Circ-ERC2 Is Involved in Melatonin Synthesis by Regulating the miR-125a-5p/MAT2A Axis
Abstract
:1. Introduction
2. Results
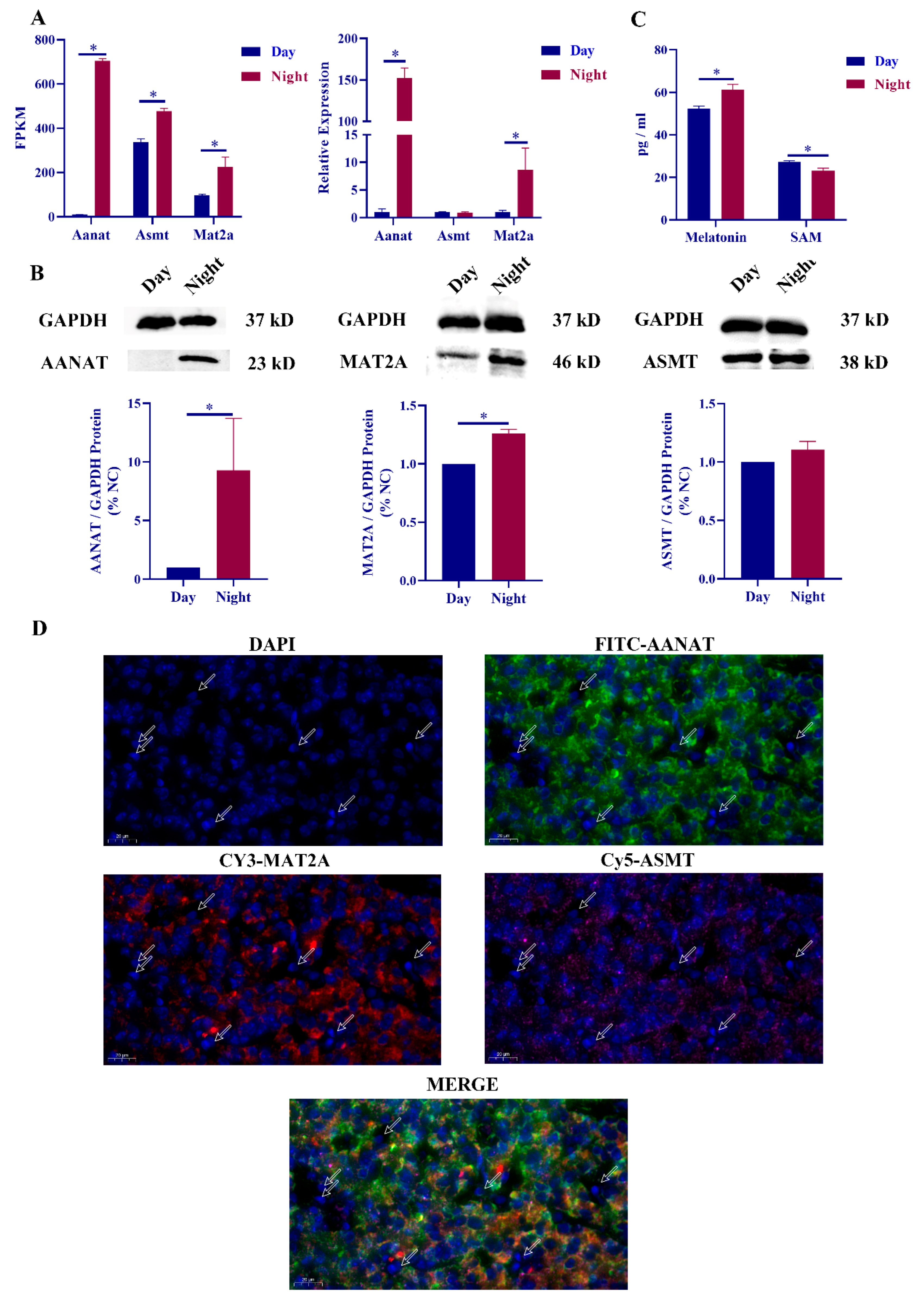
2.1. Correlation between Mat2a and Melatonin Synthesis
2.2. Mat2a Is Involved in the Synthesis of Melatonin
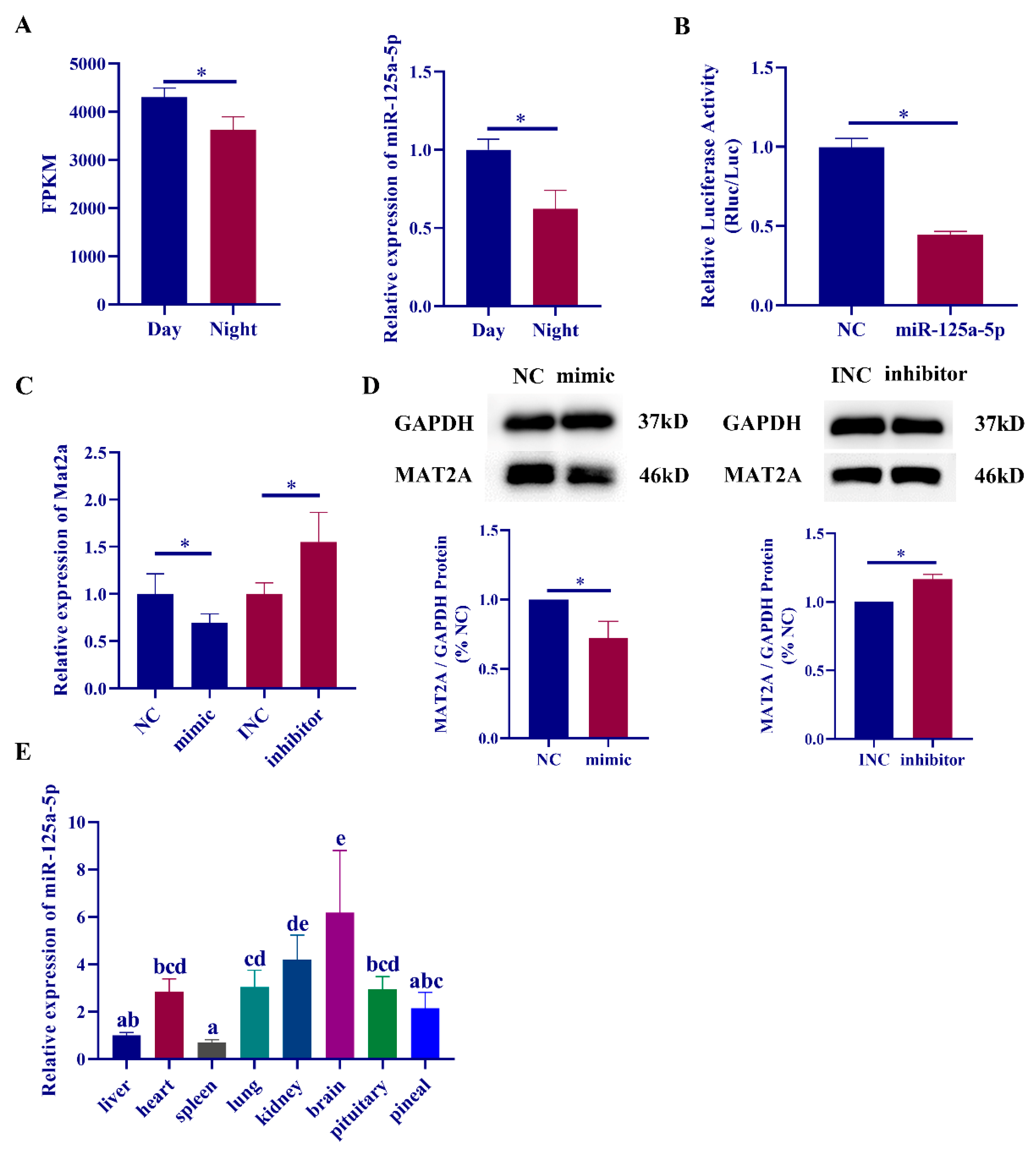
2.3. MiR-125a-5p Participates in Melatonin Synthesis by Regulating the Expression of mat2a
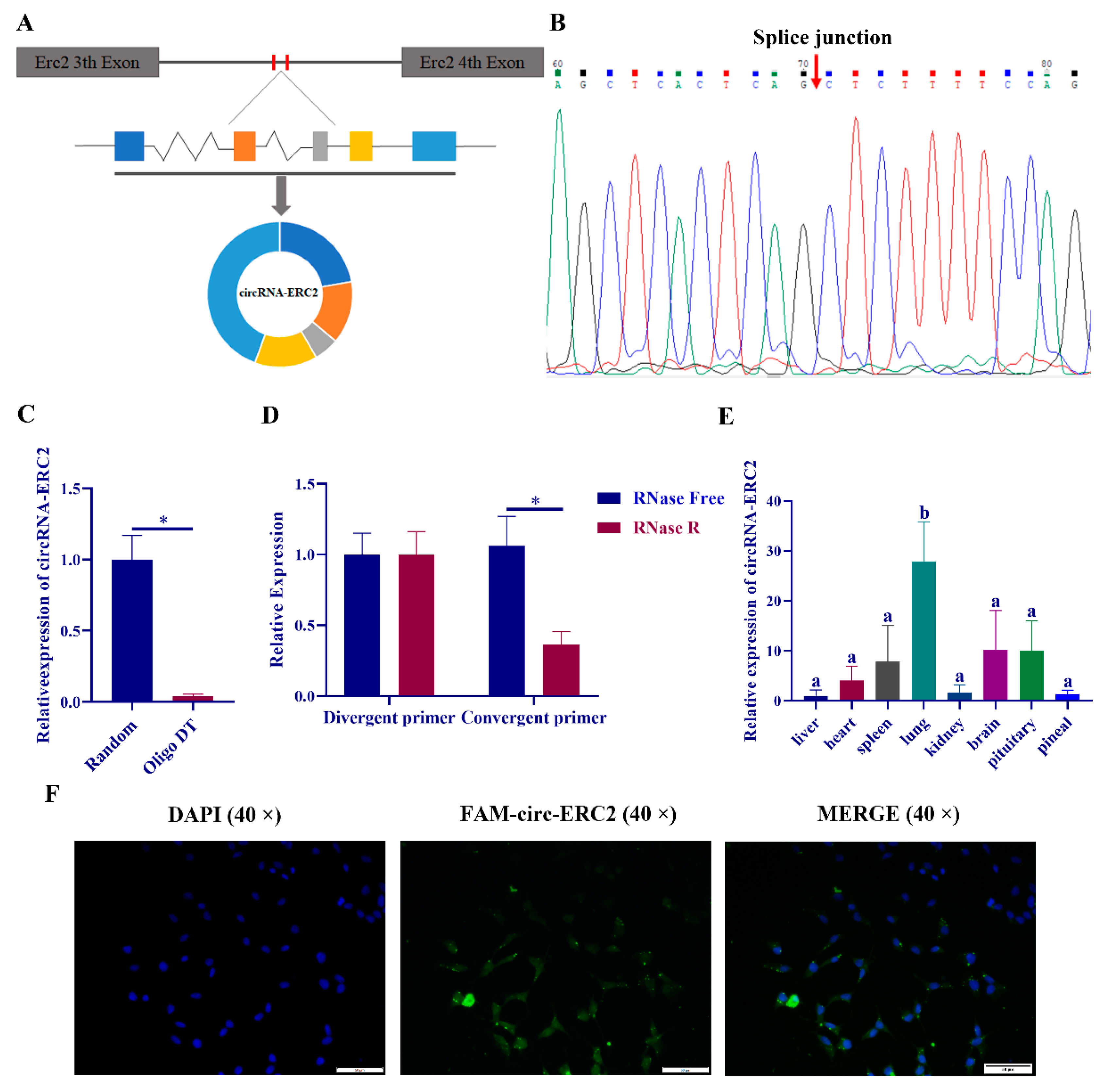
2.4. Identification of Circ-ERC2
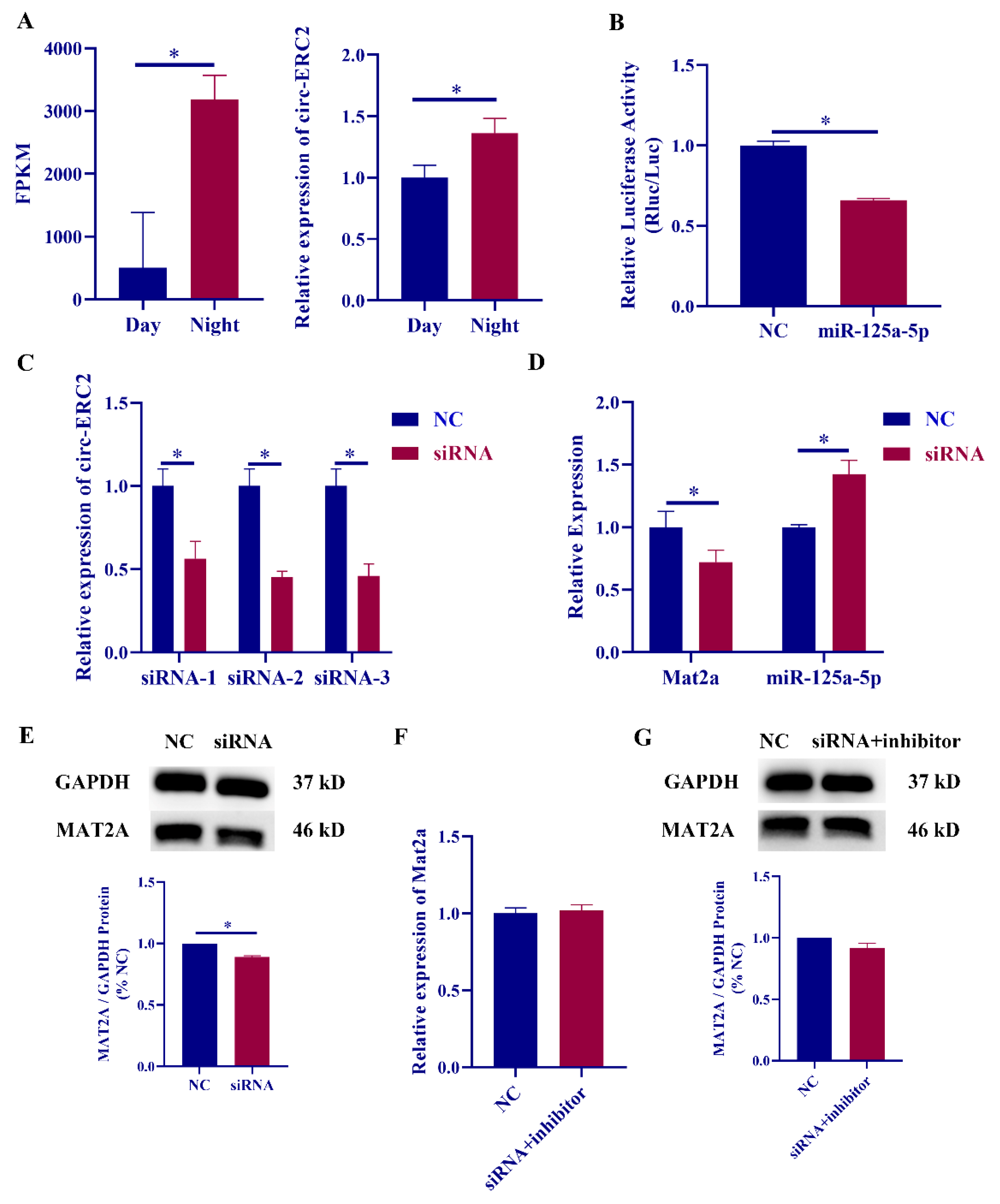
2.5. Circ-ERC2 sIs Involved in Melatonin Synthesis via the miR-125a-5p/mat2a Axis
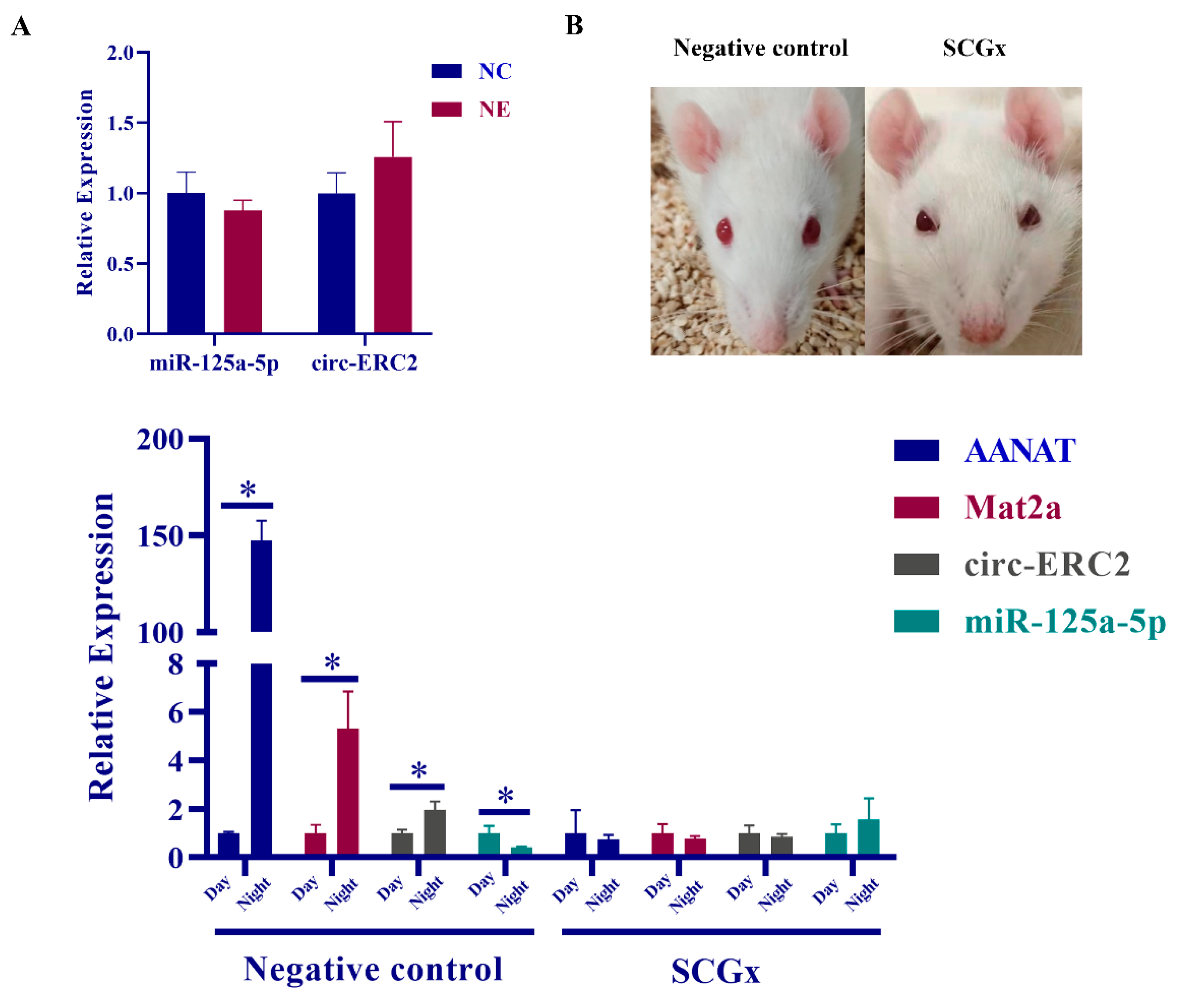
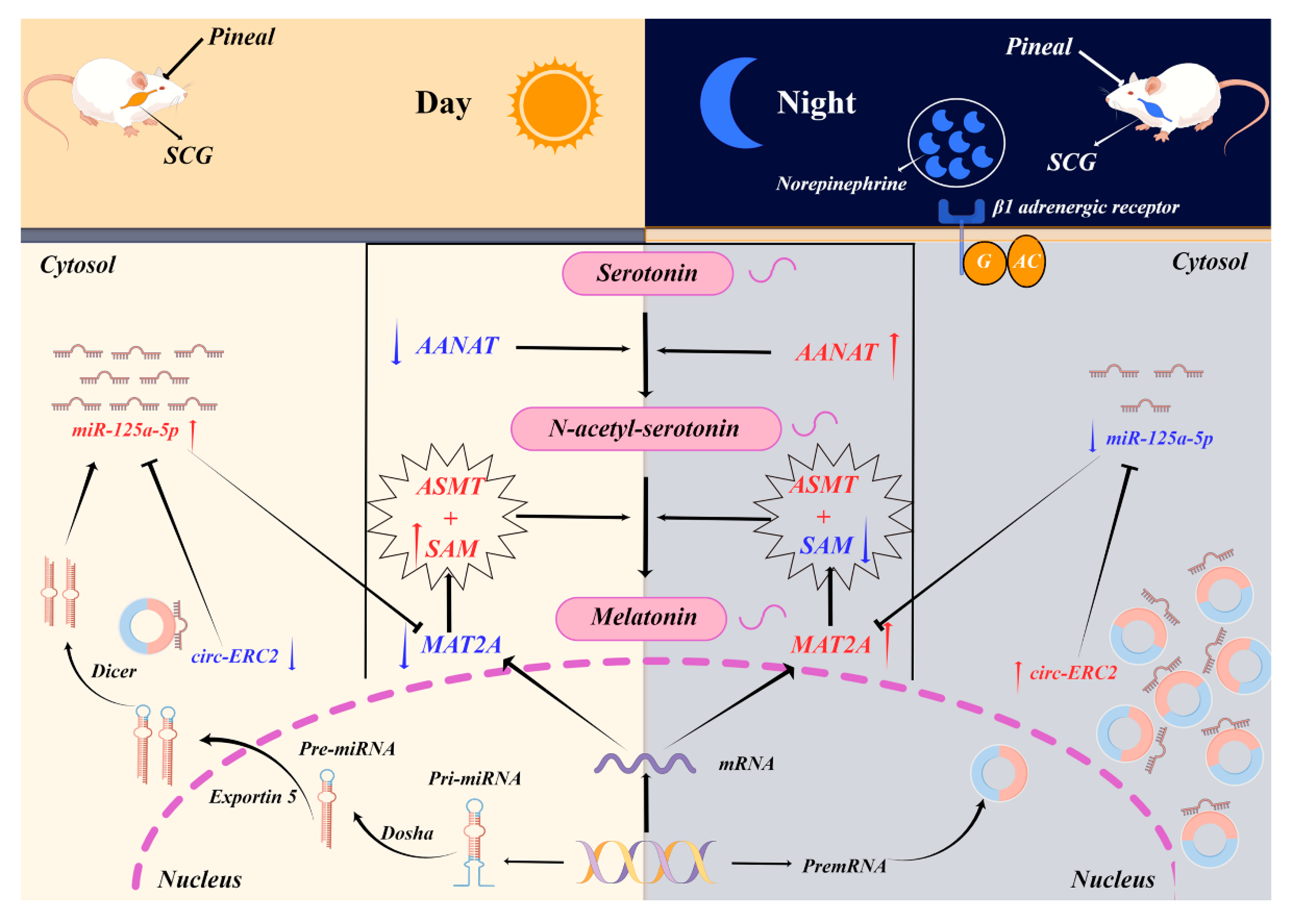
2.6. SCG Regulates Melatonin Synthesis via the circ-ERC2/miR-125a-5p/mat2a Axis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Pineal Cell Culture and Processing
4.3. Transfection with mat2a siRNA, miR-125a-5p-mimic, miR-125a-5p-inhibitor, circ-ERC2 siRNA
4.4. RNA Isolation and RT‒qPCR
4.5. ELISA Detection of Melatonin and SAM Expression Levels
4.6. Prediction of ceRNA Regulatory Networks
4.7. Dual-Luciferase Reporter Assay
4.8. Western Blotting
4.9. Sanger Sequencing and Linear RNase Treatment
4.10. Fluorescence In Situ Hybridization (FISH)
4.11. Construction of a Rat Model of Superior Cervical Ganglionectomy
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.-H.; Swaab, D.F. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal Res. 2005, 38, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004, 25, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Sapède, D.; Cau, E. The Pineal Gland from Development to Function. Curr. Top Dev. Biol. 2013, 106, 171–215. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H.; Blancas-Velazquez, A.S.; Rath, M.F. The role of homeobox gene-encoded transcription factors in regulation of phototransduction: Implementing the primary pinealocyte culture as a photoreceptor model. J. Pineal Res. 2021, 71, e12753. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Yan, Z.H.; Wang, L.; Tan, S.-J.; Liu, J.; Reiter, R.J.; Luo, S.-M.; Sun, Q.-Y.; Shen, W. A hypothetical role for autophagy during the day/night rhythm-regulated melatonin synthesis in the rat pineal gland. J. Pineal Res. 2021, 71, e12742. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H.; Carstensen, M.B.; Bering, T.; Rohde, K.; Møller, M.; Granau, A.M.; Coon, S.L.; Klein, D.C.; Rath, M.F. The Lhx4 homeobox transcript in the rat pineal gland: Adrenergic regulation and impact on transcripts encoding melatonin-synthesizing enzymes. J. Pineal Res. 2020, 68, e12616. [Google Scholar] [CrossRef]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64, e12450. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Chattoraj, A.; Liu, T.; Zhang, L.S.; Huang, Z.; Borjigin, J. Melatonin formation in mammals: In vivo perspectives. Rev. Endocr. Metab. Disord. 2009, 10, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Sugden, D. Melatonin biosynthesis in the mammalian pineal gland. Experientia 1989, 45, 922–932. [Google Scholar] [CrossRef]
