Abstract
Heme is of great significance in food nutrition and food coloring, and the successful launch of artificial meat has greatly improved the application of heme in meat products. The precursor of heme, 5-aminolevulinic acid (ALA), has a wide range of applications in the agricultural and medical fields, including in the treatment of corona virus disease 2019 (COVID-19). In this study, E. coli recombinants capable of heme production were developed by metabolic engineering and membrane engineering. Firstly, by optimizing the key genes of the heme synthesis pathway and the screening of hosts and plasmids, the recombinant strain EJM-pCD-AL produced 4.34 ± 0.02 mg/L heme. Then, the transport genes of heme precursors CysG, hemX and CyoE were knocked out, and the extracellular transport pathways of heme Dpp and Ccm were strengthened, obtaining the strain EJM-ΔCyoE-pCD-AL that produced 9.43 ± 0.03 mg/L heme. Finally, fed-batch fermentation was performed in a 3-L fermenter and reached 28.20 ± 0.77 mg/L heme and 303 ± 1.21 mg/L ALA. This study indicates that E. coli recombinant strains show a promising future in the field of heme and ALA production.
1. Introduction
Heme is a stable form of iron containing a porphyrin compound (C34H33FeN4O4) that can interact with biological membranes, existing in almost all kinds of animals, and participating in a variety of physiological and biochemical reactions including respiration, cell differentiation and signal transduction [1,2,3,4]. Heme has a strong coloring ability that can replace coloring agents and synthetic pigments in food, reduce or completely replace the use of nitrite, reduce the residue of nitrite in food and prevent food from forming nitrosamines and other carcinogens [5,6]. In the past several years, heme has been used as a good iron supplement and has shown a significant therapeutic effect in the treatment of iron-deficiency anemia [7,8]. At the same time, the heme iron complex is one of the most important cofactors in biological systems as the binding site for toxic gaseous molecules, such as NO and CO. The binding of these non-O2 gaseous molecules to the heme iron complex regulates various important physiological and pathological functions associated with O2 binding [9,10,11]. Artificial meat is one of the hottest topics in the field of food science; however, its acceptance is affected by its color. So, we hope to improve its color by using heme [12,13,14,15,16]. Heme can be extracted from animal blood by organic solvent or enzymatic hydrolysis; however, this is against animal welfare [16]. Biosynthesized heme can satisfy the need of color improvement as well as iron supplementation. Therefore, the animal-free and biotechnological production of free-heme molecules is essential for high-yield, economical and eco-friendly production.
The biosynthesis of heme has attracted extensive attention with the development of metabolic engineering and synthetic biology. Ge et al. [17] improved ALA and heme production by upregulating hemB, hemG, hemH, hemAs and hemL expression, and increasing the copy number of the genes hox1 and pcyA. [18]. Feng et al. [19] improved heme production by regulating the expression of heme peroxidase in E. coli. The regulation of the heme production pathway has always been a concern due to the complex regulatory system of heme production.
5-aminolevulinic acid (ALA) is a universal metabolite in the biosynthesis of heme, that is synthesized by one of two different routes: the C4 pathway or the C5 pathway (Figure 1). The C4 pathway, also called the ‘Shemin pathway’, is present in mammals, fungi and α-proteobacteria. The C5 pathway of heme biosynthesis is found in plants, most bacteria and archaea. Compared to the C4 pathway, the C5 pathway uses simple carbon sources with higher titer of heme as substrates; therefore, it is more suitable for the biosynthesis of heme in prokaryotes [20,21,22,23]. In the C5 pathway, glutamate was firstly catalyzed by glutamyl-tRNA synthetase (gltX) and generate glutamyl-tRNA. Secondly, glutamyl-tRNA was catalyzed by glutamyl-tRNA reductase (hemA) to generate glutamate-1-semialdehyde, which is finally catalyzed by glutamate-1-semialdehyde transaminase (hemL) to generate ALA. This is followed by the production of bilirubinogen-by-bilirubinogen synthase (hemB). Subsequently, hydroxymethyl bile is produced by bilirubinogen deaminase (hemC). Then, the uroporphyrinogen III is produced by hydroxymethyl bile through the uroporphyrinogen III synthase (hemD). Coproporphyrinogen III is then generated by decarboxylase (hemE). Protoporphyrinogen IX is produced by coproporphyrinogen III oxidase (hemF). Protoporphyrin IX is then catalyzed by protoporphyrinogen oxidase (hemG) to produce protoporphyrin IX, and finally with Fe2+ to produce heme by the action of ferrous chelatase (hemH) [24].
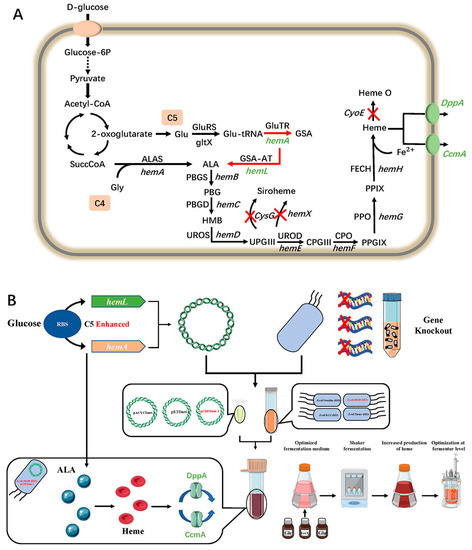
Figure 1.
