Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
- Diabetes status during pregnancy and up to 12 months postpartum (pre-existing diabetes [type 1 or type 2], impaired glucose tolerance, or GDM; adequately defined)
- Metabolic indices (continuous measurements) related to maternal glucose/lipid metabolism (e.g., glucose measurements on oral glucose tolerance test; insulin secretion/sensitivity/resistance indices; beta-cell function) during pregnancy or up to 12 months postpartum
- Obesity/body mass index, gestational weight gain
- Postpartum weight change
- Polycystic ovary syndrome
- Lipid profile
- Birthweight (absolute/centiles, macrosomia), growth restriction or placental mass in relation to pregnancies affected by maternal GDM or pre-gestational diabetes.
2.4. Study Selection and Risk of Bias Assessment
2.5. Data Extraction and Synthesis
2.6. Evidence Synthesis and Statistical Analysis
3. Results
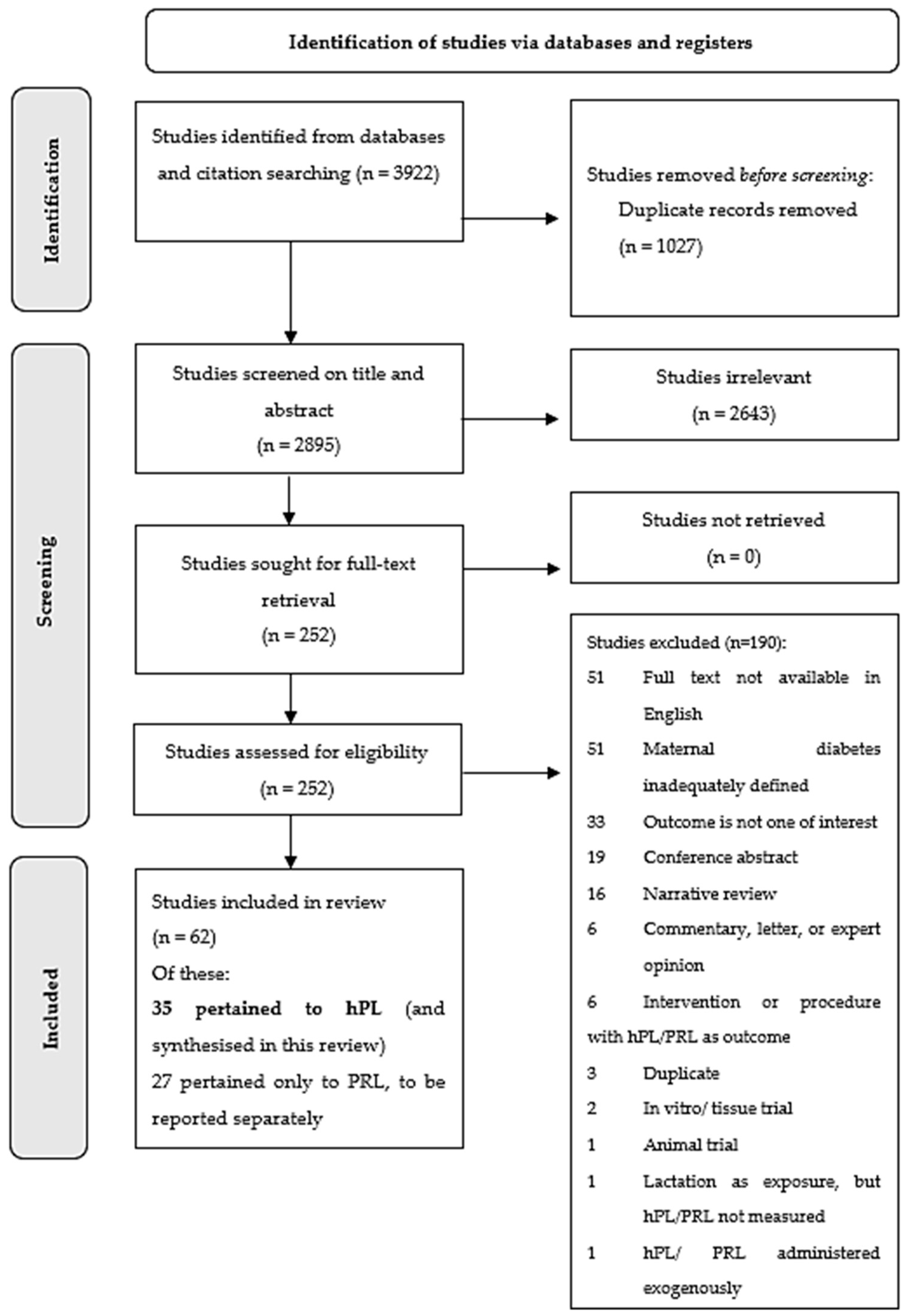
3.1. Study Selection and Characteristics
3.2. Risk of Bias of Included Studies
3.3. Synthesis of Results
3.3.1. Human Placental Lactogen in Pregnancies Affected by Pre-Gestational Metabolic Conditions
- (a)
- Differences in hPL between T1DM and control pregnancies
- (b)
- Relationship between hPL and glycaemic measures in T1DM
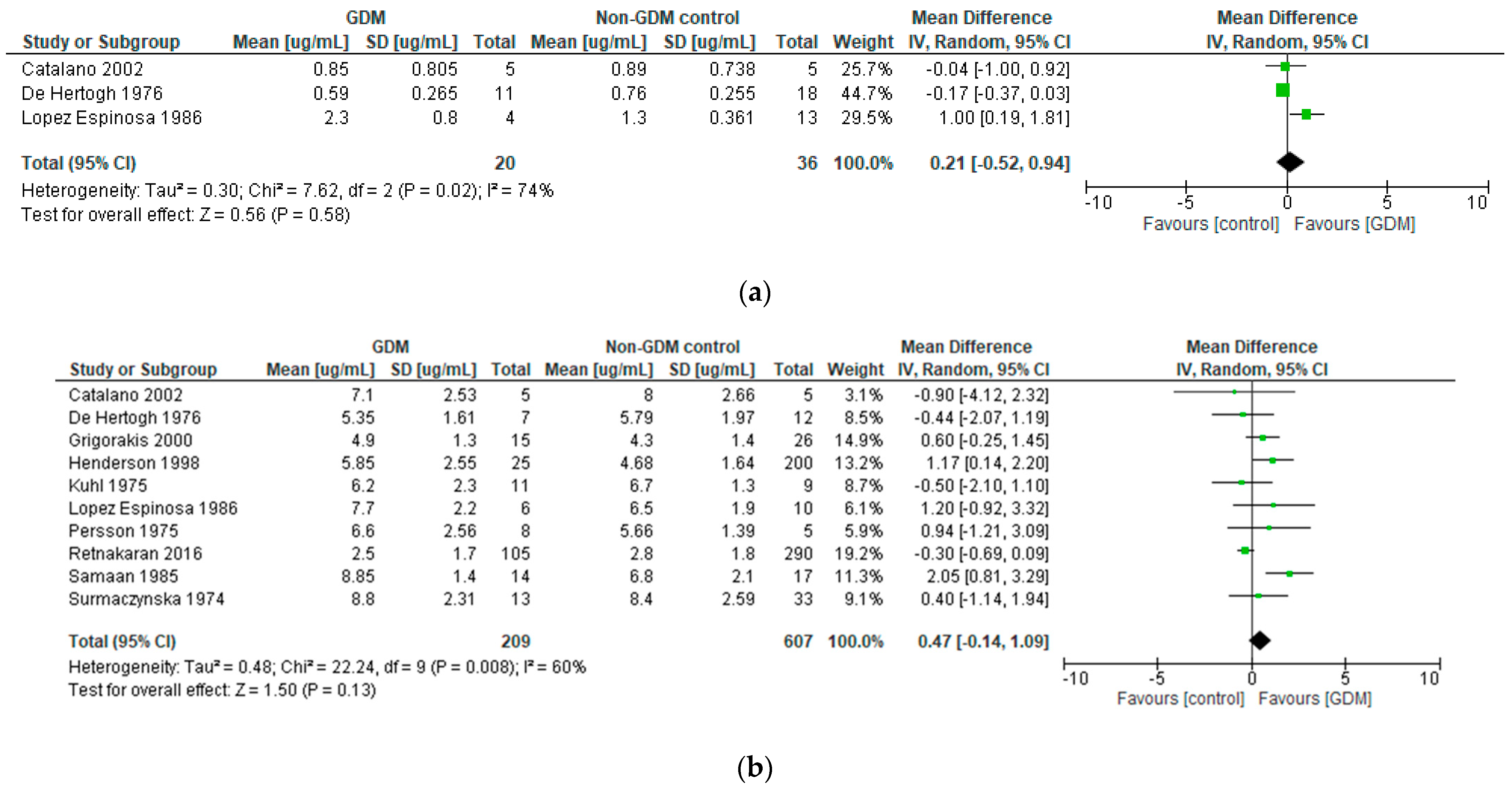
3.3.2. Human Placental Lactogen in Pregnancies Affected by Gestational Diabetes Mellitus
- (a)
- Differences in hPL between GDM and control pregnancies
- (b)
- Relationship between hPL and glycaemic measures in GDM
3.3.3. Human Placental Lactogen in Relation to Glycaemic or Insulin-Related Parameters in Healthy Pregnancies and Postpartum
3.3.4. Human Placental Lactogen in Relation to Body Mass Index or Gestational Weight Gain in Pregnancy
3.3.5. Human Placental Lactogen in Relation to Fetal, Neonatal or Placental Outcomes in Pregnancies Affected by Maternal Diabetes
4. Discussion
4.1. hPL in Pre-Gestational (Type 1) Diabetes Mellitus
4.2. hPL in Maternal Glycaemia and Gestational Diabetes Mellitus
4.3. hPL in Fetal Growth in Pregnancies Affected by Maternal Diabetes
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

| Author and Year; Country of Origin | Design | Participants and Sample Size | Methodology and hPL Pregnancy Timepoints | Metabolic Parameters Analysed | Results | Authors’ Conclusions | Risk of Bias Rating |
|---|---|---|---|---|---|---|---|
