Effects of Acidic Environments on Dental Structures after Bracket Debonding
Abstract
1. Introduction
2. Results
2.1. Shear Bond Strength
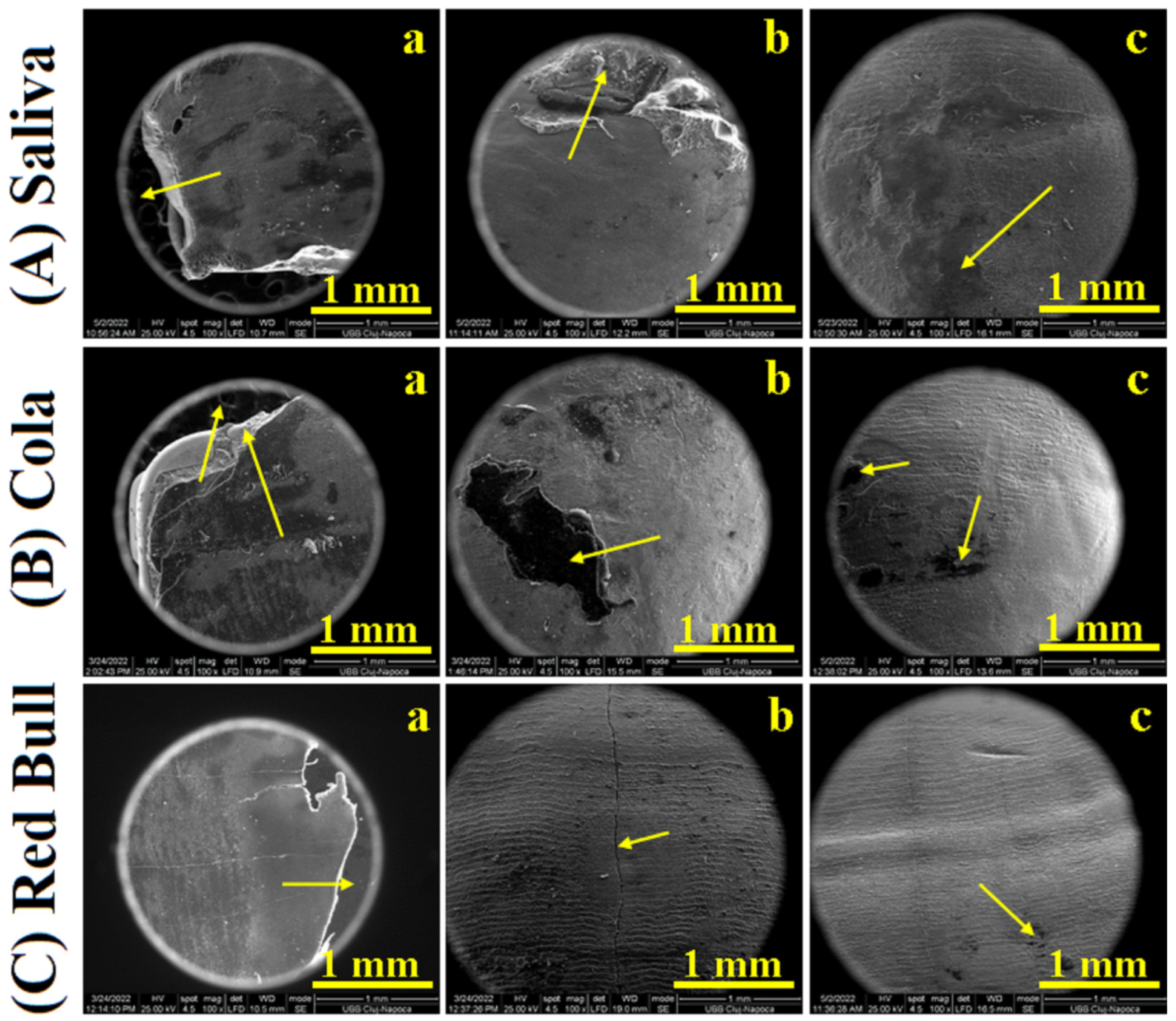
2.2. Scanning Electron Microscopy SEM
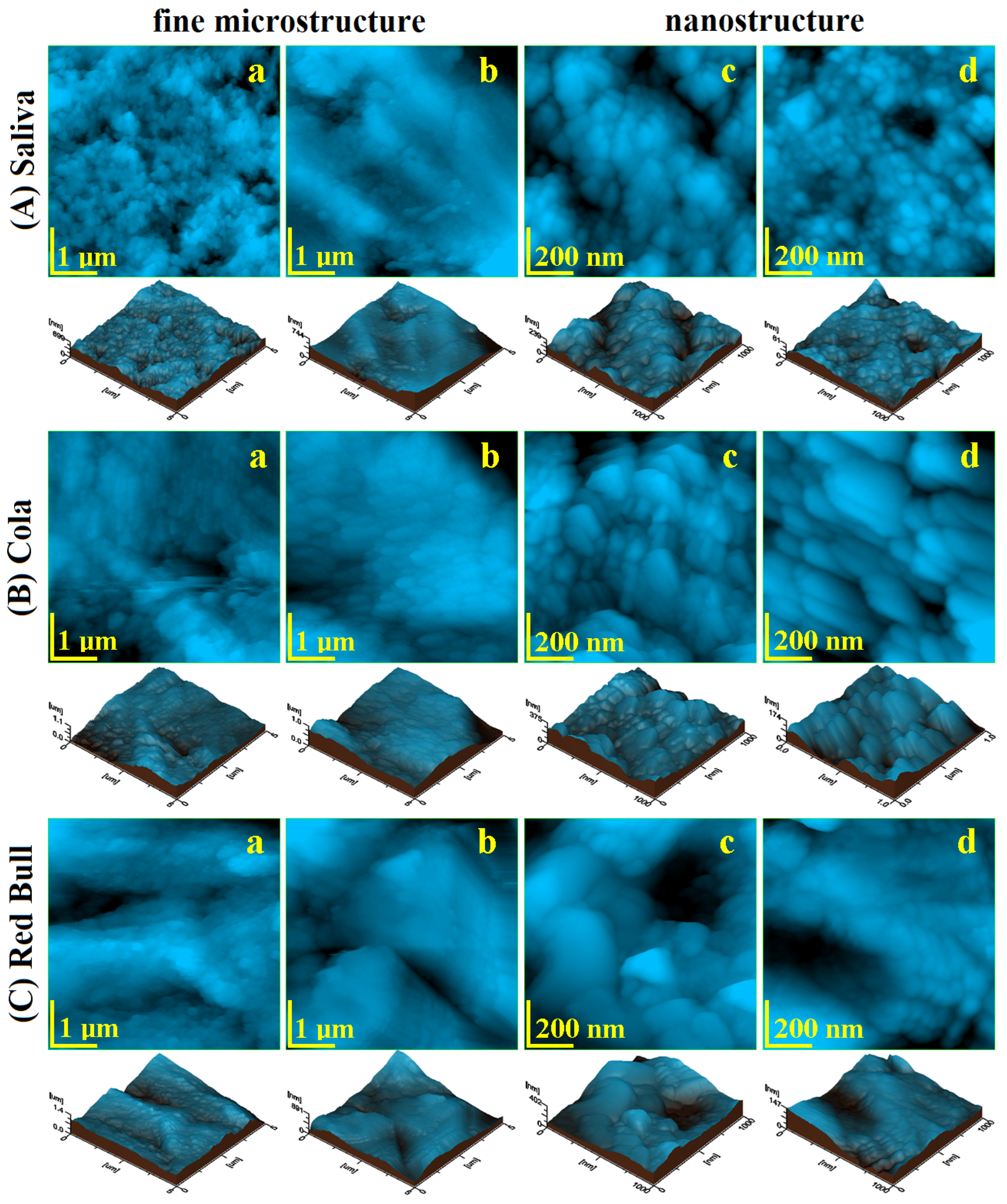
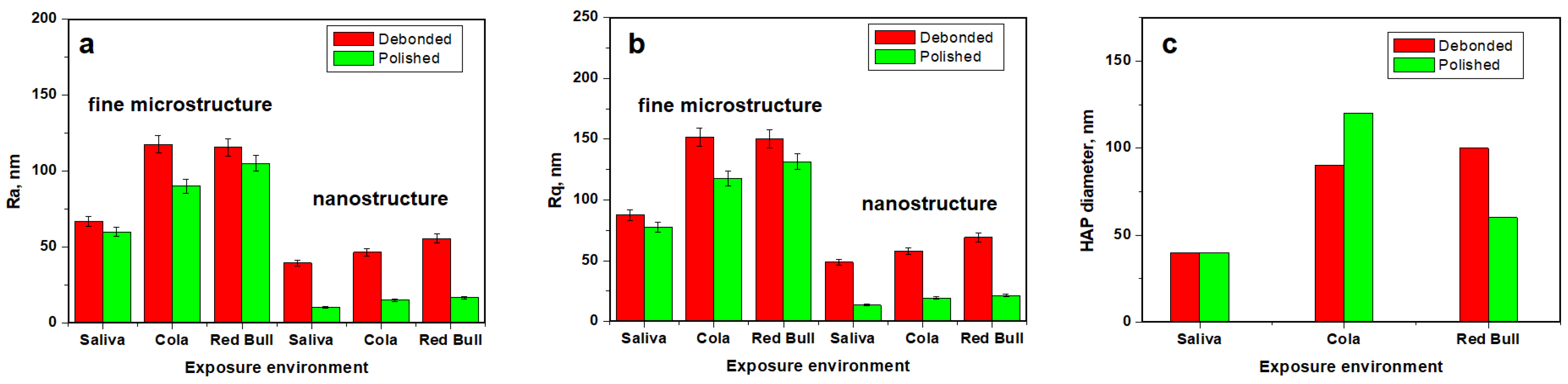
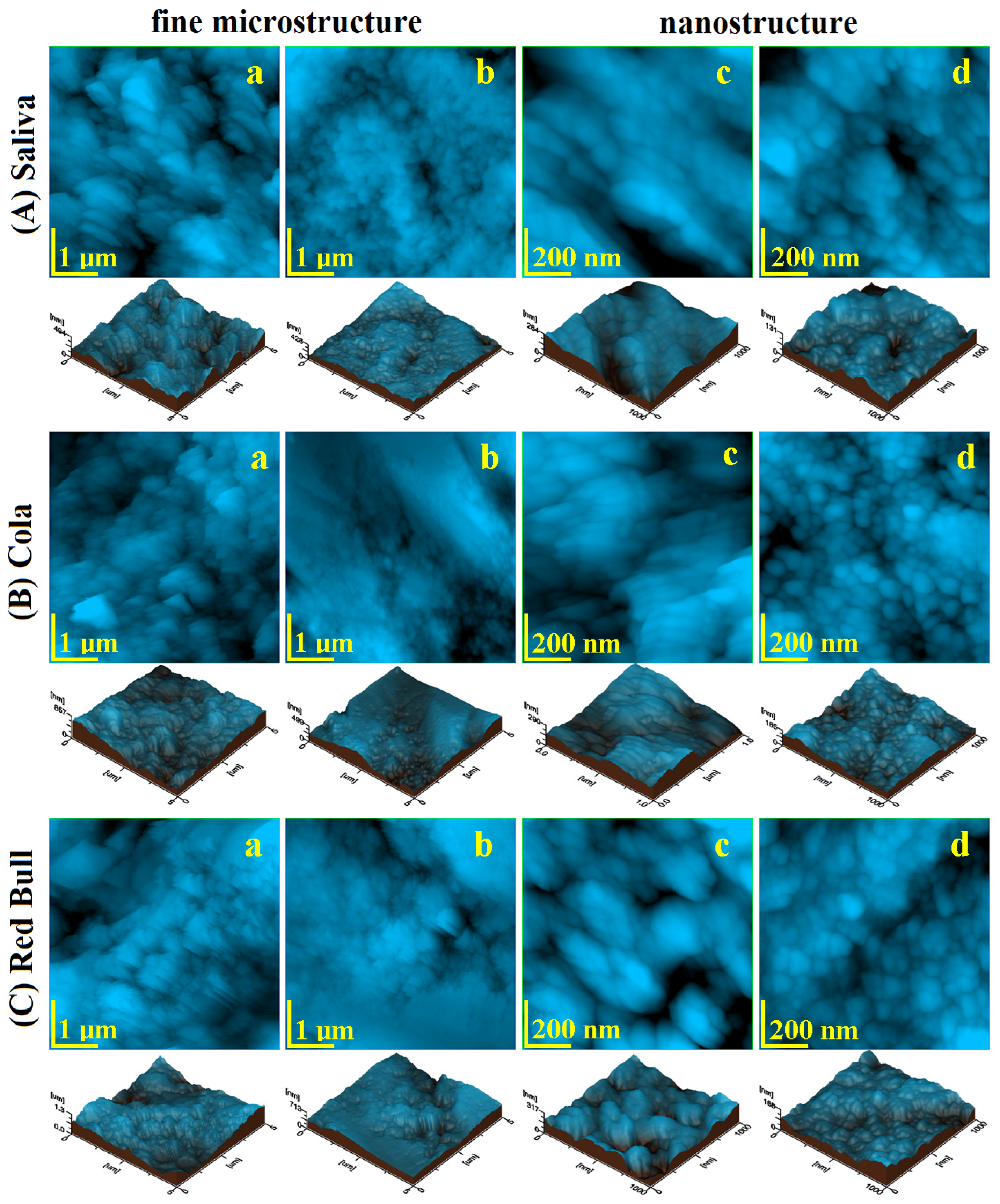
2.3. Atomic Force Microscopy AFM
3. Discussion
- -
- the first is within the bonding structure, in our case CR and RMGIC adhesive;
- -
- the second is on the enamel-adhesive interface;
- -
- and the third is on the bracket-adhesive interface.