(A) A schematic overview describing the target pathway and overall metabolic strategies for ALA and Heme production in E. coli. In this study, glucose was the only major source of energy for cell growth and porphyrin production. The green letter represents the gene overexpression in E. coli. The red X-shape denote the gene knockout. The solid-line arrow and dashed-line arrow indicate the general metabolic pathway and abbreviated pathway, respectively. The red arrow denotes enhanced metabolic flux by the combinatorial overexpression of hemA and hemL. Green ovals indicate enhanced extracellular transport pathways for heme by insertion of the target gene. (B) Strategies for modifying the biosynthesis of heme in E. coli.
In recent years, various hosts have been used to construct heme production strains, including E. coli, Corynebacterium glutamicum and Salmonella typhimurium [25,26,27,28,29]. Heme production can be effectively improved by enhancing the ALA production pathway, including accumulating succinyl CoA precursors [30], overexpressing the ALA transporter protein RhtA, a two-stage fermentation strategy [31], overexpressing transcriptional regulators DtxR [13]. Most bacteria (including E. coli) synthesize ALA and heme using glucose as a substrate by the C5 pathway, which not only simplifies the ALA and heme production pathway, but also saves costs and achieves higher yields [32,33,34]. E. coli is a universal model microorganism; in these studies, E. coli BL21(DE3) was mostly used as a host, and there is little study on heme-production potential of other kinds of E. coli.
In this study, we developed an engineered E. coli capable of de novo production of heme (Figure 1). To this end, we first screened for a host and a plasmid suitable for ALA and heme production. To improve the heme production, the genes hemA and hemL in the C5 pathway of ALA production were enhanced. Then, the genes cysG and hemX, that are involved in the precursor extracellular transport in heme production, were knocked out to improve the yield of heme from ALA. The gene cyoE was also knocked out to prevent heme conversion. Next, the Ccm and Dpp pathways were enhanced for extracellular transport of heme. Finally, fermentation optimization of recombinant E. coli was performed in 3-L bioreactors.
2. Results and Discussion
2.1. Improving HemA and HemL Expression in E. coli Host Strains
To screen a proper host for heme production in E. coli strains, the expression of genes hemA and hemL coding for glutamyl-tRNA reductase (GluTR) and glutamyl-1-hemal transaminase (GSA-AT) in the C5 pathway of ALA production were first enhanced in E. coli BL21(DE3). Plasmids pAC-hemA, pAC -hemL and pAC-hemA-hemL were constructed based on the plasmid pACYCDuet-1 (Table S1). Recombinant strains EBL-pAC-A, EBL-pAC-L and EBL-pAC-AL were obtained by introducing plasmids pAC-hemA, pAC -hemL and pAC-hemA-hemL were constructed based on the plasmid pACYCDuet-1 into E. coli BL21(DE3), EJM-pAC-A, EJM-pAC-L, EJM-pAC-AL and strain EJM-pAC-K and strain EBL-pAC-K without enhancing target genes hemA and hemL target gene were constructed by using the techniques described in Section 3.3. After shake-flask fermentation, the recombinant strain EBL-pAC-AL provided 55.48 ± 4.69 mg/L heme in 8-h fermentation. The titer of EBL-pAC-AL was 3.71-fold that of recombinant EBL-pAC-A 14.95 ± 0.05 mg/L, and was 6.80-fold that of recombinant EBL-pAC-L 8.15 ± 1.07 mg/L. The titer of EBL-pAC-AL was 49.04 mg/L, 6.24-fold that of the strain EBL-PAC-K (Figure 2A). The above results indicate that the overexpression of hemA and hemL could significantly improve the ALA production.
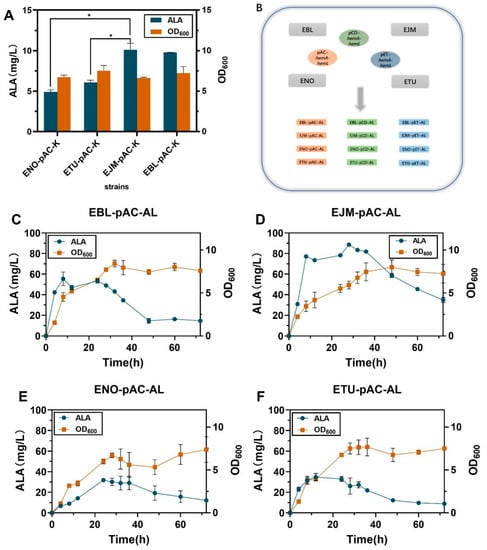
Figure 2.
(A) OD600 and ALA production of the no-loaded strains at 36 h of fermentation. (B) Recombinant plasmid construction flowchart. (C–F) Growth curve and ALA production of recombinant strain fermented for 72 h. (The recombinant strains corresponding to Figure 2C–F are EBL-pAC-AL, EJM-pAC-AL, ENO-pAC-AL, ETU-pAC-AL, respectively). All data indicate the mean of two independent biological experiments and error bars represent standard deviation. The symbol * stands for and p < 0.05, as determined by Student’s t-test.