| Botta et al., 1984 [14] Italy | Longitudinal observational | n = 15 T1DM n = 10 controls | hPL sampled at: 12 weeks 16 weeks 20 weeks 24 weeks 28 weeks 32 weeks 36 weeks | T1DM status Blood glucose | Mean hPL sig lower in T1DM women than controls at 6 of the 7 timepoints (all other than 16 weeks). In T1DM women, at 5 of the 7 timepoints, sig inverse relationship between hPL and mean blood glucose that day. In T1DM women, at 4 of the 7 timepoints, sig inverse relationship between hPL and blood glucose at the time. | hPL lower in T1DM subjects than controls across preg Indication of inverse relationship between hPL and prevailing blood glucose levels in T1DM, suggesting that hPL may be influenced by hyperglycaemia, and that normalising control may normalise hPL. | Moderate |
| Braunstein et al., 1989 [15] USA | Longitudinal observational | n = 35 T1DM n = 31 controls | hPL sampled at: 5–6 weeks 7–8 weeks 9–10 weeks 12–13 weeks 20 weeks 27–29 weeks 35–37 weeks | T1DM status Blood glucose HbA1c | Mean hPL sig lower in T1DM women than controls at 9–10 weeks and 20 weeks (2 of 7 timepoints), at other timepoints no sig difference. Mean fasting glucose, mean 1 h post-prandial glucose and HbA1c at each gestation not related to hPL in T1DM women. | Finding of lower hPL in T1DM women at 2 timepoints likely due to chance–overall, ‘no consistent finding’ of differences between T1DM and control women. No relationship between glycaemia and hPL in T1DM at any timepoint. | Moderate |
| De Hertogh et al., 1976 [16] Belgium | Longitudinal observational | n = 21 T1DM n = 22 controls | hPL sampled at: 5–8 weeks 9–12 weeks 13–16 weeks 17–20 weeks 21–24 weeks 25–28 weeks 29–32 weeks 33–36 weeks 37–40 weeks | T1DM status | Mean hPL sig lower in T1DM women than controls at 13–16, 17–20 and 21–24 weeks. No sig differences at other timepoints, although three individual T1DM women had very high (outlying) hPL values in late preg. | hPL lower in T1DM subjects than controls in early preg, possibly reflecting delayed placental development. No significant differences later in preg, although levels very high in some T1DM individuals. | High |
| Gillmer et al., 1977 [21] UK | Cross-sectional | n = 11 T1DM n = 23 controls | hPL sampled every 1–2 h over one 24 h period in at 34–35 weeks | T1DM status Blood glucose OGTT glucose | Mean hPL over 24 h was higher in T1DM than controls, but did not reach sig (T1DM vs. controls 5.9 ± 1.7 vs. 5.1 ± 1.2 µg/mL, p-value NR). No sig correlation between mean hPL over 24 h and mean glucose over 24 h in any group (T1DM or controls). In control group, no sig alteration in hPL over course of OGTT (T1DM women did not have OGTT). | NS trend to higher hPL in third trimester in T1DM women than controls. hPL varied across day in all women, but no consistent relationship to meals/fasting, etc. No sig relationship between mean glucose and hPL in either group, and no hPL alteration with OGTT in controls. | Moderate |