4. Materials and Methods
4.1. Samples Preparation
- (1)
- 37% phosphoric acid was applied for 30 s, the acid was rinsed off and the enamel was dried.
- (2)
- The primer with the adhesive was applied with a sponge for 20 s, dry easily and light-cured for 20 s.
- (3)
- The cement resin was applied on the base of the bracket and the bracket was placed and pressed on the vestibular surface of the teeth. Removing the excess around the bracket could be difficult due to the fact that the color of tooth enamel and the cement are alike. That is why a colored orthodontic cements system has been created, which changes the color during polymerization as observed by Turkkahraman et al., 2010 [13]. After the excess was removed, it was light cured for 20 s, with 5 s light incidence over each marginal side of it.
- (1)
- Thirty teeth were etched using a 10% acid polyacrilic enamel conditioner. The conditioner was applied for 20 s and the teeth were rinsed and dried. We did not use phosphoric acid with RMGIC, because this type of acid will demineralize too profoundly.
- (2)
- The capsules containing the RMGIC (Fuji Ortho LC) were activated and triturated at 4000 rpm for 8 s. Capsules were placed in the GC Capsule Applier to place the adhesive on the base of the bracket
- (3)
- RMGIC was applied and the bracket was placed on buccal enamel and pressed firmly into place. The excess of adhesive material may increase the retention of dental plaque and will determine the increase in the incidence of gingivitis and caries according to Naranjo et al., 2006 [15]. The excess was removed with a sharp scaler and the bracket was light-cured for 20 s.
4.2. Shear Bond Strength Test
4.3. Scanning Electron Microscopy SEM
4.4. Atomic Force Microscopy AFM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vorachart, W.; Sombuntham, N.; Parakonthun, K. The effect of beer and milk tea on the shear bond strength of adhesive precoated brackets: An in vitro comparative study. Heliyon 2020, 8, 10260. [Google Scholar] [CrossRef] [PubMed]
- Mocuta, D.-E.; Grad, O.; Mateas, M.; Luca, R.; Carmen Todea, D. Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study. Medicina 2022, 58, 633. [Google Scholar]
- Bucur, S.M.; Bud, A.; Gligor, A.; Vlasa, A.; Cocoș, D.I.; Bud, E.S. Observational Study Regarding Two Bonding Systems and the Challenges of Their Use in Orthodontics: An In Vitro Evaluation. Appl. Sci. 2021, 11, 7091. [Google Scholar] [CrossRef]
- Chee, S.; Mangum, J.; Teeramongkolgul, T.; Tan, S.; Schneider, P. Clinician preferences for orthodontic bracket bonding materials: A quantitative analysis. Australas. Orthod. J. 2022, 38, 173–182. [Google Scholar] [CrossRef]
- Santos, C.N.; Souza Matos, F.; Mello Rode, S.; Cesar, P.F.; Nahsan, F.P.S.; Paranhos, L.R. Effect of two erosive protocols using acidic beverages on the shear bond strength of orthodontic brackets to bovine enamel. Dent. Press J. Orthod. 2018, 23, 1–5. [Google Scholar] [CrossRef]
- Simunovic Anicic, M.; Goracci, C.; Juloski, J.; Miletic, I.; Mestrovic, S. The Influence of Resin Infiltration Pretreatment on Orthodontic Bonding to Demineralized Human Enamel. Appl. Sci. 2020, 10, 3619. [Google Scholar] [CrossRef]
- Nica, I.; Stoleriu, S.; Iovan, A.; Tărăboanță, I.; Pancu, G.; Tofan, N.; Brânzan, R.; Andrian, S. Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges. Biomedicines 2022, 10, 1755. [Google Scholar] [CrossRef]
- Padmaja, S.; Ashma, V.; Ankit, A.; Sachin, A. A comparative evaluation of metallic brackets bonded with resin-modified glass ionomer cement under different enamel preparations: A pilot study. Contemp. Clin. Dent. 2013, 4, 1–7. [Google Scholar]
- Ghubaryi, A.A.; Ingle, N.; Basser, M.A. Surface treatment of RMGIC to composite resin using different photosensitizers and lasers: A bond assessment of closed Sandwich restoration. Photodiagnosis Photodyn. Ther. 2020, 32, 101965. [Google Scholar] [CrossRef]
- Hammad, S.M.; Enan, E.T. In vivo effects of two acidic soft drinks on shear bond strength of metal orthodontic brackets with and without resin infiltration treatment. Angle Orthod. 2013, 83, 648–652. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Turp, J.P. Influence of Coca-Cola on orthodontic material. Swiss Dent. J. SSO 2020, 130, 777–780. [Google Scholar]
- Türkkahraman, H.; Adanir, N.; Gungor, A.Y.; Alkis, H. In vitro evaluation of shear bond strengths of colour change adhesives. Eur. J. Orthod. 2010, 32, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Elnafar, A.A.S.; Alam, M.K.; Hasan, R. The impact of surface preparation on shear bond strength of metallic orthodontic brackets bonded with a resin-modified glass ionimer cement. J. Orthod. 2014, 41, 201–207. [Google Scholar] [CrossRef]