Then, the recombinant plasmid pAC-hemA-hemL was converted into E. coli NovaBlue(DE3), E. coli Turner(DE3), E. coli BL21(DE3) and E. coli JM109(DE3), resulting in recombinant strains ENO-pAC-AL, ETU-pAC-AL, EBL-pAC-AL and EJM-pAC-AL. The titer of EJM-pAC-AL was 3.03-fold that of recombinant ENO-pAC-AL 29.16 ± 4.70 mg/L, and was 2.75-fold that of recombinant ETU-pAC-AL 32.97 ± 1.66 mg/L. The titer of EBL-pAC-AL was 1.87-fold that of recombinant ENO-pAC-AL 29.16 ± 4.70 mg/L, and was 1.72-fold that of recombinant ETU-pAC-AL 32.97 ± 1.66 mg/L. These results indicated that the recombinant strains EJM-pAC-AL and EBL-pAC-AL had obvious advantages in ALA synthesis (Figure 2 and Figure 3A,B).
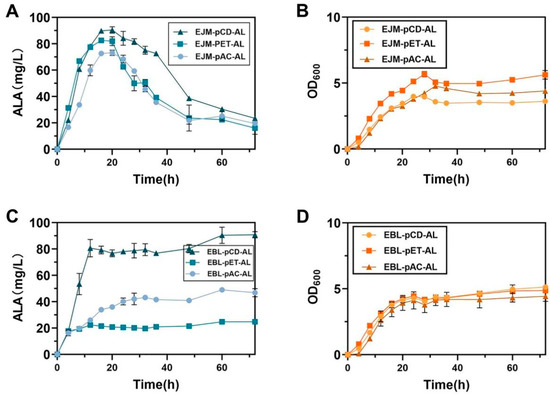
Figure 3.
(A) The recombinant plasmids pCD-hemA-hemL, pET-hemA-hemL and pAC-hemA-hemL were transformed into the host E. coli JM109(DE3) for ALA production after 72 h of fermentation. (B) Growth curves of recombinant plasmids pCD-hemA-hemL, pET-hemA-hemL and pAC-hemA-hemL were transformed into the host E. coli JM109(DE3) for 72 h fermentation. (C) The recombinant plasmids pCD-hemA-hemL, pET-hemA-hemL and pAC-hemA-hemL were transformed into the host E. coli BL21(DE3) for ALA production after 72 h of fermentation. (D) Growth curves of recombinant plasmids pCD-hemA-hemL, pET-hemA-hemL and pAC-hemA-hemL were transformed into the host E. coli BL21(DE3) for 72 h fermentation. All data indicate the mean of two independent biological experiments and error bars represent standard deviation.
In order to screen for the highest quality plasmids here we selected three recombinant plasmids namely pAC-hemA-hemL, pCD-hemA-hemL and pET-hemA-hemL, which were simultaneously transformed into host E. coli BL21(DE3) and E. coli JM109(DE3) to obtain recombinant strains EBL-pAC-AL, EBL-pCD-AL, EBL-pET-AL, EBL-pET-AL, EJM-pAC-AL, EJM-pCD-AL and EJM-pET-AL. The highest ALA titer of recombinant strain EJM-pCD-AL was 90.55 ± 1.72 mg/L (Figure 3C,D). At the same time, the maximum production of heme was 4.34 ± 0.02 mg/L produced by EJM-pCD-AL. From this, the best recombinant plasmid pCD-hemA-hemL was screened for further study. Therefore, strain EJM-pCD-AL was selected for further study.
2.2. Increasing Heme Production by Removing Competitive Pathways
To remove the competitive pathways, we knocked out CysG, hemX and CyoE genes, respectively, in E. coli JM109(DE3), resulting in EJM-ΔCysG, EJM-ΔhemX and EJM-ΔCyoE (Figure S1). Then, the plasmid pCD-hemA-hemL was transformed into the three strains, resulting recombination strains EJM-ΔCysG-pCD-AL, EJM-ΔhemX-pCD-AL and EJM-ΔCyoE-pCD-AL, respectively. The fermentation was first carried out in shaking flasks and samples were taken at 4 h intervals to determine ALA and heme titer (Figure 4A–E). By observing the color of the fermentation broth, we found that the broth changed from light yellow to dark brown after fermentation for 48 h (Figure 4F). We obtained a heme titer of 9.06 ± 0.01 mg/L for strain EJM-ΔCysG-pCD-AL, and the extracellular heme secretion titer was 4.48 ± 0.01 mg/L, while the ALA titer decreased to 39.53 ± 1.38 mg/L, suggested that the knockout of the gene CysG could increase the heme titer [35] (Figure 4A). The heme production of strain EJM-ΔhemX-pCD-AL after knockout of the gene hemX was 8.62 ± 0.08 mg/L, the increase in heme titer was not obvious, at which point the ALA production did not change much (Figure 4B). We obtained from this result that knocking out the gene hemX had less effect on the increase in heme production, and also found that there was a certain loss of precursor material production in a certain range of heme production increase. We found CyoE knockout can greatly increase heme production while keeping ALA production almost unchanged (Figure 4C). At this point the recombinant strain EJM-ΔCyoE-pCD-AL reached a maximum titer of 9.43 ± 0.03 mg/L in shake flask culture, and had a 2.17-fold increase in heme production compared with the EJM-pCD-AL strain (Figure 4F). At this time, heme extracellular production reached 4.38 ± 0.04 mg/L. This suggested that blocking the pathway from heme-to-heme O could greatly accumulate heme production. Synthesis of Siroheme catalyzed by sirohydrochlorin ferrochelatase and uroporphyrin-Ⅲ C-methyltransferase (encoded by CysG and hemX genes, respectively) can be blocked. In addition, the gene CyoE which encodes heme O synthase, was knocked out to prevent the consumption of heme.
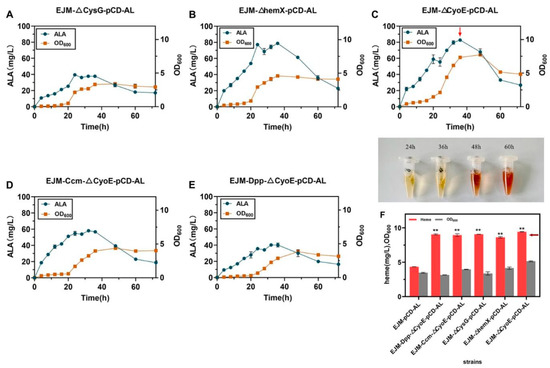
Figure 4.
(A–E) The growth curve and ALA production of the gene-edited strain within 72 h of fermentation. (F) The color change in fermentation broth at different stages of fermentation and heme titer of different recombinant strains. All data indicate the mean of two independent biological experiments and error bars represent standard deviation. The symbol ** stands for and p < 0.01, as determined by Student’s t-test.
2.3. Overexpression of Heme Exporters to Increases Heme Production
In E. coli, the effect of Ccm pathway on heme production has been demonstrated [32], but the effect of Dpp pathway on heme production has never been studied. In this study, the regulation of CcmA and DppA genes influenced the output of heme protein to further observe the influence on ALA and heme production. The recombinant strain EJM-ΔCyoE-pCD-AL screened in the early stage was transformed into the competent cells obtained by gene editing technology to obtain strains EJM-Ccm-ΔCyoE-pCD-AL and EJM-Dpp-ΔCyoE-pCD-AL. By observing the color of fermentation broth, we found that the fermentation broth changed from light yellow to dark brown after 48 h of fermentation (Figure 4F). We guessed that the heme content might be increased to some extent at this time. After the analysis in Section 3.6, the heme titer of strain EJM-Ccm-ΔCyoE-pCD-AL was 8.94 ± 0.14 mg/L, and the extracellular heme secretion was 4.43 ± 0.08 mg/L. The heme titer of strain EJM-Dpp-ΔCyoE-pCD-AL was 9.04 ± 0.06 mg/L, and the extracellular heme secretion was 4.45 ± 0.08 mg/L. The EJM-Ccm-ΔCyoE-pCD-AL strain overexpressing the CcmA gene had a 2.05-fold increase in heme production compared with the EJM-pCD-AL strain. The EJM-Dpp-ΔCyoE-pCD-AL strain overexpressing the DppA gene had a 2.08-fold increase in heme production compared with the EJM-pCD-AL strain. This was consistent with our guess that there was indeed an increase in heme production compared to the previous strain EJM-pCD-AL (Figure 4F). At this time, the output of ALA decreased to a certain extent, the output of EJM-Ccm-ΔCyoE-pCD-AL was 58.01 ± 1.38 mg/L, and the output of EJM-Dpp-ΔCyoE-pCD-AL was 40.09 ± 1.71 mg/L (Figure 4B). ALA and heme production of some recombinant strains can be seen in Table 1 and Table S3.

Table 1.
This table shows the ALA and heme production detected by fermentation of recombinant strains constructed in this study.
2.4. Improving the Heme Production in Bioreactor
2.4.1. Optimization of Fermentation Conditions
On the basis of the TB medium, we optimized the concentration of carbon source glucose (Glc), the trace element Fe2+ and the precursor glutamate (Glu), which were all required in the C5 pathway. The specific added amount can be seen in Section 3.5. As shown in (Figure 5), the increasing concentration of glucose, FeCl2 and glutamate could promote the accumulation of heme and ALA. The effect of glucose was the most obvious, which further increased the titer of heme to 10.95 ± 0.07 mg/L (Figure 5). Fe2+ was used as a metal ion chelated by heme, and the content of exogenous Fe2+ increasing within a certain range was also beneficial to the synthesis of heme. Glutamate, as the precursor of E. coli C5 pathway synthesis, can promote the accumulation of heme, and the promotion of ALA synthesis was very obvious. After optimization of the fermentation medium, the final heme titer reached up to 10.95 ± 0.07 mg/L and the ALA titer reached up to 111.98 ± 1.9 mg/L (Figure 5).
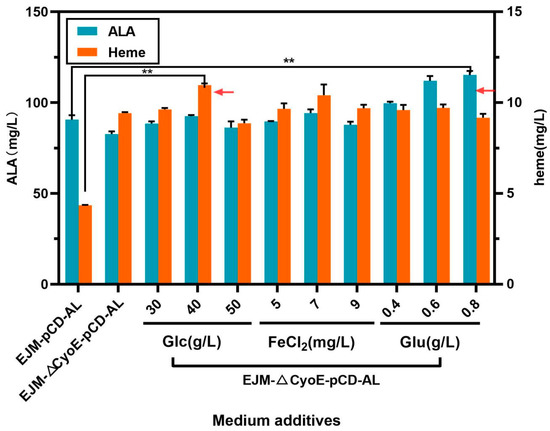
Figure 5.