| Larinkari et al., 1982 [19] Finland | Longitudinal observational | n = 57 T1DM (n = 42 with no DR, 7 with NPDR, 8 with PDR) n = 58 early preg controls n = 24 later preg controls | hPL sampled at: 7–13 weeks 14–19 weeks 20–25 weeks 26–31 weeks 32–37 weeks | T1DM status T1DM control | Mean hPL sig higher in T1DM than controls at 34–36 weeks (10.4 vs. 7.18 µg/mL, p < 0.001). Other time frames NR. T1DM patients with DR (n = 15) had higher hPL than T1DM patients without DR (n = 42) at both 14–19 weeks and 20–25 weeks (NS diff at other timepoints). This group had had worse glycaemic control in the first trimester. T1DM patients with DR in whom retinopathy progressed during preg (8 of 15, all with worse control and larger placental masses) all had hPL values at or above ULN after 28 weeks. | Higher hPL in late preg in T1DM subjects than controls. Within T1DM, patients with poor control and DR had higher hPL in second trimester than those without DR; and the subset with progressive DR had markedly elevated hPL values in the third trimester (in association with poor control and placentomegaly). | High |
| Lopez-Espinosa et al., 1986 [18] Scotland | Longitudinal observational | n = 15 T1DM n = 14 controls | For T1DM, fortnightly hPL samples 12–32 weeks and then weekly until delivery For controls, hPL monthly | T1DM status T1DM severity/complications T1DM duration Blood glucose Insulin requirements | Mean hPL in T1DM sig higher than controls in 2nd trimester (mean ± SEM 1.7 ± 0.1 vs. 1.3 ± 0.1 µg/mL, p < 0.01), NS diff in early 3rd trimester (6.4 ± 0.4 vs. 5.4 ± 0.5, NS), and sig higher again at 37–40 weeks (8.4 ± 0.3 vs. 6.5 ± 0.3, p < 0.01). No differences in hPL between T1DM subjects with differing T1DM severity/complications (White’s classes B, C and D). No relationship of hPL to duration of T1DM. No relationship of hPL to either plasma glucose levels or insulin requirements across preg in T1DM. | hPL appears higher in T1DM than control patients across late preg. No apparent relationship between hPL and T1DM plasma glucose, insulin requirements, disease duration or severity. | Moderate |
| Madsen et al., 1983 [23] Denmark | Cross-sectional | n = 42 T1DM n = 20 controls | One-off hPL sample at 30–36 weeks | T1DM status | Median hPL higher in T1DM than controls in 3rd trimester, 6.9 vs. 6 µg/mL; unclear if sig (p-value NR). | Median hPL value higher in T1DM than control women in third trimester, but unclear if sig. | High |
