Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers
Abstract
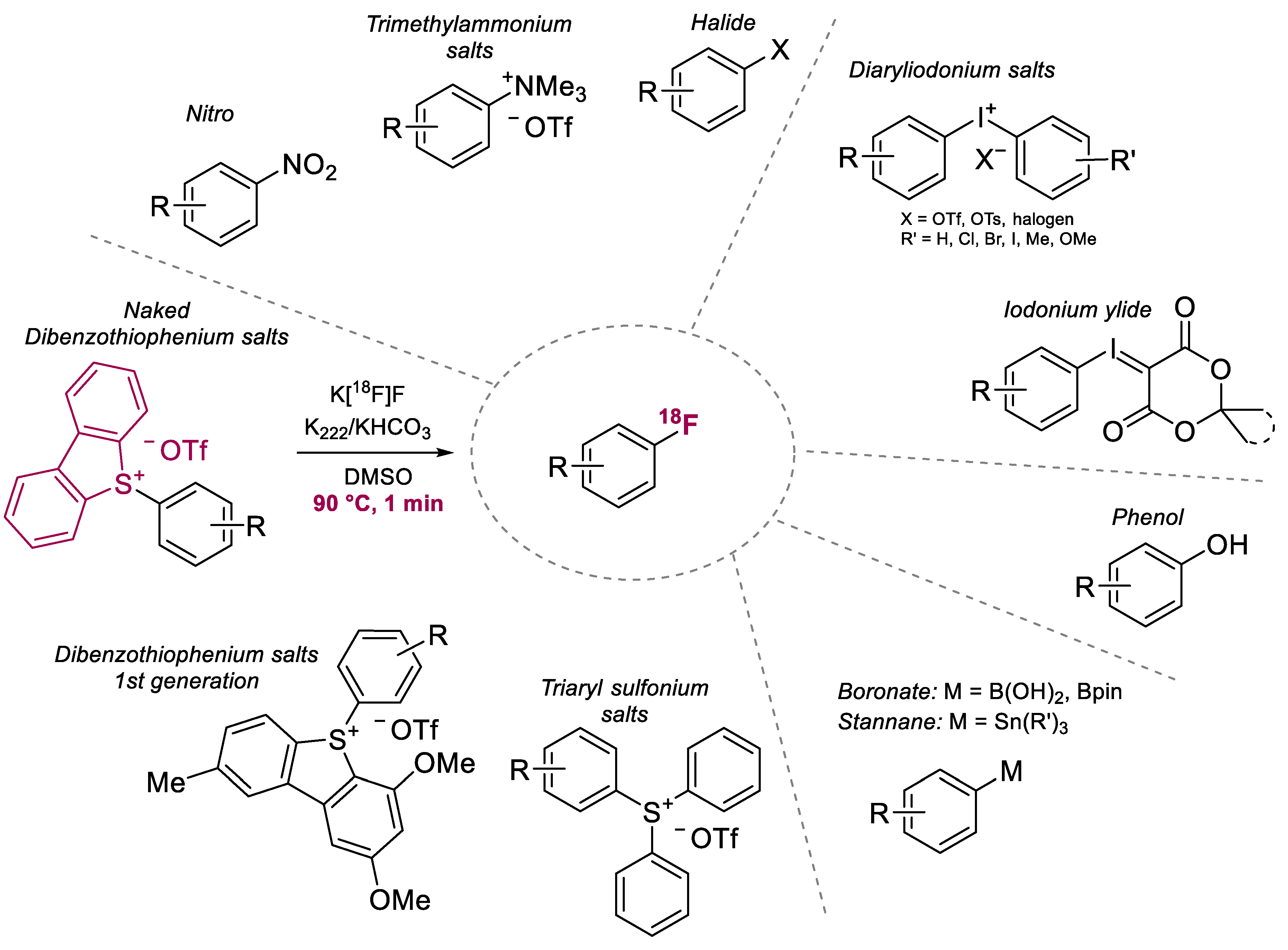
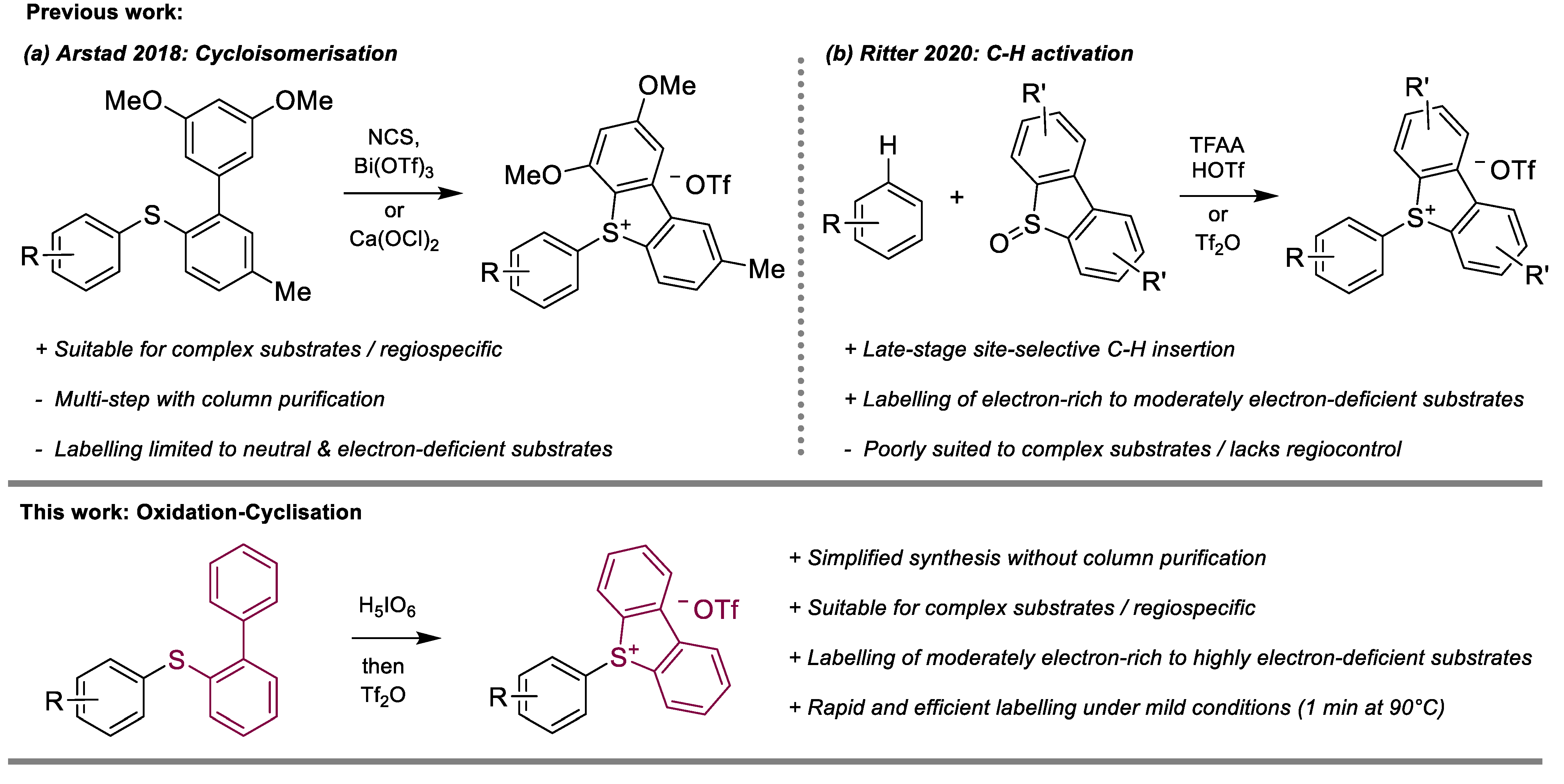
1. Introduction
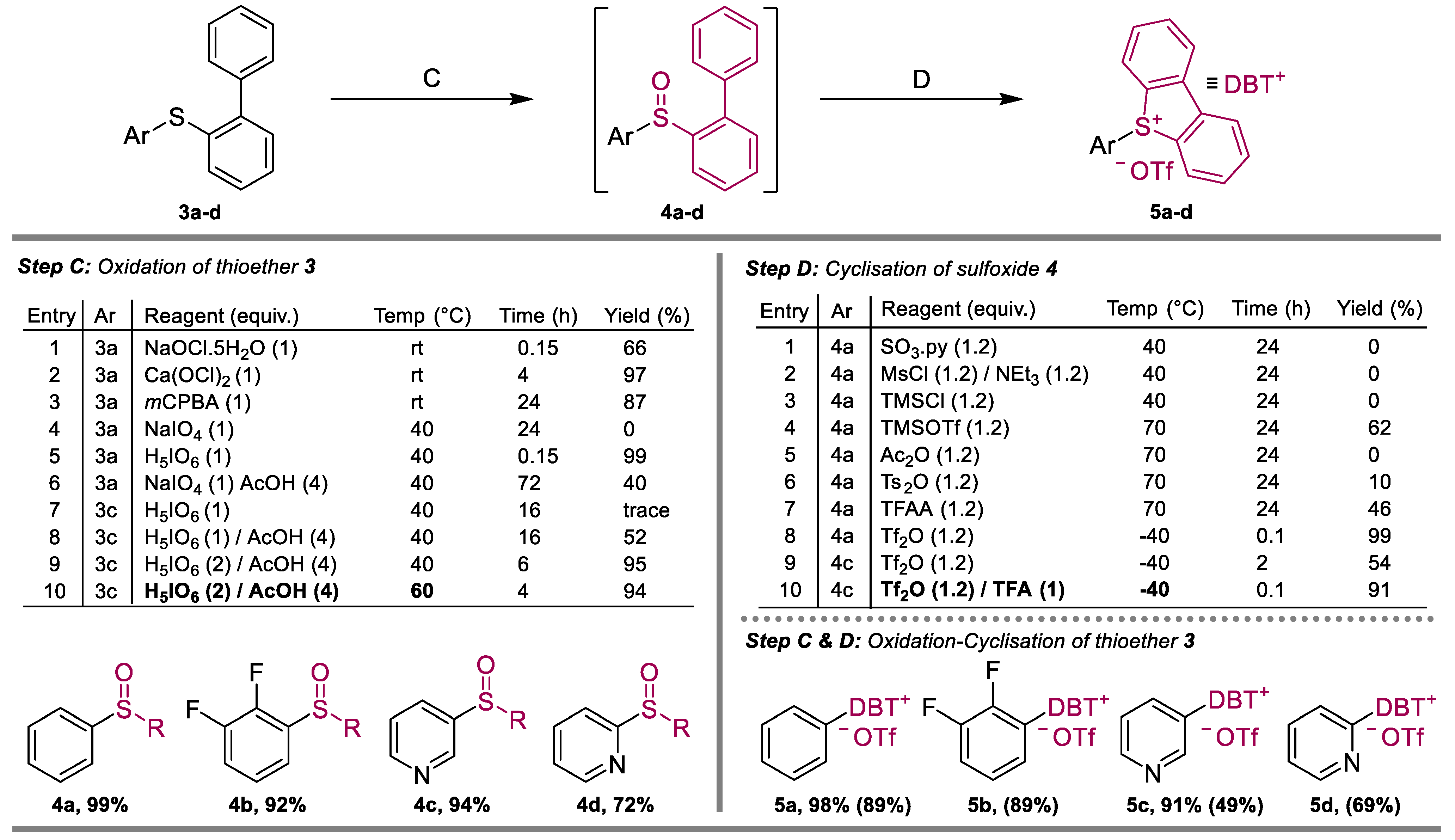
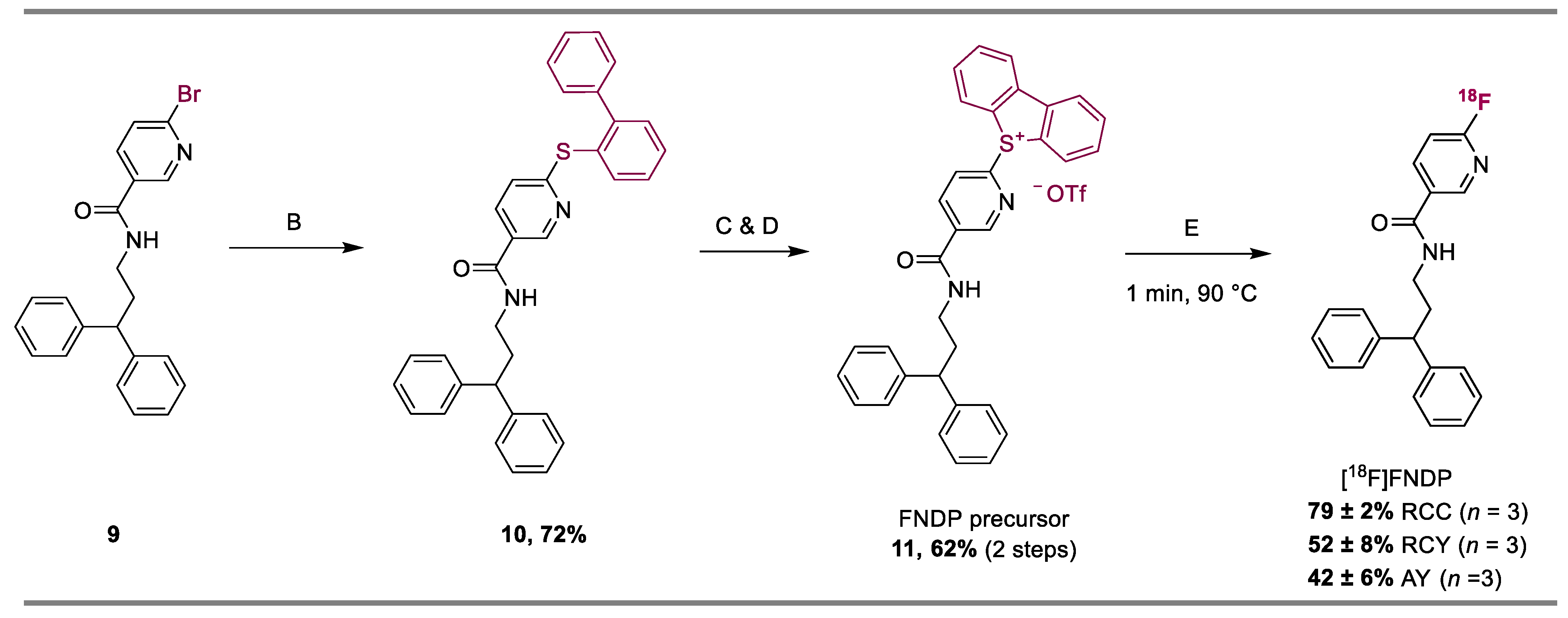
2. Results
3. Discussion
4. Materials and Methods
4.1. Procedure for Step A
4.2. Procedure for Step B
4.3. Procedure for Step C
4.4. Procedure for Step D
4.5. One-Pot Procedure for Steps C & D
4.6. Procedure for Step E
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Nerella, S.G.; Singh, P.; Sanam, T.; Digwal, C.S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med. 2022, 9, 812270. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, S.P.; Plisson, C.; Rabiner, E.A.; Howes, O. Advances in CNS