A Greek National Cross-Sectional Study on Myotonic Dystrophies
Abstract
1. Introduction
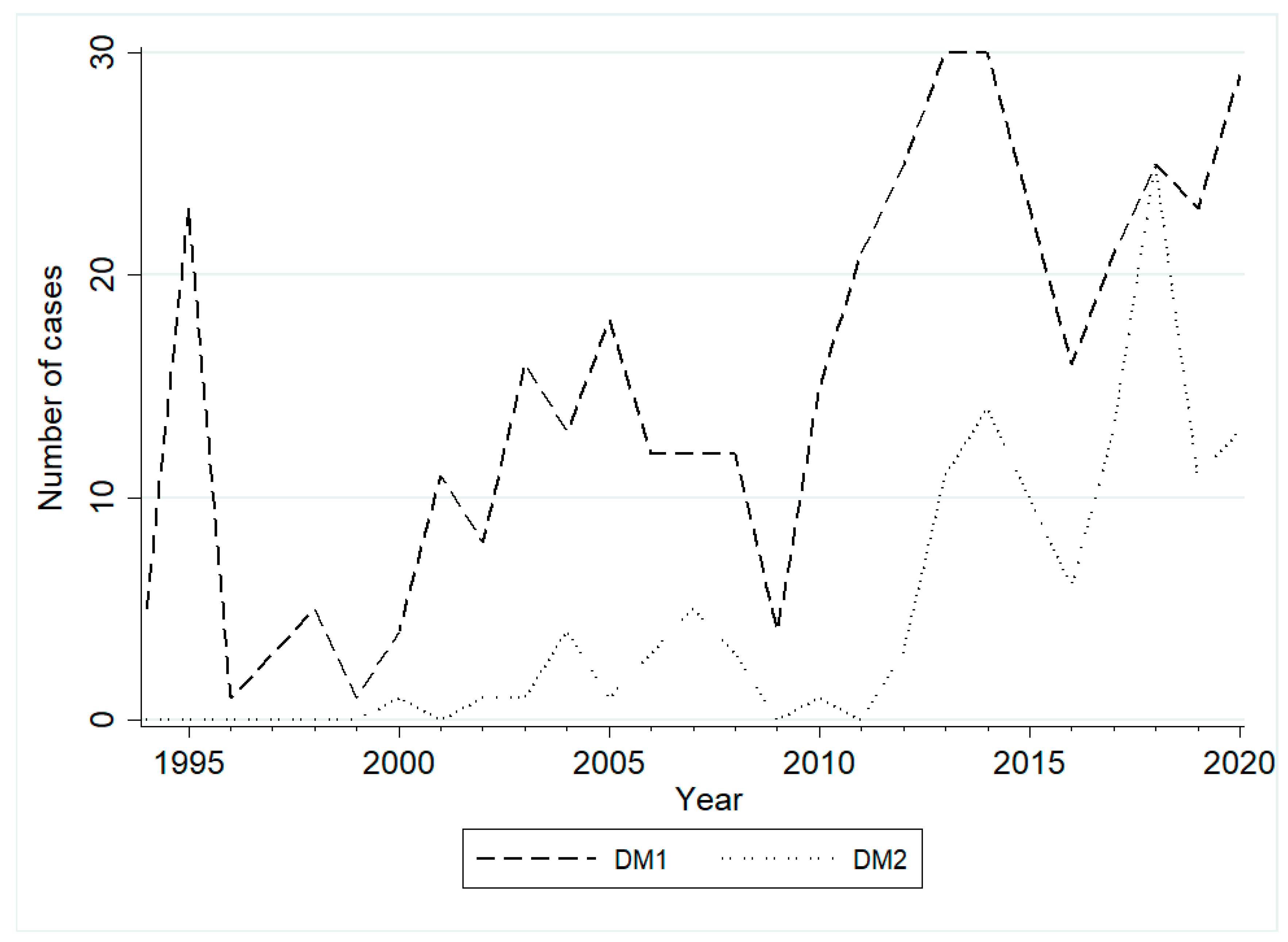
2. Results
3. Discussion
4. Materials and Methods
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, C.A. Myotonic dystrophy. Neurol. Clin. 2014, 32, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.E. Myotonic Muscular Dystrophies. Continuum 2019, 25, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Meola, G.; Cardani, R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim. Biophys. Acta 2015, 1852, 594–606. [Google Scholar] [CrossRef]
- Turner, C.; Hilton-Jones, D. Myotonic dystrophy: Diagnosis, management and new therapies. Curr. Opin. Neurol. 2014, 27, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Rodrigues, M.; Roxburgh, R.; Balalla, S.; Higgins, C.; Bhattacharjee, R.; Jones, K.; Krishnamurthi, R.; Feigin, V. Prevalence of muscular dystrophies: A systematic literature review. Neuroepidemiology 2014, 43, 259–268. [Google Scholar] [CrossRef]
- Mahadevan, M.; Tsilfidis, C.; Sabourin, L.; Shutler, G.; Amemiya, C.; Jansen, G.; Neville, C.; Narang, M.; Barceló, J.; O’Hoy, K.; et al. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992, 255, 1253–1255. [Google Scholar] [CrossRef]
- Ranum, L.P.; Rasmussen, P.F.; Benzow, K.A.; Koob, M.D.; Day, J.W. Genetic mapping of a second myotonic dystrophy locus. Nat. Genet. 1998, 19, 196–198. [Google Scholar] [CrossRef]
- Liquori, C.L.; Ricker, K.; Moseley, M.L.; Jacobsen, J.F.; Kress, W.; Naylor, S.L.; Day, J.W.; Ranum, L.P. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 2001, 293, 864–867. [Google Scholar] [CrossRef]
- Suominen, T.; Bachinski, L.; Auvinen, S.; Hackman, P.; Baggerly, K.A.; Angelini, C.; Peltonen, L.; Krahe, R.; Udd, B. Population frequency of myotonic dystrophy: Higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland. Eur. J. Hum. Genet. 2011, 19, 776–782. [Google Scholar] [CrossRef]
- Mahyera, A.S.; Schneider, T.; Halliger-Keller, B.; Schrooten, K.; Hörner, E.M.; Rost, S.; Kress, W. Distribution and Structure of DM2 Repeat Tract Alleles in the German Population. Front. Neurol. 2018, 9, 463. [Google Scholar] [CrossRef]
- Dogan, C.; De Antonio, M.; Hamroun, D.; Varet, H.; Fabbro, M.; Rougier, F.; Amarof, K.; Arne Bes, M.C.; Bedat-Millet, A.L.; Behin, A.; et al. Gender as a Modifying Factor Influencing Myotonic Dystrophy Type 1 Phenotype Severity and Mortality: A Nationwide Multiple Databases Cross-Sectional Observational Study. PLoS ONE 2016, 11, e0148264. [Google Scholar] [CrossRef] [PubMed]