| Pedersen et al., 1998 [17] Denmark | Cross-sectional | n = 79 T1DM n = 93 controls | One-off hPL sample at 8–13 weeks | T1DM status T1DM severity/complications HbA1c | hPL value, as MoM for exact gestation, sig lower in T1DM than controls at 8–13 weeks: median difference 0.34, p < 0.00001 No differences in hPL between T1DM subjects with differing T1DM severity/complications (White’s classes B, C and D). No relationship of hPL to HbA1c in T1DM subjects. | hPL sig lower (for gestation) in T1DM than control preg in first trimester. Authors suggest that this may reflect delayed placental development/depressed trophoblast function in T1DM preg; and/or effect of hyperglycaemia on hPL secretion. No apparent relationship between hPL and T1DM severity/class, nor HbA1c. | Moderate |
| Persson et al., 1975 [22] Sweden | Cross-sectional | n = 7 T1DM n = 5 controls | Five samples of hPL over one 8 h period at 34–37 weeks | T1DM status Blood glucose FFAs/glycerol Ketones Insulin | Mean hPL over 8 h sampling period was not sig different between T1DM vs. controls. More variability in hPL noted in T1DM. hPL changes over the sampling period bore no relationship to changes in glucose, FFAs, ketones, or insulin over sampling period. | hPL in over 8 h in third trimester not sig different between T1DM and controls; although possibly more variable in T1DM. hPL not clearly related to insulin or glucose dynamics over an 8 h period in T1DM or controls. | High |
| Schmitz et al., 1985 [25] Denmark | Longitudinal observational | n = 6 T1DM | hPL sampled in early preg (~13 weeks) and late preg (~34 weeks) | T1DM insulin sensitivity (glucose disposal via clamp) | Increase in hPL from early to late preg, ΔhPL, calculated. ΔhPL inversely proportional to the degree of insulin sensitivity by late preg, ie those with larger ΔhPL were less insulin sensitive in late preg; r = −0.84, p < 0.04. | hPL increase inversely proportional to late preg insulin sensitivity in small T1DM clamp study cohort. Authors conclude that hPL seems to be a major factor causing impaired insulin action in late gestation. | Moderate |
| Spellacy et al., 1973 [24] USA | Longitudinal observational | n = 22 T1DM | Frequent hPL sampling from early first trimester to delivery | T1DM insulin requirements | Insulin requirement increase across pregnancy in T1DM was individually variable and not consistently related to hPL increase. | hPL rise across pregnancy showed no consistent relationship to rising insulin requirements in T1DM. Authors suggest that hPL may not be the key diabetogenic stress factor of pregnancy as previously postulated, rather increasing insulin resistance with gestation likely to be multifactorial/synergistic. | High |