PET: The state-of-the-art for new imaging targets for pathophysiology and drug development. Eur. J. Med. Mol. Imaging 2020, 47, 451–489. [Google Scholar] [CrossRef] [PubMed]
- Duclos, V.; Iep, A.; Gomez, L.; Goldfard, L.; Besson, F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021, 22, 4159. [Google Scholar] [CrossRef] [PubMed]
- Ermert, J.; Coenen, H.H. 18F-labelling innovations and their potential for clinical application. Clin. Transl. Imaging 2018, 6, 169–193. [Google Scholar]
- Ritter, T.; Halder, R. 18F-Fluorination: Challenge and Opportunity for Organic Chemists. J. Org. Chem. 2021, 86, 13873–13884. [Google Scholar]
- Campbell, M.G.; Mercier, J.; Genicot, C.; Gouverneur, V.; Hooker, J.M.; Ritter, T. Bridging the gaps in 18F PET tracer development. Nat. Chem. 2017, 9, 1–3. [Google Scholar] [CrossRef]
- Tredwell, M.; Gouverneur, V. 18F Labeling of Arenes. Angew. Chem. Int. Ed. 2012, 51, 11426–11437. [Google Scholar] [CrossRef]
- Kilbourn, M.R.; Scott, P.J.H. Handbook of Radiopharmaceuticals: Methodology and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 251–290. [Google Scholar]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for Positron Emission Tomography: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chem. Int. Ed. 2019, 58, 2580–2605. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Liu, T. Recent Advances in Synthetic Methodologies to Form C-18F Bonds. Front. Chem. 2022, 10, 883866. [Google Scholar] [CrossRef]