- Condò, R.; Mampieri, G.; Pasquantonio, G.; Giancotti, A.; Pirelli, P.; Cataldi, M.E.; La Rocca, S.; Leggeri, A.; Notargiacomo, A.; Maiolo, L.; et al. In Vitro Evaluation of Structural Factors Favouring Bacterial Adhesion on Orthodontic Adhesive Resins. Materials 2021, 14, 2485. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.-Y.; Oh, S.; Lim, B.-S.; Lee, H.-S.; Chung, S.H. Effect of Simplified Bonding on Shear Bond Strength between Ceramic Brackets and Dental Zirconia. Materials 2019, 12, 1640. [Google Scholar] [CrossRef]
- Panpan, L.; Chungik, O.; Hongjun, K.; Chen-Glasser, M.; Park, G.; Jetybayeva, A.; Yeom, J.; Kim, H.; Ryu, J.; Hong, S. Nanoscaleeffects of beverages on enamel surface of human teeth: An atomic force microscopy study. J. Mech. Behav. Biomed. Mater. 2020, 110, 103930. [Google Scholar]
- Torres-Gallegos, I.; Zavala-Alonso, V.; Patino-Marin, N.; Martinez-Castanon, G.A.; Anusavice, K.; Loyola-Rodriguez, J.P. Enamel roughness and depth profile after phosphoric acid etching of healthy and fluorotic enamel. Aust. Dent. J. 2012, 57, 1–6. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, L.; Xiao, H.; Zheng, J.; Zhou, Z. Wear mechanism of human tooth enamel: The role of interfacial protein bonding between HA crystals. J. Mech. Behav. Biomed. Mater. 2020, 110, 103845. [Google Scholar] [CrossRef]
- Agrawal, N.; Shashikiran, N.D.; Singla, S.; Ravi, K.S.; Kulkarni, V.K. Atomic force microscopic comparison of remineralization with casein-phosphopeptide amorphous calcium phosphate paste, acidulated phosphate fluoride gel and iron supplement in primary and permanent teeth: An in-vitro study. Contemp. Clin. Dent. 2014, 5, 75–80. [Google Scholar] [CrossRef]
- Urichianu, M.; Makowka, S.; Covell, D., Jr.; Warunek, S.; Al-Jewair, T. Shear Bond Strength and Bracket Base Morphology of New and Rebonded Orthodontic Ceramic Brackets. Materials 2022, 15, 1865. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Pascadopoli, M.; Gallo, S.; Ricaldone, F.; Kramp, D.D.; Valla, M.; Gandini, P.; Scribante, A. Effect of Enamel Pretreatment with Pastes Presenting Different Relative Dentin Abrasivity (RDA) Values on Orthodontic Bracket Bonding Efficacy of Microfilled Composite Resin: In Vitro Investigation and Randomized Clinical Trial. Materials 2022, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, A.-C.; Serbanoiu, D.-C.; Ghiga, D.-V.; Moldovan, M.; Cuc, S.; Pollmann, M.C.F.; Pacurar, M. Comparative Evaluation of Two Bracket Systems’ Kinetic Friction: Conventional and Self-Ligating. Materials 2022, 15, 4304. [Google Scholar] [CrossRef]
- Ga, Y.; Okamoto, Y.; Matsuya, S. The effects of treated time of acidulated phosphate fluoridesolutions on enamel erosion. Pediatr. Dent. J. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Cerci, B.B.; Roman, L.S.; Guariza-Filho, O.; Camargo, E.S.; Tanaka, O.M. Dental enamel roughness with different acid etching times: Atomic force microscopy study. Eur. J. Gen. Dent. 2012, 1, 187–191. [Google Scholar] [CrossRef]
- Pulgaonkar, R.; Chitra, P. Stereomicroscopic analysis of microleakage, evaluation of shear bond strengths and adhesive remnants beneath orthodontic brackets under cyclic exposure to commonly consumed commercial “soft” drinks. Indian J. Dent. Res. 2021, 32, 98–103. [Google Scholar]
- Zheng, B.-W.; Cao, S.; Ali-Somairi, M.A.; He, J.; Liu, Y. Effect of enamel-surface modifications on shear bond strength using different adhesive materials. BMC Oral Health 2022, 22, 224. [Google Scholar] [CrossRef]
- Ghoubril, V.; Ghoubril, J.; Khoury, E. A comparison between RMGIC and composite with acid-etch preparation or hypochlorite on the adhesion of a premolar metal bracket by testing SBS and ARI: In vitro study. Int. Orthod. 2020, 18, 127–136. [Google Scholar] [CrossRef]
- Farshidfar, N.; Agharokh, M.; Ferooz, M.; Bagheri, R. Microtensile bond strength of glass ionomer cements to a resin composite using universal bonding agents with and without acid etching. Heliyon 2022, 8, 08858. [Google Scholar] [CrossRef]
- Wiegand, A.; Lechte, C.; Kanzow, P. Adhesion to eroded enamel and dentin: Systematic review and meta-analysis. Dent. Mater. 2021, 37, 1845–1853. [Google Scholar] [CrossRef]
- Yang., H.; Hong, D.-W.; Attin, T.; Cheng, T.; Yu, H. Erosion of CAD/CAM restorative materials and human enamel: An in situ/in vivo study. J. Mech. Behav. Biomed. Mater. 2020, 110, 103903. [Google Scholar] [CrossRef]
- Sabău, E.; Bere, P.; Moldovan, M.; Petean, I.; Miron-Borzan, C. Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment. Polymers 2021, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Mazurek, A.; Siudem, P.; Kowalska, V.; Paradowska, K. Qualitative and quantitative analysis of energy drinks using 1H NMR and HPLC methods. J. Pharm. Biomed. Anal. 2022, 213, 114682. [Google Scholar] [CrossRef] [PubMed]
- Frias, F.J.L. The “big red bull” in the esports room: Anti-doping, esports, and energy drinks. Perform. Enhanc. Health 2022, 10, 100205. [Google Scholar] [CrossRef]
- Mokkarala, P.; Shekarabi, A.; Wiah, S.; Rawls, M. Energy drink produces aversive effects in planarians. Physiol. Behav. 2022, 225, 113933. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Meena, A. A comparative study of the effect of fillers and monomer on dental restorative material. Mater. Today Proc. 2021, 44, 5023–5027. [Google Scholar] [CrossRef]
- Caixeta, R.V.; Berger, S.B.; Lopes, M.B.; Paloco, E.A.C.; Faria-Junior, E.M.; Contreras, E.F.R.; Gonini-Junior, A.; Guiraldo, R.D. Evaluation of enamel roughness after the removal of brackets bonded with different materials: In vivo study. Braz. Dent. J. 2021, 32, 30–40. [Google Scholar] [CrossRef]
- Tisler, C.E.; Moldovan, M.; Petean, I.; Buduru, S.D.; Prodan, D.; Sarosi, C.; Leucuţa, D.-C.; Chifor, R.; Badea, M.E.; Ene, R. Human Enamel Fluorination Enhancement by Photodynamic Laser Treatment. Polymers 2022, 14, 2969. [Google Scholar] [CrossRef]
- Voina, C.; Delean, A.; Muresan, A.; Valeanu, M.; Mazilu Moldovan, A.; Popescu, V.; Petean, I.; Ene, R.; Moldovan, M.; Pandrea, S. Antimicrobial activity and the effect of green tea experimental gels on teeth surfaces. Coatings 2020, 10, 537. [Google Scholar] [CrossRef]
- Chisnoiu, A.M.; Moldovan, M.; Sarosi, C.; Chisnoiu, R.M.; Rotaru, D.I.; Delean, A.G.; Pastrav, O.; Muntean, A.; Petean, I.; Tudoran, L.B.; et al. Marginal adaptation assessment for two composite layering techniques using dye penetration, AFM, SEM and FTIR: An In-Vitro comparative study. Appl. Sci. 2021, 11, 5657. [Google Scholar]
- Chisnoiu, R.M.; Moldovan, M.; Prodan, D.; Chisnoiu, A.M.; Hrab, D.; Delean, A.G.; Muntean, A.; Rotaru, D.I.; Pastrav, O.; Pastrav, M. In-Vitro comparative adhesion evaluation of bioceramic and dual-cure resin endodontic sealers using SEM, AFM, Push-Out and FTIR. Appl. Sci. 2021, 11, 4454. [Google Scholar] [CrossRef]


| Mechanical Properties | Saliva | Coca-ColaTM | Red BullTM | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| CR | ||||||
| Bond Strength, MPa | 5.20774 | 1.96879 | 2.72076 | 0.77658 | 2.90572 | 1.49381 |
| Maximum Load, N | 67.5786 | 23.91672 | 32.96726 | 22.66505 | 35.92676 | 18.51022 |
| Breaking Load, N | 60.34059 | 31.61737 | 30.044 | 18.14057 | 31.888897 | 15.86262 |
| RMGIC | ||||||
| Bond Strength, MPa | 7.65976 | 2.3793 | 3.53586 | 1.10649 | 3.62436 | 2.38791 |
| Maximum Load, N | 86.92681 | 24.5682 | 41.42943 | 14.38154 | 53.40967 | 36.83688 |
| Breaking Load, N | 82.48911 | 26.11797 | 31.19834 | 10.23796 | 50.90918 | 38.59967 |
| CR + Saliva | CR + Cola | CR + Red Bull | RMGIC + Saliva | RMGIC + Cola | RMGIC + Red Bull | |
|---|---|---|---|---|---|---|
| Enamel fine microstructure | ||||||
| Ra, nm | 66.90 | 117.66 | 115.56 | 86.90 | 116.83 | 133.30 |
| P1 | 0.08 | 0.08 | 0.46 | 0.46 | ||
| Rq, nm | 87.63 | 151.66 | 150 | 105.50 | 145.66 | 170.30 |
| P2 | 0.06 | 0.06 | 0.36 | 0.36 | ||
| Enamel nanostructure | ||||||
| Ra, nm | 39.43 | 46.6 | 55.7 | 25.56 | 35.26 | 51.26 |
| P1 | 0.24 | 0.24 | 0.21 | 0.21 | ||
| Rg, nm | 48.63 | 57.83 | 69 | 36 | 42.53 | 63.83 |
| P2 | 0.24 | 0.24 | 0.25 | 0.25 | ||
| CR + Saliva | CR + Cola | CR + Red Bull | RMGIC + Saliva | RMGIC + Cola | RMGIC + Red Bull | |
|---|---|---|---|---|---|---|
| Enamel fine microstructure | ||||||
| Ra, nm | 66 | 90.03 | 105.13 | 69.53 | 75.2 | 133.43 |
| P1 | 0.40 | 0.40 | 0.24 | 0.24 | ||
| Rq, nm | 77.56 | 117.63 | 131.33 | 86.43 | 94.7 | 164.66 |
| P2 | 0.41 | 0.41 | 0.20 | 0.20 | ||
| Enamel nanostructure | ||||||
| Ra, nm | 10.4 | 15.16 | 16.70 | 14.26 | 18.23 | 21.23 |
| P1 | 0.22 | 0.22 | 0.29 | 0.29 | ||
| Rg, nm | 13.28 | 19.10 | 21.23 | 18.03 | 23.10 | 26.26 |
| P2 | 0.22 | 0.22 | 0.28 | 0.28 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosif, C.; Cuc, S.; Prodan, D.; Moldovan, M.; Petean, I.; Badea, M.E.; Sava, S.; Tonea, A.; Chifor, R. Effects of Acidic Environments on Dental Structures after Bracket Debonding. Int. J. Mol. Sci. 2022, 23, 15583. https://doi.org/10.3390/ijms232415583
Iosif C, Cuc S, Prodan D, Moldovan M, Petean I, Badea ME, Sava S, Tonea A, Chifor R. Effects of Acidic Environments on Dental Structures after Bracket Debonding. International Journal of Molecular Sciences. 2022; 23(24):15583. https://doi.org/10.3390/ijms232415583
Chicago/Turabian StyleIosif, Cristina, Stanca Cuc, Doina Prodan, Marioara Moldovan, Ioan Petean, Mîndra Eugenia Badea, Sorina Sava, Andrada Tonea, and Radu Chifor. 2022. "Effects of Acidic Environments on Dental Structures after Bracket Debonding" International Journal of Molecular Sciences 23, no. 24: 15583. https://doi.org/10.3390/ijms232415583
APA StyleIosif, C., Cuc, S., Prodan, D., Moldovan, M., Petean, I., Badea, M. E., Sava, S., Tonea, A., & Chifor, R. (2022). Effects of Acidic Environments on Dental Structures after Bracket Debonding. International Journal of Molecular Sciences, 23(24), 15583. https://doi.org/10.3390/ijms232415583