| Stewart et al., 1989 [20] UK | Longitudinal observational | n = 40 T1DM n = 69 controls | Frequent hPL sampling between 6 and 38 weeks in T1DM subjects | T1DM status T1DM insulin requirements | hPL levels in T1DM subjects not sig different from those of controls across preg, including when T1DM sub-grouped according to study site and/or glycaemic control. Insulin requirement increase across pregnancy in T1DM not consistently related to hPL. | hPL NS different between T1DM and controls across gestation, and not related to increase in insulin requirements across gestation in T1DM. Authors state that hormonal response of T1DM women to pregnancy is not different to that of normal controls to any marked extent, and other factors likely explain their increasing insulin requirements and tendency to macrosomia. | Low |
| Author and Year; Country of Origin | Design | Participants and Sample Size | Methodology and hPL Pregnancy Timepoints | Metabolic Parameters Analysed | GDM Definition Used | Results | Authors’ Conclusions | Risk of Bias Rating |
|---|---|---|---|---|---|---|---|---|
| Al Busaidi et al., 2004 [27] Oman | Longitudinal observational | n = 200, of which n = 15 developed GDM | One-off hPL sample at 11–13 weeks | GDM status | NR | Mean 11–13 week hPL in women who developed GDM (n = 15) NS diff to that of women with other preg complications (eg. PIH, IUGR) and/or control women with no complications | Early (11–13 week) hPL NS different between GDM women and controls. Early hPL does not appear to be a useful biomarker for prediction of GDM risk. | High |
| Al-Hussein et al., 2021 [30] † Iraq | Cross-sectional | n = 40 GDM (20 male fetus, 20 female) n = 40 controls (20 male fetus, 20 female) | One-off hPL sample, exact timepoint NR (presume >24–28 weeks after OGTT) | GDM status Fasting glucose HOMA-IR | NR | hPL highest in GDM women pregnant with female fetus, then control with female fetus, then GDM with male fetus, then control with male fetus (all sig). hPL levels NS related to fasting glucose in all 4 groups. hPL levels inversely related to HOMA-IR in all 4 groups, but sig only in non-GDM women with female fetus (r = −0.790, p = 0.001). | hPL highest in GDM women carrying female infants (note groups not matched for BMI or other key baseline characteristics). No clear overall relationships between hPL and fasting glucose. Suggestion of inverse relationship between hPL and IR but sig only in one subgroup. | High |