- Pike, V.W. Hypervalent aryliodine compounds as precursors for radiofluorination. J. Label. Compd. Radiopharm. 2018, 61, 196–227. [Google Scholar] [CrossRef]
- Liang, S.H.; Wang, L.; Stephenson, N.A.; Rotstein, B.H.; Vasdev, N. Facile 18F labeling of non-activated arenes via a spirocyclic iodonium (III) ylide method and its application in the synthesis of the mGluR5 PET radiopharmaceutical [18F]FBEB. Nat. Protoc. 2019, 14, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.J.; Emer, E.; Preshlock, S.; Schelder, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. [Google Scholar] [CrossRef] [PubMed]
- Guibbal, F.; Isenegger, P.; Wilson, T.C.; Pacelli, A.; Mahaut, D.; Sap, J.B.I.; Taylor, N.J.; Verhoog, S.; Preshlock, S.; Hueting, R.; et al. Manual and automated Cu-mediated radiosynthesis of the PARP inhibitor [18F]olaparib. Nat. Protoc. 2020, 15, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Shoup, T.M.; Moon, S.-H.; Brownell, A.-L. A concise method for fully automated radiosynthesis of [18F]JNJ-46356479 and [18F]FITM via Cu-mediated 18F-fluorination of organoboranes. RSC Adv. 2020, 10, 25223–25227. [Google Scholar] [CrossRef] [PubMed]
- Barbosa de Lemos, C.M.; Ferrão, F.M.; Graceli, J.B. Organtin Compounds Toxicity: Focus on Kidney. Front. Endocrinol. 2018, 9, 256. [Google Scholar] [CrossRef]
- European Medicines Agency; Committee for Human Medicinal Products. ICH Guidelines for Element Impurities Q3D(R1); EMA/CHMP/ICH/353369/2013, 28 March 2019; European Medicines Agency: Amsterdam, The Netherlands, 2019.