Heme and ALA production of recombinant strain EJM-∆CyoE-pCD-AL under medium-optimized conditions. The abscissa shows the heme and ALA production of control strain EJM-pCD-AL and the yield of recombinant strain EJM-∆CyoE-pCD-AL under optimized conditions, respectively. The symbol ** stands for and p < 0.01, as determined by Student’s t-test.
2.4.2. Fed-Batch Fermentation of Heme by Recombinant Strains
To test the high-level ALA and heme production potential of engineered strains, fed-batch fermentations were conducted in 3-L bioreactors using the optimized producer strain EJM-∆CyoE-pCD-AL and the primary producer strain EJM-pCD-AL as a control. Overall, strain EJM-∆CyoE-pCD-AL was significantly better than strain EJM-pCD-AL in terms of cell growth, heme production and ALA production (Figure 6). After 48 h for fermentation, 303 ± 1.21 mg/L of ALA was produced by strain EJM-∆CyoE-pCD-AL, representing a 39.6% improvement compared to strain EJM-pCD-AL (224.17 ± 3.08 mg/L) (Figure 6). At the same time, 28.20 ± 0.77 mg/L of heme was produced by strain EJM-∆CyoE-pCD-AL, representing a 35.2% improvement compared to strain EJM-pCD-AL (20.27 ± 0.17 mg/L) (Figure 6). The above results indicate that by enhancing the expression of key genes hemA and hemL, and simultaneously knocking out the gene CyoE that decomposes ALA, played an important role in the efficient production of heme. This also provided an effective strategy for the industrial production of heme.
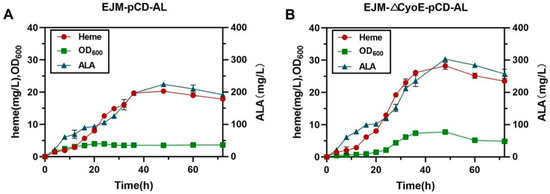
Figure 6.
Fed-batch fermentation results of engineered strains. (A) Fed-batch fermentation profiles of the strain EJM-pCD-AL during 72 h. (B) Fed-batch fermentation profiles of the strain EJM-△CyoE-pCD-AL during 72 h. The red circle represents heme production, the green square represents OD600 and the blue triangle represents ALA production.
3. Materials and Methods
3.1. Strains and Plasmids
All plasmids and bacterial strains constructed and used in this study are listed in Table S1. E. coli DH5α was used as template for the amplification of target genes hemA and hemL. E. coli JM109 was used for plasmid construction and preservation. E. coli JM109(DE3), E. coli BL21(DE3), E. coli Turner(DE3) and E. coli NovaBlue(DE3) were used as host for ALA and heme production. The genomic DNA of E. coli JM109 was used for the amplification of CysG, CyoE, HemX, CcmA and DppA. Plasmids pKD13 and pKD46 were used for genome editing of E. coli.
3.2. Culture Conditions
For plasmids construction, strains were cultured in a 250 mL shake flask containing 50 mL of Luria-Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl) at 37 °C 220 rpm. The solid medium was prepared by adding 15 g/L agar to the liquid LB medium. Chloramphenicol (20 μg/mL), streptomycin (100 μg/mL), ampicillin (100 μg/mL) and kanamycin (100 μg/mL) were added to the medium depending on the situation. Single colonies harboring different plasmids were picked up and cultured in 5 mL LB media at 37 ℃ overnight with continuous shaking. Then, 5.0% inoculum of the seed was transferred to a 250 mL flask with about 50 mL Terrific Broth (TB) medium (11.8 g/L tryptone, 23.6 g/L yeast extract, 9.4 g/L K2HPO4, 2.2 g/L KH2PO4, 4 mL glycerol) for ALA and heme production. All recombinant strains were cultured at 37 °C, 220 rpm. Chloramphenicol, streptomycin, ampicillin, or kanamycin was used for plasmids selection. Gene expression was induced with initial addition of isopropyl-β-D-thiogalactopyranoside (IPTG, 0.1 mM).
For genome modification, SOB medium (20 g/L tryptone, 5 g/L yeast extract, 0.5 g/L NaCl, 5 g/L MgSO4·7H2O) as a nutritionally rich growth medium was used for the preparation of competent cells to improve the transfection efficiency. In addition, L-arabinose (10 mM) was also added to induce the expression of Red-recombinase. SOC medium (20 g/L tryptone, 5 g/L yeast extract, 0.5 g/L NaCl, 5 g/L MgSO4·7H2O, 3.6 g/L D-Glucose) was used in the last stage of transformation. As plasmid pKD46 is temperature sensitive, cells used for genome editing should be cultured at 30 °C.