| Catalano et al., 1993 [37] USA | Cross-sectional | n = 38 women with abnormal screening GCT | OGTT performed twice, 1 week apart, at 27–30 weeks; hPL sampled alongside | GDM status | NDDG, but deemed abnormal if only one value exceeded thresholds | Study focused on how gestational hormones may influence OGTT reproducibility. hPL NS diff at either first or second OGTT in either women with ‘definite’ non-GDM (2 normal results), ‘definite’ GDM (2 abnormal results) or those with discordant results (one normal and one abnormal result). | hPL does not appear to be a hormonal factor influencing OGTT reproducibility. | Low |
| Catalano et al., 2002 [26] USA | Longitudinal observational | n = 5 obese GDM n = 4 obese controls | hPL sampled at: 12–14 weeks 34–36 weeks | GDM status | Carpenter-Coustan | hPL NS diff between GDM women and controls: in both early preg (mean ±SEM of GDM vs. controls 0.85 ± 0.36 vs. 0.89 ± 0.33 µg/mL) and late preg (GDM vs. controls 7.1 ± 1.13 vs. 8.00 ± 1.19 µg/mL), p = 0.3 for both. | hPL NS diff between GDM women and control women in either early or late preg. | High |
| De Hertogh et al., 1976 [16] Belgium | Longitudinal observational | n = 19 GDM n = 22 controls | hPL sampled at: 5–8 weeks 9–12 weeks 13–16 weeks 17–20 weeks 21–24 weeks 25–28 weeks 29–32 weeks 33–36 weeks 37–40 weeks | GDM status | 100 g OGTT. 0, 30, 60, 120, 180 min; thresholds 5.0/8.9/8.3/6.7/5.5 mmol/L. ≥2 high for dx | Mean hPL sig lower in GDM than control group at 17–20 weeks only. At all other timepoints, hPL NS diff between GDM and controls. | hPL not consistently sig diff between GDM and control women in serial preg sampling. | High |
| Grigorakis et al., 2000 [31] Greece | Cross-sectional | n = 15 GDM n = 26 controls | One-off hPL sample at 28–32 weeks at time of OGTT | GDM status | ADA | Mean hPL NS diff between GDM and controls (GDM 4.9 ± 1.3 vs. controls 4.3 ± 1.4 µg/mL). | hPL NS diff between GDM and controls in late preg. Any contribution of hPL to GDM pathophysiology ‘likely to be weak’. | Moderate |
| Henderson et al., 1998 [34] USA | Cross-sectional | n = 257 women, of whom n = 57 had abnormal screening GCT; and n = 25 then had abnormal OGTT (i.e., GDM) | One-off hPL sample at time of GCT (exact timepoint NR but presume early 3rd trimester) | GDM status | Carpenter-Coustan | Of women who had an abnormal GCT, hPL sig higher in women who proceeded to abnormal OGTT than in those who had a subsequent normal OGTT; 5.85 ± 2.55 µg/mL vs. 3.38 ± 1.40, p = 0.034. NS diff in hPL between women with normal GCT (4.68 ± 1.64 µg/mL), and those who had abnormal GCT but ultimately went on to have normal OGTT. n = 11 women had normal GCT but subsequently delivered an infant > 4 kg, in them mean hPL at time of GCT had been similar to the GDM group (5.83 ± 1.29 µg/mL). | hPL in appeared a helpful adjunct to GCT, and seemed to help with predicting those who would go on to have positive OGTT. hPL in normoglycaemic women who proceeded to deliver macrosomic infants had retrospectively been similar to that of GDM women–potential for hPL as a risk predictor for macrosomia, or of GDM ‘missed’ by OGTT? | Moderate |