- Sander, K.; Gendron, T.; Yiannaki, E.; Kalber, T.L.; Lythgoe, M.F.; Årstad, E. Sulfonium Salts as Leaving Groups for Aromatic Labelling of Drug-like Small Molecules with Fluorine-18. Sci. Rep. 2015, 5, 9941. [Google Scholar] [CrossRef]
- Gendron, T.; Sander, K.; Cybulska, K.; Benhamou, L.; Sin, P.K.B.; Khan, A.; Wood, M.; Porter, M.J.; Årstad, E. Ring-Closing Synthesis of Dibenzothiophene Sulfonium Salts and Their Use as Leaving Groups for Aromatic 18F-Fluorination. J. Am. Chem. Soc. 2018, 140, 11125–11132. [Google Scholar] [CrossRef]
- Sirindil, F.; Årstad, E.; Sander, K.; Awais, R.; Twyman, F.; Marcolan, C.; Glaser, M. Automated production of [18F]AldoView: First translation of a sulfonium salt precursor to GMP. Nucl. Med. Biol. 2022, 108, S137. [Google Scholar] [CrossRef]
- Varlow, C.; Murerell, E.; Holland, J.P.; Kassenbrock, A.; Shannon, W.; Liang, S.H.; Vasdev, N.; Stephenson, N.A. Revisiting the Radiosynthesis of [18F]FPEB and Preliminary PET Imaging in a Mouse Model of Alzheimer’s Disease. Molecules 2020, 25, 982. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, D.; Berger, F.; Hamad, A.; Rickmeier, J.; Petzold, R.; Kondratiuk, M.; Bohdan, K.; Ritter, T. Site-Selective Late-Stage Aromatic [18F]Fluorination via Aryl Sulfonium Salts. Angew. Chem. Int. Ed. 2020, 59, 1956–1960. [Google Scholar] [CrossRef]
- Soria-Castro, S.M.; Penenory, A.B. Efficient Cu-catalyzed base-free C-S coupling under conventional and microwave heating. A simple access to S-heterocycles and sulfides. Beilstein J. Org. Chem. 2013, 9, 467–475. [Google Scholar] [CrossRef] [PubMed]
- 2-iodobiphenyl can also be obtained in high yield from inexpensive 2-aminobiohenyl: Boelke, A.; Kuczmera, T.J.; Caspers, L.D.; Lork, E.; Nachtsheim, B.J. Iodolopyrazolium Salts: Synthesis, Derivatizations, and Applications. Org. Lett. 2020, 22, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, U.; Schlapbach, A. A general palladium-catalysed synthesis of aromatic and heteroaromatic thioethers. Tetrahedron 2001, 57, 3069–3073. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Soukhakian, S.; Jazebizadeh, M.H. Selected methods for the synthesis of sulfoxides and sulfones with emphasis on oxidative protocols. Phosphorus Sulfur Silicon Relat. Elem. 2019, 195, 181–193. [Google Scholar] [CrossRef]
- Tong, Q.L.; Fan, Z.F.; Yang, J.W.; Li, Q.; Chen, Y.X.; Cheng, M.S.; Liu, Y. The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid. Catalysts 2019, 9, 791. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, H.W.; Pu, X.L.; Qian, Z.P.; Xiao, Y. Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts 2020, 10, 1339. [Google Scholar] [CrossRef]
- Golchoubian, H.; Hosseinpoor, F. Effective Oxidation of Sulfides to Sulfoxides with Hydrogen Peroxide under Transition-Metal-Free Conditions. Molecules 2007, 12, 304–311. [Google Scholar] [CrossRef]
- Kim, S.S.; Nehru, K.; Kim, S.S.; Kim, D.W.; Jung, H.C. A Mild and Highly Efficient Oxidation of Sulfides to Sulfoxides with Periodic Acid Catalyzed by FeCl3. Synthesis 2002, 17, 2484–2486. [Google Scholar] [CrossRef]
- Kirihara, M.; Okada, T.; Sugiyama, Y.; Akiyoshi, M.; Matsunaga, T.; Kimura, Y. Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis. Org. Process. Res. Dev. 2017, 21, 1925–1937. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Green, I.R.; Ahmed, I.; Abbas, G.; Rehman, N.U. meta-Chloroperbenzoic acid (mCPBA): A versatile reagent in organic synthesis. RSC Adv. 2014, 4, 12882–12917. [Google Scholar] [CrossRef]
- Horn, A.; Kazmaier, U. Purified mCPBA, a Useful Reagent for the Oxidation of Aldehydes. Eur. J. Org. Chem. 2018, 2018, 2531–2536. [Google Scholar] [CrossRef]
- Ruff, F.; Fábián, A.; Farkas, Ö.; Kucsman, Á. Mechanism for the Oxidation of Sulfides and Sulfoxides with Periodates: Reactivity of the Oxidizing Species. Eur. J. Org. Chem. 2009, 2009, 2102–2111. [Google Scholar] [CrossRef]
- Iwasaki, T.; Kohinata, Y.; Nishide, H. Poly(thiaheterohelicene): A Stiff Conjugated Helical Polymer Comprised of Fused Benzothiophene Rings. Org. Lett. 2005, 7, 755–758. [Google Scholar] [CrossRef]
- Haryono, A.; Miyatake, K.; Natori, J.; Tsuchida, E. Synthesis of a Novel Oligo(p-phenylene) Ladder by Sulfide and Sulfonio Groups. Macromolecules 1999, 32, 3146–3149. [Google Scholar] [CrossRef]
- Vasu, D.; Yorimitsu, H.; Osuka, A. Palladium-Assisted “Aromatic Metamorphosis” of Dibenzothiophenes into Triphenylenes. Angew. Chem. Int. Ed. 2015, 54, 7126–7166. [Google Scholar] [CrossRef]
- Altundas, B.; Kumar, C.V.S.; Fleming, F.F. Acetonitrile-Hexane Extraction Route to Pure Sulfonium Salts. ACS Omega 2020, 5, 13384–13388. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; Gendron, T.; Cybulska, K.A.; Sirindil, F.; Zhou, J.; Kalber, T.L.; Lythoge, M.F.; Kurzawinski, T.R.; Brown, M.J.; Williams, B.; et al. Development of [18F]AldoView as the First Highly Selective Aldosterone Synthase PET Tracer for Imaging of Primary Hyperaldosteronism. J. Med. Chem. 2021, 64, 9321–9329. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Wang, Y.; Minn, I.; Lan, X.; Wang, J.; Koehler, R.C.; Alkayed, N.J.; Dannals, R.F.; Pomper, M.G. 18F-FNDP for PET Imaging of Soluble Epoxide Hydrolase. J. Nuc. Med. 2016, 57, 1817–1822. [Google Scholar] [CrossRef]
- Azad, B.B.; Holt, D.P.; Ravert, H.T.; Horti, A.G.; Dannals, R.F. An optimized radiosynthesis of [18 F]FNDP, a positron emission tomography radiotracer for imaging soluble epoxide hydrolase (sEH). J. Label. Comp. Radiopharm. 2018, 61, 567–572. [Google Scholar] [CrossRef]
- Horti, A.; Pomper, M.G.; Alayed, N.J. 18F-FNDP for PET Imaging of Soluble Epoxide Hydrolase (sEH). WO 2017/192854 A1, 9 November 2017. [Google Scholar]
- Nabulsi, N.B.; Mercier, J.; Holden, D.; Carré, S.; Najafzadeh, S.; Vandergeten, M.-C.; Lin, S.; Deo, A.; Price, N.; Wood, M.; et al. Synthesis and Preclinical Evaluation of 11C-UCB-J as a PET Tracer for Imaging the Synaptic Vesicle Glycoprotein 2A in the Brain. J. Nuc. Med. 2016, 57, 777–784. [Google Scholar] [CrossRef]
- Mercier, J.; Provins, L.; Valde, A. Discovery and development of SV2A PET tracers: Potential for imaging synaptic density and clinical applications. Drug Discov. Today Technol. 2017, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, S.; Matuskey, D.; Nabulsi, N.; Huang, Y. PET imaging of synaptic density: A new tool for investigation of neuropsychiatric diseases. Neurosci. Lett. 2019, 691, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Dammicco, S.; Bahri, M.A.; Salmon, E. The Rise of Synaptic Density PET Imaging. Molecules 2020, 25, 2303. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, R.S.; Mecca, A.P.; Chen, M.K.; Naganawa, M.; Toyonaga, T.; Lu, Y.; Godek, T.A.; Harris, J.E.; Barlett, H.H.; Banks, E.R.; et al. Association of Aβ deposition and regional synaptic density in early Alzheimer’s disease: A PET imaging study with [11C]UCB-J. Alz. Res. Ther. 2021, 13, 11. [Google Scholar] [CrossRef]
- Li, S.; Cai, Z.; Zhang, W.; Holden, D.; Lin, S.; Finnema, S.; Shirali, A.; Ropchan, J.; Carre, S.; Mercier, J.; et al. Synthesis and in vivo evaluation of [18F]UCB-J for PET imaging of synaptic vesicle glycoprotein 2A (SV2A). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1952–1965. [Google Scholar] [CrossRef]
- Constantinescu, C.C.; Tresse, C.; Zheng, M.; Gouasmat, A.; Carroll, V.M.; Mistico, L.; Alagille, D.; Sandiego, C.M.; Papin, C.; Marek, K.; et al. Development and In Vivo Preclinical Imaging of Fluorine-18-Labeled Synaptic Vesicle Protein 2A (SV2A) PET Tracers. Mol. Imaging Biol. 2019, 21, 509–518. [Google Scholar] [CrossRef]
- Sergeev, M.; Lazari, M.; Morgia, F.; Collins, J.; Javed, M.R.; Sergeeva, O.; Jones, J.; Phelps, M.E.; Lee, J.T.; Keng, P.Y.; et al. Performing radiosynthesis in microvolumes to maximize molar activity of tracers for positron emission tomography. Commun. Chem. 2018, 1, 10. [Google Scholar] [CrossRef]
- Link, J.M.; Shoner, S.C.; Krohn, K.A. Sources of carrier F-19 in F-18 fluoride. AIP Conf. Proc. 2012, 1509, 61–65. [Google Scholar]
- Sephton, S.M.; Miklovicz, T.; Russell, J.J.; Doke, A.; Li, L.; Boros, I.; Aigbirhio, F.I. Automated radiosynthesis of [11C]UCB-J for imaging synaptic density by positron emission tomography. J. Label. Compd. Radiopharm. 2020, 63, 151–158. [Google Scholar] [CrossRef]
- Pauton, M.; Aubert, C.; Bluet, G.; Gruss-Leleu, F.; Roy, S.; Perrio, C. Development, Optimization, and Scope of the Radiosynthesis of 3/5-[18F]Fluoropyridines from Readily Prepared Aryl(pyridinyl) Iodonium Salts: The Importance of TEMPO and K2CO3. Org. Process Res. Dev. 2019, 23, 900–911. [Google Scholar] [CrossRef]
- Clamp, J.R.; Hough, L. Some Observations on the Periodate Oxidation of Amino Compounds. Biochem. J. 1966, 101, 120–126. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirindil, F.; Maher, S.; Schöll, M.; Sander, K.; Årstad, E. Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers. Int. J. Mol. Sci. 2022, 23, 15481. https://doi.org/10.3390/ijms232415481
Sirindil F, Maher S, Schöll M, Sander K, Årstad E. Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers. International Journal of Molecular Sciences. 2022; 23(24):15481. https://doi.org/10.3390/ijms232415481
Chicago/Turabian StyleSirindil, Fatih, Sinead Maher, Michael Schöll, Kerstin Sander, and Erik Årstad. 2022. "Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers" International Journal of Molecular Sciences 23, no. 24: 15481. https://doi.org/10.3390/ijms232415481
APA StyleSirindil, F., Maher, S., Schöll, M., Sander, K., & Årstad, E. (2022). Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers. International Journal of Molecular Sciences, 23(24), 15481. https://doi.org/10.3390/ijms232415481