- Ganguly, S.; Coon, S.L.; Klein, D.C. Control of melatonin synthesis in the mammalian pineal gland: The critical role of serotonin acetylation. Cell Tissue Res. 2002, 309, 127–137. [Google Scholar] [CrossRef]
- Ganguly, S.; Gastel, J.A.; Weller, J.L.; Schwartz, C.; Jaffe, H.; Namboodiri, M.A.A.; Coon, S.L.; Hickman, A.B.; Rollag, M.; Obsil, T.; et al. Role of a pineal cAMP-operated arylalkylamine N- acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 8083–8088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melke, J.; Goubran-Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelrod, J.; Weissbach, H. Purification and properties of hydroxyindole-O-methyl transferase. J. Biol. Chem. 1961, 236, 211–213. [Google Scholar] [CrossRef]
- Sitaram, B.R.; Sitaram, M.; Traut, M.; Chapman, C.B. Nyctohemeral Rhythm in the Levels of S-Adenosylmethionine in the Rat Pineal Gland and Its Relationship to Melatonin Biosynthesis. J. Neurochem. 1995, 65, 1887–1894. [Google Scholar] [CrossRef]
- Kim, J.-S.; Coon, S.L.; Blackshaw, S.; Cepko, C.L.; Møller, M.; Mukda, S.; Zhao, W.-Q.; Charlton, C.G.; Klein, D. Methionine Adenosyltransferase: Adrenergic-cAMP Mechanism Regulates a Daily Rhythm in Pineal Expression. J. Biol. Chem. 2005, 280, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Mato, J.M.; Alvarez, L.; Ortiz, P.; Pajares, M.A. S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications. Pharmacol. Ther. 1997, 73, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, C.L.; Kaiser, S.E.; Bolaños, B.; Nowlin, D.; Grantner, R.; Karlicek-Bryant, S.; Feng, J.L.; Jenkinson, S.; Freeman-Cook, K.; Dann, S.G.; et al. Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nat. Chem. Biol. 2017, 13, 785–792. [Google Scholar] [CrossRef]
