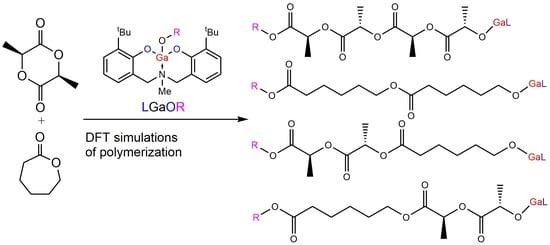
The Novel Gallium Aminobisphenolate Initiator of the Ring-Opening Copolymerization of L-Lactide and ε-Caprolactone: A Computational Study
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Dhanasekaran, N.P.D.; Muthuvelu, K.S.; Arumugasamy, S.K. Recent Advancement in Biomedical Applications of Polycaprolactone and Polycaprolactone-Based Materials. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 795–809. [Google Scholar]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Chapter 1 PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 1–36. [Google Scholar]
- Panchal, S.S.; Vasava, D.V. Biodegradable Polymeric Materials: Synthetic Approach. ACS Omega 2020, 5, 4370–4379. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Carpentier, J.-F.; Guillaume, C.; Guillaume, S.M.; Helou, M.; Poirier, V.; Sarazin, Y.; Trifonov, A. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 2010, 39, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Stirling, E.; Champouret, Y.; Visseaux, M. Catalytic metal-based systems for controlled statistical copolymerisation of lactide with a lactone. Polym. Chem. 2018, 9, 2517–2531. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Nomura, N.; Akita, A.; Ishii, R.; Mizuno, M. Random Copolymerization of ε-Caprolactone with Lactide Using a Homosalen−Al Complex. J. Am. Chem. Soc. 2010, 132, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Ma, H. Copolymerization of l-lactide and ε-caprolactone catalyzed by mono-and dinuclear salen aluminum complexes bearing bulky 6,6′-dimethylbipheyl-bridge: Random and tapered copolymer. RSC Adv. 2016, 6, 47402–47409. [Google Scholar] [CrossRef]
- Shi, T.; Luo, W.; Liu, S.; Li, Z. Controlled random copolymerization of rac-lactide and ɛ-caprolactone by well-designed phenoxyimine Al complexes. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 611–617. [Google Scholar] [CrossRef]
- Li, G.; Lamberti, M.; Pappalardo, D.; Pellecchia, C. Random Copolymerization of ε-Caprolactone and Lactides Promoted by Pyrrolylpyridylamido Aluminum Complexes. Macromolecules 2012, 45, 8614–8620. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Copolymerization of Cyclic Esters Controlled by Chiral NNO-Scorpionate Zinc Initiators. Organometallics 2016, 35, 189–197. [Google Scholar] [CrossRef]
- Maruta, Y.; Abiko, A. Random copolymerization of ε-caprolactone and l-lactide with molybdenum complexes. Polym. Bull. 2014, 71, 989–999. [Google Scholar] [CrossRef]
- Harinath, A.; Bhattacharjee, J.; Sarkar, A.; Nayek, H.P.; Panda, T.K. Ring Opening Polymerization and Copolymerization of Cyclic Esters Catalyzed by Group 2 Metal Complexes Supported by Functionalized P–N Ligands. Inorg. Chem. 2018, 57, 2503–2516. [Google Scholar] [CrossRef]
- Ouyang, H.; Yuan, D.; Nie, K.; Zhang, Y.; Yao, Y.; Cui, D. Synthesis and Characterization of Dinuclear Salan Rare-Earth Metal Complexes and Their Application in the Homo- and Copolymerization of Cyclic Esters. Inorg. Chem. 2018, 57, 9028–9038. [Google Scholar] [CrossRef]
- Chandanabodhi, D.; Nanok, T. A DFT study of the ring-opening polymerization mechanism of l-lactide and ε-caprolactone using aluminium salen-type initiators: Towards an understanding of their reactivities in homo- and copolymerization. Mol. Catal. 2017, 436, 145–156. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Coordination Ring-Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117. [Google Scholar] [CrossRef]
- Jehanno, C.; Mezzasalma, L.; Sardon, H.; Ruipérez, F.; Coulembier, O.; Taton, D. Benzoic Acid as an Efficient Organocatalyst for the Statistical Ring-Opening Copolymerization of ε-Caprolactone and L-Lactide: A Computational Investigation. Macromolecules 2019, 52, 9238–9247. [Google Scholar] [CrossRef]
- Cross, E.D.; Tennekone, G.K.; Decken, A.; Shaver, M.P. Aluminum amine-(bis)phenolate complexes for ring-opening polymerization of rac-lactide and ε-caprolactone. Green Mater. 2013, 1, 79–86. [Google Scholar] [CrossRef]
- Alcazar-Roman, L.M.; O'Keefe, B.J.; Hillmyer, M.A.; Tolman, W.B. Electronic influence of ligand substituents on the rate of polymerization of ε-caprolactone by single-site aluminium alkoxide catalysts. Dalton Trans. 2003, 15, 3082–3087. [Google Scholar] [CrossRef]
- Chen, C.-T.; Huang, C.-A.; Huang, B.-H. Aluminium metal complexes supported by amine bis-phenolate ligands as catalysts for ring-opening polymerization of ε-caprolactone. Dalton Trans. 2003, 3799–3803. [Google Scholar] [CrossRef]
- Chen, C.-T.; Huang, C.-A.; Huang, B.-H. Aluminum Complexes Supported by Tridentate Aminophenoxide Ligand as Efficient Catalysts for Ring-Opening Polymerization of ε-Caprolactone. Macromolecules 2004, 37, 7968–7973. [Google Scholar] [CrossRef]
- Phomphrai, K.; Chumsaeng, P.; Sangtrirutnugul, P.; Kongsaeree, P.; Pohmakotr, M. Reverse orders of reactivities in the polymerization of cyclic esters using N2O2 aluminium alkoxide complexes. Dalton Trans. 2010, 39, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, E.A.; Zaitsev, K.V.; Mamedova, F.A.; Churakov, A.V.; Zaitseva, G.S.; Lemenovsky, D.A.; Karlov, S.S. Synthesis, structure, and catalytic activity of new aluminum and titanium complexes based on aminobisphenolate ligands containing bulky substituents. Russ. Chem. Bull. 2016, 65, 1743–1749. [Google Scholar] [CrossRef]
- Vieira, I.d.S.; Whitelaw, E.L.; Jones, M.D.; Herres-Pawlis, S. Synergistic Empirical and Theoretical Study on the Stereoselective Mechanism for the Aluminum Salalen Complex Mediated Polymerization of rac-Lactide. Chem. Eur. J. 2013, 19, 4712–4716. [Google Scholar] [CrossRef]
- Chang, M.-C.; Lu, W.-Y.; Chang, H.-Y.; Lai, Y.-C.; Chiang, M.Y.; Chen, H.-Y.; Chen, H.-Y. Comparative Study of Aluminum Complexes Bearing N,O- and N,S-Schiff Base in Ring-Opening Polymerization of ε-Caprolactone and l-Lactide. Inorg. Chem. 2015, 54, 11292–11298. [Google Scholar] [CrossRef]
- Walshe, A.; Fang, J.; Maron, L.; Baker, R.J. New Mechanism for the Ring-Opening Polymerization of Lactones? Uranyl Aryloxide-Induced Intermolecular Catalysis. Inorg. Chem. 2013, 52, 9077–9086. [Google Scholar] [CrossRef]
- Della Monica, F.; Luciano, E.; Roviello, G.; Grassi, A.; Milione, S.; Capacchione, C. Group 4 Metal Complexes Bearing Thioetherphenolate Ligands. Coordination Chemistry and Ring-Opening Polymerization Catalysis. Macromolecules 2014, 47, 2830–2841. [Google Scholar] [CrossRef]
- Jitonnom, J.; Molloy, R.; Punyodom, W.; Meelua, W. Theoretical Studies on Aluminum Trialkoxide-Initiated Lactone Ring-Opening Polymerizations: Roles of Alkoxide Substituent and Monomer Ring Structure. Comput. Theor. Chem. 2016, 1097, 25–32. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef]
- Gesslbauer, S.; Savela, R.; Chen, Y.; White, A.J.P.; Romain, C. Exploiting Noncovalent Interactions for Room-Temperature Heteroselective rac-Lactide Polymerization Using Aluminum Catalysts. ACS Catal. 2019, 9, 7912–7920. [Google Scholar] [CrossRef]
- Meduri, A.; Mazzeo, M.; Lamberti, M.; Capacchione, C.; Milione, S. Electronic influence of ligand substituents in the ring-opening polymerization of l-Lactide promoted by OSSO-type zirconium complexes. Mol. Catal. 2019, 471, 54–59. [Google Scholar] [CrossRef]
- Marshall, E.L.; Gibson, V.C.; Rzepa, H.S. A Computational Analysis of the Ring-Opening Polymerization of rac-Lactide Initiated by Single-Site в-Diketiminate Metal Complexes: Defining the Mechanistic Pathway and the Origin of Stereocontrol. J. Am. Chem. Soc. 2005, 127, 6048–6051. [Google Scholar] [CrossRef]
- Lawan, N.; Muangpil, S.; Kungwan, N.; Meepowpan, P.; Lee, V.S.; Punyodom, W. Tin (IV) alkoxide initiator design for poly (d-lactide) synthesis using DFT calculations. Comput. Theor. Chem. 2013, 1020, 121–126. [Google Scholar] [CrossRef]
- Wang, L.; Kefalidis, C.E.; Sinbandhit, S.; Dorcet, V.; Carpentier, J.-F.; Maron, L.; Sarazin, Y. Heteroleptic Tin(II) Initiators for the Ring-Opening (Co)Polymerization of Lactide and Trimethylene Carbonate: Mechanistic Insights from Experiments and Computations. Chem. Eur. J. 2013, 19, 13463–13478. [Google Scholar] [CrossRef]
- Tabthong, S.; Nanok, T.; Sumrit, P.; Kongsaeree, P.; Prabpai, S.; Chuawong, P.; Hormnirun, P. Bis(pyrrolidene) Schiff Base Aluminum Complexes as Isoselective-Biased Initiators for the Controlled Ring-Opening Polymerization of rac-Lactide: Experimental and Theoretical Studies. Macromolecules 2015, 48, 6846–6861. [Google Scholar] [CrossRef]
- Lewiński, J.; Horeglad, P.; Wójcik, K.; Justyniak, I. Chelation Effect in Polymerization of Cyclic Esters by Metal Alkoxides: Structure Characterization of the Intermediate Formed by Primary Insertion of Lactide into the Al−OR Bond of an Organometallic Initiator. Organometallics 2005, 24, 4588–4593. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.A. Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zabalov, M.V.; Tiger, R.P. The supermolecule method, as applied to studies of liquid-phase reaction mechanisms taking cyclocarbonate aminolysis in dioxane as an example: Specific features. Russ. Chem. Bull. 2016, 65, 631–639. [Google Scholar] [CrossRef]
- Zabalov, M.V.; Tiger, R.P. Specificities of application of the supermolecule method to the calculation of reaction mechanisms in a protonodonor medium. Ethylene carbonate aminolysis in methanol. Theor. Chem. Acc. 2017, 136, 95. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Pimerzin, A.A. The thermodynamic properties of DL- and L-lactides. Russ. J. Phys. Chem. 2009, 83, 2013–2021. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Stepurko, E.N.; Roganov, G.N.; Georgieva, M.K. Thermodynamical properties of ε-caprolactone. Russ. J. Phys. Chem. 2010, 84, 356–363. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabalov, M.V.; Mankaev, B.N.; Egorov, M.P.; Karlov, S.S. The Novel Gallium Aminobisphenolate Initiator of the Ring-Opening Copolymerization of L-Lactide and ε-Caprolactone: A Computational Study. Int. J. Mol. Sci. 2022, 23, 15523. https://doi.org/10.3390/ijms232415523
Zabalov MV, Mankaev BN, Egorov MP, Karlov SS. The Novel Gallium Aminobisphenolate Initiator of the Ring-Opening Copolymerization of L-Lactide and ε-Caprolactone: A Computational Study. International Journal of Molecular Sciences. 2022; 23(24):15523. https://doi.org/10.3390/ijms232415523
Chicago/Turabian StyleZabalov, Maxim V., Badma N. Mankaev, Mikhail P. Egorov, and Sergey S. Karlov. 2022. "The Novel Gallium Aminobisphenolate Initiator of the Ring-Opening Copolymerization of L-Lactide and ε-Caprolactone: A Computational Study" International Journal of Molecular Sciences 23, no. 24: 15523. https://doi.org/10.3390/ijms232415523
APA StyleZabalov, M. V., Mankaev, B. N., Egorov, M. P., & Karlov, S. S. (2022). The Novel Gallium Aminobisphenolate Initiator of the Ring-Opening Copolymerization of L-Lactide and ε-Caprolactone: A Computational Study. International Journal of Molecular Sciences, 23(24), 15523. https://doi.org/10.3390/ijms232415523