3.3. Construction of Recombinant Plasmids and Strains
Standard molecular genetic techniques were used for DNA manipulation [36]. The recombinant plasmids and strains constructed and used in this study are listed in Table S1. The primers used for constructing plasmid and Red-based recombineering are listed in Table S2. The genomic DNA of E. coli DH5α was used to amplify the hemA and hemL. pACYCuet-1, pCDFDuet-1 and pETDuet-1 vectors were used for E. coli for the overexpression of target genes. HemA and hemL genes were amplified using primers hemA-F/hemA-R and hemL-F/hemL-R to construct plasmid pAC-hemA-hemL, pCD-hemA-hemL and pET-hemA-hemL. The linear plasmid and coding sequence were assembled using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). The recombinant plasmids pAC-hemA-hemL, pCD-hemA-hemL and pET-hemA-hemL were then transformed into host receptor cells E. coli JM109(DE3), E. coli BL21(DE3), E. coli Turner(DE3) and E. coli NovaBlue(DE3), respectively, to construct recombinant strains EBL-pAC-AL, EBL-pCD-AL, EBL-pET-AL, EJM-pAC-AL, EJM-pCD-AL, EJM-pET-AL, ENO-pAC-AL, ENO-pCD-AL, ENO-pET-AL, ETU-pAC-AL, ETU-pCD-AL and ETU- pET-AL.
3.4. Red/ET-Based Recombineering
In order to knock out the genes CysG, CyoE and HemX on the E. coli genome, the Red/ET homologous recombination system was applied here to design three knockout frames consisting of two homologous regions (one 550 bp upstream and one 500 bp downstream of the gene to be knocked out) and one region replacing the pKD13 resistance gene fragment of the gene to be knocked out [37]. These three regions were amplified and purified using the primers in Table S2 (kanR-F/R, kanR-YZ, cysG-up-F/R, cysG-down-F/R, cyoE-up-F/R, cyoE-down-F/R, hemX-up-F/R and hemX-down-F/R) and then subjected to fusion polymerase chain reaction (PCR). To enhance the expression of the heme extracellular transport pathway, two insertion frames were designed here by the Red/ET homologous recombination system, where the primers from Table S2 (kanR-F/R, kanR-YZ, dppA-up-F/R, dppA-T7-F/R, dppA-F/R, ccmA-up-F/R, ccmA-T7-F/R and ccmA-F/R) were separately amplified and purified for fusion the insertion frames CcmAup-KanR-T7-CcmA and DppAup-KanR-T7-DppA were obtained by polymerase chain reaction (PCR).
3.5. Fed-Batch Fermentation
The culture used in fed-batch fermentation was optimized based on TB medium with different concentrations of glucose (30 g/L, 40 g/L and 50g/L), FeCl2 (5 mg/L, 7 mg/L and 9 mg/L) and glutamate (0.4g /L, 0.6 g/L and 0.8 g/L).
Fed-batch fermentations for ALA and heme production were performed as follows: 40 mL seed of the engineered strain cultured in LB medium at 37 °C, 220 rpm for 16 h was inoculated to 3-L fermenter with approximately 1.5 L of fermentation medium (11.8 g/L tryptone, 23.6 g/L yeast extract, 9.4 g/L K2HPO4, 2.2 g/L KH2PO4, 6 mL glycerol, 40 g/L glucose and 7 mg/L FeCl2). The culture conditions were maintained as follows: temperature 37 °C, airflow rate 1.0 vvm, agitation speed 500 rpm. pH was automatically controlled at 7.0.
3.6. Analytical Methods
Cell growth was measured at OD600 using a UV-vis spectrophotometer (Yuanxi Co, Shanghai, China) after the culture was diluted to a proper volume with distilled water. ALA concentration was measured using the colorimetric called Ehrlich’s reagent. Take 1 mL cultured cells were centrifuged at 12000 rpm for 5 min. An amount of 300 μL of the supernatant was chemically reacted with 400 μL of sodium acetate buffer (PH 4.6) and 35 μL acetylacetone at 100 °C for 15 min. After cooling to room temperature, 440 μL Modified Ehrlich’s reagent was added for 10 min and the absorbance value was measured at 554 nm using a spectrophotometer [38]. For the measurement of intracellular heme, 1 mL cultured cells were centrifuged at 4000 rpm for 6 min. After separating supernatant, the cell pellet was disrupted using-modified actone: HCl extraction methods described by Espinas et al. [39]. After 1mL of acetone: HCl (95:5) buffer was added to the cell-harvested tube, the mixture was vortexed and diluted with 1 mL of 1 M NaOH. The intracellular sample was disrupted, and the supernatant was filtered using an MCE filter for concentration analysis. For the measurement of extracellular heme, the supernatant from the cell culture was mixed with 1 M NaOH at a 1:1 ratio and filtered using an MCE filter for concentration analysis. Heme concentration was determined using a high-performance liquid chromatography (HPLC) system (Agilent Co, Palo Alto, CA, USA). The filtered sample was separated in a Symmetry® C18 HPLC Column 5 μm particle size, 4.6 × 250 mm. Solvent A is a 10:90 (v/v) HPLC grade methanol: acetonitrile mixture, and solvent B is a 0.5% (v/v) trifluoroacetic acid (TFA) in HPLC grade water. The flow rate was 0.6 mL/min for 40 min, and the absorbance was determined at 405 nm [13,20]. The HPLC chromatograms of heme were shown in Figure S2. In this experiment, differences of two groups of data were determined by a two-tailed Student’s t test, and the statistical significance is indicated as * for p < 0.05 and ** for p < 0.01.