| Kirwan et al., 2002 [39] USA | Longitudinal observational | n = 5 obese GDM n = 5 lean controls n = 5 obese controls | hPL sampled at: pre-conception 10–12 weeks 34–36 weeks | Insulin sensitivity | Carpenter-Coustan | Insulin sensitivity measured via clamp in early and late preg. Late preg insulin sensitivity found to be NS related to hPL levels (r = −0.24, p = 0.39). | hPL levels in late preg did not appear to be sig related to the degree of preg-induced insulin resistance in GDM or non-GDM women (main sig findings of the trial related to TNF-α, which did emerge as sig related to insulin resistance). | Moderate |
| Kuhl et al., 1975 [36] Denmark | Cross-sectional | n = 11 GDM n = 9 controls | One-off hPL sample at 34–35 weeks at time of OGTT | GDM status OGTT glucose | At least 2 values on OGTT that were >3 SD above mean of authors’ previous normal preg population | Mean fasting hPL NS diff in GDM vs. controls (6.2 ± 2.3 vs. 6.7 ± 1.3 µg/mL). Mean hPL at 3 h of OGTT NS diff in GDM vs. controls (6.1 ± 2.6 vs. 6.2 ± 1.1 µg/mL). No sig alteration in hPL over course of OGTT in either group. No difference in shape of hPL curves alongside OGTT in GDM vs. controls. | hPL NS diff between GDM and controls. Previous literature suggestive of high late preg hPL values in T1DM patients may not apply to mild GDM. hPL does not appear to be sig altered by minor physiological fluctuations in plasma glucose, such as with OGTT. | Moderate |
| Lopez-Espinoza et al., 1986 [18] Scotland | Longitudinal observational | n = 8 early GDM on insulin n = 14 controls | For GDM, hPL sampled fortnightly 12–32 wk, then weekly until delivery. For controls, hPL sampled monthly | GDM status Plasma glucose Insulin requirements | WHO 1980 | Mean hPL higher in GDM than controls in 2nd trimester (mean ±SEM 2.3 ± 0.4 vs. 1.3 ± 0.1 µg/mL, p < 0.05). In early and late third trimester, hPL also showed a trend to being higher in GDM than controls (early 3rd trimester 6.3 ± 0.7 vs. 5.4 ± 0.5; late 7.7 ± 0.9 vs. 6.5 ± 0.6) but did not achieve sig. hPL not related to plasma glucose or insulin requirements in GDM. | Suggestion of higher hPL levels in GDM than controls across gestation in this study, although sig only in second trimester. No apparent relationship between hPL and either plasma glucose or insulin requirements in GDM. | Moderate |
| Luthman et al., 1994 [38] