanov SK, Mikhalchik EV, Urmantaeva NT, Salimov EL, Ragimov AA, Khlebnikova TM, Timashev PS. Redox-Activation of Neutrophils Induced by Pericardium Scaffolds. International Journal of Molecular Sciences. 2022; 23(24):15468. https://doi.org/10.3390/ijms232415468
Chicago/Turabian StyleVlasova, Irina I., Shakir K. Suleimanov, Elena V. Mikhalchik, Nailya T. Urmantaeva, Emin L. Salimov, Aligeydar A. Ragimov, Tatyana M. Khlebnikova, and Peter S. Timashev. 2022. "Redox-Activation of Neutrophils Induced by Pericardium Scaffolds" International Journal of Molecular Sciences 23, no. 24: 15468. https://doi.org/10.3390/ijms232415468