4. Conclusions
In this study, we engineered E. coli for improving the production of ALA and heme. Here we chose the C5 pathway of heme biosynthesis and demonstrated that co-expression of the genes hemA and hemL can significantly promoted the accumulation of ALA and heme. We also knocked out the genes CysG, hemX and CyoE, which may prevent intracellular degradation of ALA hinder heme biosynthesis, and found that knocking out CysG was effective in further increasing heme accumulation, and CyoE knockout can greatly increase heme production while keeping ALA production almost unchanged. Finally, membrane engineering was attempted by enhanced putative heme exporters. Ths enhanced the dipeptide transport system substrate-binding protein and heme exporter protein A of heme outward transport regulated by the genes DppA and CcmA. It was demonstrated that overexpression of the genes CcmA and DppA encoding heme export proteins also enhanced heme production. The results of this study improved the heme production and its yield from ALA by preventing the degradation of ALA and heme and enhancing the heme transport. However, the titer and yield of heme was still not qualified for large-scale production, therefore, further modifications are needed to maximize the potential of the engineered strains to fit the industrial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415524/s1.
Author Contributions
Z.G., J.D. and B.X. conceived and designed the experiments; Z.G., J.D. and J.G. performed the experiments; Z.G., J.D. and B.X. analyzed the data; Z.G. and J.D. wrote the paper; Z.G., J.D., W.C., H.Z. and B.X. interpreted the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Anhui Provincial Natural Science Foundation of China (grant number 2108085QC148), the Major Science and Technology Program of Anhui, China (grant number 2021d06050001), and the National Key R&D Program of China (grant number 2021YFD2100804).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mukherjee, M.; Dey, A. Rejigging Electron and Proton Transfer to Transition between Dioxygenase, Monooxygenase, Peroxygenase, and Oxygen Reduction Activity: Insights from Bioinspired Constructs of Heme Enzymes. JACS Au 2021, 1, 1296–1311. [Google Scholar] [CrossRef]
- Zamarreño Beas, J.; Videira, M.A.M.; Saraiva, L.M. Regulation of bacterial haem biosynthesis. Coord. Chem. Rev. 2022, 452. [Google Scholar] [CrossRef]
- Donegan, R.K.; Moore, C.M.; Hanna, D.A.; Reddi, A.R. Handling heme: The mechanisms underlying the movement of heme within and between cells. Free Radic. Biol. Med. 2019, 133, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.G.; Willoughby, M.M.; Hamza, I.; Reddi, A.R. One ring to bring them all and in the darkness bind them: The trafficking of heme without deliverers. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118881. [Google Scholar] [CrossRef] [PubMed]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef]
- Gamage, S.M.K.; Dissabandara, L.; Lam, A.K.; Gopalan, V. The role of heme iron molecules derived from red and processed meat in the pathogenesis of colorectal carcinoma. Crit. Rev. Oncol. Hematol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Abu-Faza, M.; Shikanova, S.; Zhurabekova, G.; Maghrabi, M.M. Heme-bound iron in treatment of pregnancy-associated iron deficiency anemia. J. Fam. Med. Prim. Care 2018, 7, 1434–1438. [Google Scholar] [CrossRef]
- Buzała, M.; Janicki, B.; Buzała, M.; Słomka, A. Heme iron in meat as the main source of iron in the human diet. J. Elem. 2016, 21, 303–314. [Google Scholar] [CrossRef]
- Shimizu, T.; Huang, D.; Yan, F.; Stranava, M.; Bartosova, M.; Fojtikova, V.; Martinkova, M. Gaseous O2, NO, and CO in signal transduction: Structure and function relationships of heme-based gas sensors and heme-redox sensors. Chem. Rev. 2015, 115, 6491–6533. [Google Scholar] [CrossRef]
- Shimizu, T.; Lengalova, A.; Martinek, V.; Martinkova, M. Heme: Emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef]
- Shisaka, Y.; Shoji, O. Bridging the gap: Unveiling novel functions of a bacterial haem-acquisition protein capturing diverse synthetic porphyrinoids. Coord. Chem. Rev. 2022, 472. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Odagiri, H.; Nishimura, T.; Hattori, A. Quantitative determination of Zn protoporphyrin IX, heme and protoporphyrin IX in Parma ham by HPLC. Meat Sci. 2009, 82, 139–142. [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, M.; You, S.K.; Shin, S.K.; Chang, J.; Choi, H.J.; Jeong, W.Y.; Lee, M.E.; Hwang, D.H.; Han, S.O. Animal-free heme production for artificial meat in Corynebacterium glutamicum via systems metabolic and membrane engineering. Metab. Eng. 2021, 66, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, Z.; Shen, L.; Xiao, H. Synthetic biology: A new frontier in food production. Trends Biotechnol. 2022, 40, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Badar, I.H.; Xia, X.; Kong, B.; Chen, Q. Prospects of artificial meat: Opportunities and challenges around consumer acceptance. Trends Food Sci. Technol. 2021, 116, 434–444. [Google Scholar] [CrossRef]