- Joosten, I.B.T.; Hellebrekers, D.M.E.I.; de Greef, B.T.A.; Smeets, H.J.M.; de Die-Smulders, C.E.M.; Faber, C.G.; Gerrits, M.M. Parental repeat length instability in myotonic dystrophy type 1 pre- and protomutations. Eur. J. Hum. Genet. 2020, 28, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Salehi, L.B.; Bonifazi, E.; Stasio, E.D.; Gennarelli, M.; Botta, A.; Vallo, L.; Iraci, R.; Massa, R.; Antonini, G.; Angelini, C.; et al. Risk prediction for clinical phenotype in myotonic dystrophy type 1: Data from 2650 patients. Genet. Test. 2007, 11, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, P. Myotonic Dystrophies: A Genetic Overview. Genes 2022, 13, 367. [Google Scholar] [CrossRef]
- Meola, G. Myotonic dystrophy type 2: The 2020 update. Acta Myol. 2020, 39, 222–234. [Google Scholar]
- Wenninger, S.; Montagnese, F.; Schoser, B. Core Clinical Phenotypes in Myotonic Dystrophies. Front. Neurol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Young, N.P.; Daube, J.R.; Sorenson, E.J.; Milone, M. Absent, unrecognized, and minimal myotonic discharges in myotonic dystrophy type 2. Muscle Nerve 2010, 41, 758–762. [Google Scholar] [CrossRef]
- Suokas, K.I.; Haanpää, M.; Kautiainen, H.; Udd, B.; Hietaharju, A.J. Pain in patients with myotonic dystrophy type 2: A postal survey in Finland. Muscle Nerve 2012, 45, 70–74. [Google Scholar] [CrossRef]
- Wenninger, S.; Stahl, K.; Montagnese, F.; Schoser, B. Utility and Results from a Patient-Reported Online Survey in Myotonic Dystrophies Types 1 and 2. Eur. Neurol. 2020, 83, 523–533. [Google Scholar] [CrossRef]
- Heatwole, C.; Johnson, N.; Bode, R.; Dekdebrun, J.; Dilek, N.; Hilbert, J.E.; Luebbe, E.; Martens, W.; McDermott, M.P.; Quinn, C.; et al. Patient-Reported Impact of Symptoms in Myotonic Dystrophy Type 2 (PRISM-2). Neurology 2015, 85, 2136–2146. [Google Scholar] [CrossRef]
- van Vliet, J.; Tieleman, A.A.; Verrips, A.; Timmerman, H.; van Dongen, R.T.M.; van Engelen, B.G.M.; Wilder-Smith, O.H.G. Qualitative and Quantitative Aspects of Pain in Patients With Myotonic Dystrophy Type 2. J. Pain 2018, 19, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Hilton-Jones, D. The myotonic dystrophies: Diagnosis and management. J. Neurol. Neurosurg. Psychiatry 2010, 81, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Kekou, K.; Xirou, S.; Kitsiou-Tzeli, S.; Kararizou, E.; Papadimas, G.K. Early onset posterior subscapular cataract in a series of myotonic dystrophy type 2 patients. Eye 2018, 32, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Pagoulatos, D.; Kapsala, Z.; Makri, O.E.; Georgakopoulos, C.D. Christmas tree cataract and myotonic dystrophy type 1. Eye 2018, 32, 1794–1795. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Mann, D.L.; Pinto, Y.; Bhakta, D.; Tomaselli, G.; Nazarian, S.; Groh, W.J.; Tamura, T.; Duboc, D.; Itoh, H.; et al. Clinical Care Recommendations for Cardiologists Treating Adults With Myotonic Dystrophy. J. Am. Heart Assoc. 2020, 9, e014006. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Allard, P.; Potvin, L.; Prévost, C.; Bégin, P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999, 52, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.A.; Gagnon, C.; Participants of the 207th ENMC Workshop. 207th ENMC Workshop on Chronic Respiratory Insufficiency in Myotonic Dystrophies: Management and Implications for Research, 27–29 June 2014, Naarden, The Netherlands. Neuromuscul. Disord. 2015, 25, 432–442. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Runte, M.; Heidbreder, A.; Dreher, M.; Young, P.; Brix, T.; Boentert, M. Sleep-disordered breathing and effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with myotonic dystrophy type I. Neuromuscul. Disord. 2019, 29, 302–309. [Google Scholar] [CrossRef]
- Romigi, A.; Maestri, M.; Nicoletta, C.; Vitrani, G.; Caccamo, M.; Siciliano, G.; Bonanni, E.; Centonze, D.; Sanduzzi, A. Sleep Complaints, Sleep and Breathing Disorders in Myotonic Dystrophy Type 2. Curr. Neurol. Neurosci. Rep. 2019, 19, 9. [Google Scholar] [CrossRef]
- Subramony, S.H.; Wymer, J.P.; Pinto, B.S.; Wang, E.T. Sleep disorders in myotonic dystrophies. Muscle Nerve 2020, 62, 309–320. [Google Scholar] [CrossRef]
- Perna, A.; Maccora, D.; Rossi, S.; Nicoletti, T.F.; Zocco, M.A.; Riso, V.; Modoni, A.; Petrucci, A.; Valenza, V.; Grieco, A.; et al. High Prevalence and Gender-Related Differences of Gastrointestinal Manifestations in a Cohort of DM1 Patients: A Perspective, Cross-Sectional Study. Front. Neurol. 2020, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, J.R.; Ørngreen, M.C.; Witting, N.; Vissing, J. Endocrine function over time in patients with myotonic dystrophy type 1. Eur. J. Neurol. 2015, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, P.V.; Venetsanopoulou, A.I.; Kalpourtzi, N.; Gavana, M.; Vantarakis, A.; Hadjichristodoulou, C.; Chlouverakis, G.; Trypsianis, G.; Alamanos, Y.; Touloumi, G.; et al. Thyroid dysfunction in Greece: Results from the national health examination survey EMENO. PLoS ONE 2022, 17, e0264388. [Google Scholar] [CrossRef] [PubMed]
- Alsaggaf, R.; St George, D.M.M.; Zhan, M.; Pfeiffer, R.M.; Wang, Y.; Wagner, K.R.; Greene, M.H.; Amr, S.; Gadalla, S.M. Cancer Risk in Myotonic Dystrophy Type I: Evidence of a Role for Disease Severity. JNCI Cancer Spectr. 2018, 2, pky052. [Google Scholar] [CrossRef]
- Wang, Y.; Pfeiffer, R.M.; Alsaggaf, R.; Meeraus, W.; Gage, J.C.; Anderson, L.A.; Bremer, R.C.; Nikolenko, N.; Lochmuller, H.; Greene, M.H.; et al. Risk of skin cancer among patients with myotonic dystrophy type 1 based on primary care physician data from the U.K. Clinical Practice Research Datalink. Int. J. Cancer. 2018, 142, 1174–1181. [Google Scholar] [CrossRef]
- Weber, Y.G.; Roebling, R.; Kassubek, J.; Hoffmann, S.; Rosenbohm, A.; Wolf, M.; Steinbach, P.; Jurkat-Rott, K.; Walter, H.; Reske, S.N.; et al. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology 2010, 74, 1108–1117. [Google Scholar] [CrossRef]
- Greek legislation/Recital 50 of the new EU General Data Protection Regulation Directive 95/46/EC.
- Kamsteeg, E.J.; Kress, W.; Catalli, C.; Hertz, J.M.; Witsch-Baumgartner, M.; Buckley, M.F.; van Engelen, B.G.; Schwartz, M.; Scheffer, H. Best practice guidelines and recommendations on the molecular diagnosis of myotonic dystrophy types 1 and 2. Eur. J. Hum. Genet. 2012, 20, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Papadimas, G.K.; Kekou, K.; Papadopoulos, C.; Kararizou, E.; Kanavakis, E.; Manta, P. Phenotypic variability and molecular genetics in proximal myotonic myopathy. Muscle Nerve 2015, 51, 686–691. [Google Scholar] [CrossRef]
- Warner, J.P.; Barron, L.H.; Goudie, D.; Kelly, K.; Dow, D.; Fitzpatrick, D.R.; Brock, D.J. A general method for the detection of large CAG repeat expansions by fluorescent PCR. J. Med. Genet. 1996, 33, 1022–1026. [Google Scholar] [CrossRef]
- Shelbourne, P.; Davies, J.; Buxton, J.; Anvret, M.; Blennow, E.; Bonduelle, M.; Schmedding, E.; Glass, I.; Lindenbaum, R.; Lane, R.; et al. Direct diagnosis of myotonic dystrophy with a disease-specific DNA marker. N. Engl. J. Med. 1993, 328, 471–475. [Google Scholar] [CrossRef]

| Myotonic Dystrophy Type 1 | Myotonic Dystrophy Type 2 | |
|---|---|---|
| Total (N (%)) | 434 (100.0) | 127 (100.0) |
| Gender (N (%)) | ||
| Male | 209 (48.2) | 56 (44.1) |
| Female | 225 (51.8) | 71 (55.9) |
| Age of onset in years (mean ± SD) | 27.1 ± 14.7 | 44.4 ± 17.0 |
| Diagnostic delay in years (Median (IQR; 25–75)) | 8 (2–17.5) | 6 (2–17) |
| Parental Origin * | DM1 Premutations 36–50 CTG Repeats | DM1 Protοmutations 51–80 CTG Repeats | Full-Sized DM1 Mutations >80 CTG Repeats |
|---|---|---|---|
| Paternal | 1 (contraction) | 3 | 118 |
| Maternal | 2 | 9 + 1 fetus | 101 |
| Unknown | 4 | 13 | 183 |
| Total | 7 | 25 | 402 |
| Symptom | Myotonic Dystrophy Type 1 N (%) | Myotonic Dystrophy Type 2 N (%) |
|---|---|---|
| Muscle Weakness | 134 (30.9) | 79 (62.2) |
| Myotonia | 135 (31.1) | 12 (9.4) |
| Hypotonia | 18 (4.2) | 0 (0.0) |
| Cataract | 7 (1.6) | 2 (1.6) |
| Dysarthria | 6 (1.4) | 0 (0.0) |
| Cardiological complications | 4 (0.9) | 0 (0.0) |
| Cognitive difficulties | 4 (0.9) | 0 (0.0) |
| Dysphagia | 4(0.9) | 0 (0.0) |
| Nasal speech | 3 (0.7) | 0 (0.0) |
| Fatigue | 2 (0.5) | 0 (0.0) |
| Ptosis | 2 (0.5) | 0 (0.0) |
| Pes valgus | 1 (0.2) | 0 (0.0) |
| Respiratory complications | 1 (0.2) | 0 (0.0) |
| Hypogonadism | 1 (0.2) | 0 (0.0) |
| Rhabdomyolysis | 1 (0.2) | 0 (0.0) |
| Cognitive impairment | 1 (0.2) | 0 (0.0) |
| Myalgia | 0 (0.0) | 7 (5.5) |
| Increased CPK | 0 (0.0) | 5 (3.9) |
| Somnolence | 0 (0.0) | 2 (1.6) |
| Stiffness | 0 (0.0) | 2 (1.6) |
| Cramp | 0 (0.0) | 2 (1.6) |
| Fatigue | 0 (0.0) | 1 (0.8) |
| Unknown | 110 (25.4) | 15 (11.8) |
| Total | 434 (100.0) | 127 (100.0) |
| Myotonic Dystrophy Type 1 | Myotonic Dystrophy Type 2 | |
|---|---|---|
| Comorbidity (N (%)) | 434 (100.0) | 127 (100.0) |
| Muscle weakness Yes No Unknown | 315 (72.6) 26 (6.0) 93 (21.4) | 96 (75.6) 14 (11.0) 17 (13.4) |
| Myotonia Yes No Unknown | 275 (63.4) 25 (5.8) 134 (30.9) | 75 (59.1) 10 (7.9) 42 (33.1) |
| Fatigue Yes No Unknown | 68 (15.7) 63 (14.5) 303 (69.8) | 35 (27.6) 16 (12.6) 76 (59.8) |
| Cataract Yes No Unknown | 123 (28.3) 124 (28.6) 187 (43.1) | 39 (30.7) 49 (38.6) 39 (30.7) |
| Rhythm abnormalities/and or pacemaker Yes No Unknown | 83 (19.1) 129 (29.7) 222 (51.2) | 10 (7.9) 73 (57.5) 44 (34.6) |
| Cardiomyopathy Yes No Unknown | 21 (4.8) 128 (29.5) 285 (65.7) | 2 (1.6) 81 (63.8) 44 (34.6) |
| Respiratory inv Yes No Unknown | 35 (8.1) 80 (18.4) 319 (73.5) | 1 (0.8) 27 (21.3) 99 (77.9) |
| Daytime sleepiness Yes No Unknown | 25 (5.8) 63 (14.5) 346 (79.7) | 7 (5.5) 0 (0.0) 120 (94.5) |
| Sleep apnea Yes No Unknown | 26 (6.0) 48 (11.1) 360 (82.9) | 0 (0.0) 12 (9.4) 115 (90.6) |
| Ventilation Yes No Unknown | 15 (3.5) 72 (16.6) 332 (76.5) | 0 (0.0) 0 (0.0) 127 (100.0) |
| Thyroid dysfunction Yes No Unknown | 47 (10.8) 157 (36.2) 230 (53.0) | 20 (15.7) 57 (44.9) 50 (39.4) |
| Glucose intolerance Yes No Unknown | 19 (4.4) 181 (41.7) 234 (53.9) | 19 (15.0) 48 (37.8) 60 (47.2) |
| Dysphagia Yes No Unknown | 37 (8.5) 65 (15.0) 332 (76.5) | 0 (0.0) 11 (8.7) 116 (91.3) |
| Cancer Yes No Unknown | 4 (0.9) 216 (49.8) 214 (49.3) | 12 (9.4) 5 (3.9) 110 (86.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimas, G.K.; Papadopoulos, C.; Kekou, K.; Kartanou, C.; Kladi, A.; Nitsa, E.; Sofocleous, C.; Tsanou, E.; Sarmas, I.; Kaninia, S.; et al. A Greek National Cross-Sectional Study on Myotonic Dystrophies. Int. J. Mol. Sci. 2022, 23, 15507. https://doi.org/10.3390/ijms232415507
Papadimas GK, Papadopoulos C, Kekou K, Kartanou C, Kladi A, Nitsa E, Sofocleous C, Tsanou E, Sarmas I, Kaninia S, et al. A Greek National Cross-Sectional Study on Myotonic Dystrophies. International Journal of Molecular Sciences. 2022; 23(24):15507. https://doi.org/10.3390/ijms232415507
Chicago/Turabian StylePapadimas, Georgios K., Constantinos Papadopoulos, Kyriaki Kekou, Chrisoula Kartanou, Athina Kladi, Evangelia Nitsa, Christalena Sofocleous, Evangelia Tsanou, Ioannis Sarmas, Stefania Kaninia, and et al. 2022. "A Greek National Cross-Sectional Study on Myotonic Dystrophies" International Journal of Molecular Sciences 23, no. 24: 15507. https://doi.org/10.3390/ijms232415507
APA StylePapadimas, G. K., Papadopoulos, C., Kekou, K., Kartanou, C., Kladi, A., Nitsa, E., Sofocleous, C., Tsanou, E., Sarmas, I., Kaninia, S., Chroni, E., Tsivgoulis, G., Kimiskidis, V., Arnaoutoglou, M., Stefanis, L., Panas, M., Koutsis, G., Karadima, G., & Traeger-Synodinos, J. (2022). A Greek National Cross-Sectional Study on Myotonic Dystrophies. International Journal of Molecular Sciences, 23(24), 15507. https://doi.org/10.3390/ijms232415507