- Kotb, M.; Mudd, S.; Mato, J.; Geller, A.M.; Kredich, N.M.; Chou, J.Y.; Cantoni, G.L. Consensus nomenclature for the mammalian methionine adenosyltransferase genes and gene products. Trends Genet. 1997, 13, 51–52. [Google Scholar] [CrossRef]
- Frau, M.; Feo, F.; Pascale, R.M. Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J. Hepatol. 2013, 59, 830–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D.C.; Bailey, M.J.; Carter, D.A.; Kim, J.-S.; Shi, Q.; Ho, A.K.; Chik, C.L.; Gaildrat, P.; Morin, F.; Ganguly, S.; et al. Pineal function: Impact of microarray analysis. Mol. Cell. Endocrinol. 2010, 314, 170–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Jiang, H.; Wang, H.-Q.; Guo, H.-X.; Han, D.-X.; Huang, Y.-J.; Gao, Y.; Yuan, B.; Zhang, J.-B. Identifying daily changes in circRNAs and circRNA-associated-ceRNA networks in the rat pineal gland. Int. J. Med. Sci. 2021, 18, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Shingle, D.L.; Green, C.B. Post-transcriptional control of circadian rhythms. J. Cell Sci. 2011, 124, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemière, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wang, H.-Q.; Guo, H.-X.; Xie, H.-L.; Zhang, W.-D.; Han, D.-X.; Jiang, H.; Yuan, B.; Zhang, J.-B. CircRNA-WNK2 Acts as a ceRNA for miR-328a-3p to Promote AANAT Expression in the Male Rat Pineal Gland. Endocrinology 2022, 163, bqab255. [Google Scholar] [CrossRef]
- Clokie, S.; Lau, P.; Kim, H.H.; Coon, S.L.; Klein, D.C. MicroRNAs in the Pineal Gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase. J. Biol. Chem. 2012, 287, 25312–25324. [Google Scholar] [CrossRef] [Green Version]
- Ben-Moshe, Z.; Alon, S.; Mracek, P.; Faigenbloom, L.; Tovin, A.; Vatine, G.D.; Eisenberg, E.; Foulkes, N.S.; Gothilf, Y. The light-induced transcriptome of the zebrafish pineal gland reveals complex regulation of the circadian clockwork by light. Nucleic Acids Res. 2014, 42, 3750–3767. [Google Scholar] [CrossRef]
- Sha, N.; Wang, H.-W.; Sun, B.; Gong, M.; Miao, P.; Jiang, X.-L.; Yang, X.-F.; Li, M.; Xu, L.-X.; Feng, C.-X.; et al. The role of pineal microRNA-325 in regulating circadian rhythms after neonatal hypoxic-ischemic brain damage. Neural Regen. Res. 2021, 16, 2071–2077. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, B.; Huang, J.; Xu, L.; Pan, J.; Fang, C.; Li, M.; Li, G.; Tao, Y.; Yang, X.; et al. Up-regulation of miR-325-3p suppresses pineal aralkylamine N-acetyltransferase (Aanat) after neonatal hypoxia–ischemia brain injury in rats. Brain Res. 2017, 1668, 28–35. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, T.; Chattoraj, A.; Ahmed, S.; Wang, M.M.; Deng, J.; Sun, X.; Borjigin, J. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J. Pineal Res. 2008, 45, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F.; Coon, S.L.; Amaral, F.G.; Weller, J.L.; Møller, M.; Klein, D.C. Melatonin Synthesis: Acetylserotonin O-Methyltransferase (ASMT) Is Strongly Expressed in a Subpopulation of Pinealocytes in the Male Rat Pineal Gland. Endocrinology 2016, 157, 2028–2040. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.K.; Chik, C.L. Modulation of Aanat gene transcription in the rat pineal gland. J. Neurochem. 2010, 112, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Price, D.; Terriff, D.; Chik, C. Timing of mitogen-activated protein kinase (MAPK) activation in the rat pineal gland. Mol. Cell. Endocrinol. 2006, 252, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Wurtman, R.J.; Snyder, S.H. Control of Hydroxyindole O-Methyltransferase Activity in the Rat Pineal Gland by Environmental Lighting. J. Biol. Chem. 1965, 240, 949–954. [Google Scholar] [CrossRef]
- Sandrock, A.W.; Leblanc, G.G.; Wong, D.L.; Ciaranello, R.D. Regulation of Rat Pineal Hydroxyindole-O-Methyltransferase: Evidence of S-Adenosylmethionine-Mediated Glucocorticoid Control. J. Neurochem. 1980, 35, 536–543. [Google Scholar] [CrossRef]
- Chan, Y.; Cheung, Y.; Pang, S. Rhythmic Release Pattern of Pineal Melatonin in Rodents. Neuroendocrinology 1991, 53, 60–67. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, J.; Zhou, Y.; Li, X.; Li, H.; Liu, J.; Gou, K.; Zhao, J.; Cui, S. MicroRNA-7 inhibits melatonin synthesis by acting as a linking molecule between leptin and norepinephrine signaling pathways in pig pineal gland. J. Pineal Res. 2019, 66, e12552. [Google Scholar] [CrossRef]
- Chen, H.; Gu, B.; Zhao, X.; Zhao, Y.; Huo, S.; Liu, X.; Lu, H. Circular RNA hsa_circ_0007364 increases cervical cancer progression through activating methionine adenosyltransferase II alpha (MAT2A) expression by restraining microRNA-101-5p. Bioengineered 2020, 11, 1269–1279. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Du, Q.-R.; He, Y.-J.; Chen, W.-S.; Jiang, W.-Y.; Gui, Q.; Xu, C.-C.; Wang, W.; Cheng, H.-Y. Circ_0044516 Regulates miR-136/MAT2A Pathway to Facilitate Lung Cancer Development. J. Immunol. Res. 2021, 2021, 5510869. [Google Scholar] [CrossRef]
- Liu, N.-Z.; Li, T.; Liu, C.-M.; Liu, F.-R.; Wang, Y.-X. Hsa_circ_0000337 promotes proliferation, migration and invasion in glioma by competitively binding miRNA-942-5p and thus upregulates MAT2A. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12251–12257. [Google Scholar] [PubMed]
- Tie, W.; Ge, F. MALAT1 Inhibits Proliferation of HPV16-Positive Cervical Cancer by Sponging miR-485-5p to Promote Expression of MAT2A. DNA Cell Biol. 2021, 40, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, P.H.; Coon, S.L.; Baler, R.; McCune, S.K.; Weller, J.L.; Klein, D.C. Melatonin synthesis: Analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology 1996, 137, 3033–3045. [Google Scholar] [CrossRef] [Green Version]
- Savastano, L.E.; Castro, A.E.; Fitt, M.R.; Rath, M.F.; Romeo, H.E.; Munoz, E.M. A standardized surgical technique for rat superior cervical ganglionectomy. J. Neurosci. Methods 2010, 192, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, H.H.; Fleitas, M.F.G.; Aranda, M.L.; Calanni, J.S.; Sarmiento, M.I.K.; Chianelli, M.S.; Alaimo, A.; Sande, P.H.; Romeo, H.E.; Rosenstein, R.E.; et al. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J. Pineal Res. 2020, 68, e12643. [Google Scholar] [CrossRef]
- Carstensen, M.B.; Hertz, H.; Bering, T.; Møller, M.; Rohde, K.; Klein, D.C.; Coon, S.L.; Rath, M.F. Circadian regulation and molecular role of the Bsx homeobox gene in the adult pineal gland. J. Pineal Res. 2020, 68, e12629. [Google Scholar] [CrossRef]
- Wang, C.-J.; Gao, F.; Huang, Y.-J.; Han, D.-X.; Zheng, Y.; Wang, W.-H.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.-B. circAkap17b acts as a miR-7 family molecular sponge to regulate FSH secretion in rat pituitary cells. J. Mol. Endocrinol. 2020, 65, 135–148. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.-X.; Zheng, Y.; Zhao, G.-K.; Wang, H.-Q.; Yu, S.; Gao, F.; Zhang, J.-B.; Zhang, Y.-H.; Yuan, B. Circ-ERC2 Is Involved in Melatonin Synthesis by Regulating the miR-125a-5p/MAT2A Axis. Int. J. Mol. Sci. 2022, 23, 15477. https://doi.org/10.3390/ijms232415477
Guo H-X, Zheng Y, Zhao G-K, Wang H-Q, Yu S, Gao F, Zhang J-B, Zhang Y-H, Yuan B. Circ-ERC2 Is Involved in Melatonin Synthesis by Regulating the miR-125a-5p/MAT2A Axis. International Journal of Molecular Sciences. 2022; 23(24):15477. https://doi.org/10.3390/ijms232415477
Chicago/Turabian StyleGuo, Hai-Xiang, Yi Zheng, Guo-Kun Zhao, Hao-Qi Wang, Song Yu, Fei Gao, Jia-Bao Zhang, Yong-Hong Zhang, and Bao Yuan. 2022. "Circ-ERC2 Is Involved in Melatonin Synthesis by Regulating the miR-125a-5p/MAT2A Axis" International Journal of Molecular Sciences 23, no. 24: 15477. https://doi.org/10.3390/ijms232415477