- Palmer, C. Engineer Dinner—Save the Environment? Engineering 2020, 6, 213–215. [Google Scholar] [CrossRef]
- Ge, B.; Chen, Y.; Yu, Q.; Lin, X.; Li, J.; Qin, S. Regulation of the heme biosynthetic pathway for combinational biosynthesis of phycocyanobilin in Escherichia coli. Process Biochem. 2018, 71, 23–30. [Google Scholar] [CrossRef]
- Celis, A.I.; Choby, J.E.; Kentro, J.; Skaar, E.P.; DuBois, J.L. Control of Metabolite Flux during the Final Steps of Heme b Biosynthesis in Gram-Positive Bacteria. Biochemistry 2019, 58, 5259–5270. [Google Scholar] [CrossRef]
- Feng, C.; Pan, M.; Tang, L. 5-Aminolevulinic acid level and dye-decolorizing peroxidase expression regulate heme synthesis in Escherichia coli. Biotechnol. Lett. 2022, 44, 271–277. [Google Scholar] [CrossRef]
- Ko, Y.J.; Joo, Y.C.; Hyeon, J.E.; Lee, E.; Lee, M.E.; Seok, J.; Kim, S.W.; Park, C.; Han, S.O. Biosynthesis of organic photosensitizer Zn-porphyrin by diphtheria toxin repressor (DtxR)-mediated global upregulation of engineered heme biosynthesis pathway in Corynebacterium glutamicum. Sci. Rep. 2018, 8, 14460. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Du, G.; Chen, J. Recent Advances in the Microbial Synthesis of Hemoglobin. Trends Biotechnol. 2021, 39, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Richtová, J.; Sheiner, L.; Gruber, A.; Yang, S.-M.; Kořený, L.; Striepen, B.; Oborník, M. Using Diatom and Apicomplexan Models to Study the Heme Pathway of Chromera velia. Int. J. Mol. Sci. 2021, 22, 6495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Qian, X.; Chen, D.; Ge, M. Role of two 5-aminolevulinic acid biosynthetic pathways in heme and secondary metabolite biosynthesis in Amycolatopsis orientalis. J. Basic Microbiol. 2018, 58, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Hubner, I.; Shapiro, J.A.; Hossmann, J.; Drechsel, J.; Hacker, S.M.; Rather, P.N.; Pieper, D.H.; Wuest, W.M.; Sieber, S.A. Broad Spectrum Antibiotic Xanthocillin X Effectively Kills Acinetobacter baumannii via Dysregulation of Heme Biosynthesis. ACS Cent. Sci. 2021, 7, 488–498. [Google Scholar] [CrossRef]
- Steinbrink, J.M.; Myers, R.A.; Hua, K.; Johnson, M.D.; Seidelman, J.L.; Tsalik, E.L.; Henao, R.; Ginsburg, G.S.; Woods, C.W.; Alexander, B.D.; et al. The host transcriptional response to Candidemia is dominated by neutrophil activation and heme biosynthesis and supports novel diagnostic approaches. Genome Med. 2021, 13, 108. [Google Scholar] [CrossRef]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Su, T.; Guo, Q.; Zheng, Y.; Liang, Q.; Wang, Q.; Qi, Q. Fine-Tuning of hemB Using CRISPRi for Increasing 5-Aminolevulinic Acid Production in Escherichia coli. Front. Microbiol. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Fiege, K.; Frankenberg-Dinkel, N. Construction of a new T7 promoter compatible Escherichia coli Nissle 1917 strain for recombinant production of heme-dependent proteins. Microb. Cell Factories 2020, 19, 190. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Cheng, X.; Wang, J.; Wang, Q.; Qi, Q. A New Strategy for Production of 5-Aminolevulinic Acid in Recombinant Corynebacterium glutamicum with High Yield. Appl. Environ. Microbiol. 2016, 82, 2709–2717. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of delta-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Choi, K.R.; Lee, S.Y. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat. Catal. 2018, 1, 720–728. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, Z.; Zhang, J.; Zheng, H.; Jiang, X.; Gong, K.; Liang, Q.; Wang, Q.; Qi, Q. Stable and Efficient Biosynthesis of 5-Aminolevulinic Acid Using Plasmid-Free Escherichia coli. J. Agric. Food Chem. 2019, 67, 1478–1483. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Esterbauer, H.; Kozlov, A.V. Role of Heme Oxygenase as a Modulator of Heme-Mediated Pathways. Antioxidants 2019, 8, 475. [Google Scholar] [CrossRef]
- Testa, G.; Schaft, J.; van der Hoeven, F.; Glaser, S.; Anastassiadis, K.; Zhang, Y.; Hermann, T.; Stremmel, W.; Stewart, A.F. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 2004, 38, 151–158. [Google Scholar] [CrossRef]
- Rivero-Muller, A.; Lajic, S.; Huhtaniemi, I. Assisted large fragment insertion by Red/ET-recombination (ALFIRE)—An alternative and enhanced method for large fragment recombineering. Nucleic Acids Res. 2007, 35, e78. [Google Scholar] [CrossRef]
- Zhang, J.; Weng, H.; Zhou, Z.; Du, G.; Kang, Z. Engineering of multiple modular pathways for high-yield production of 5-aminolevulinic acid in Escherichia coli. Bioresour. Technol. 2019, 274, 353–360. [Google Scholar] [CrossRef]
- Mauzerall, D.; Granick, S. The Occurrence and Determination of δ-Aminolevulinic Acid and Porphobilinogen in Urine. J. Biol. Chem. 1956, 219, 435–446. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Takahashi, S.; Mochizuki, N.; Masuda, T. Evaluation of unbound free heme in plant cells by differential acetone extraction. Plant Cell Physiol. 2012, 53, 1344–1354. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).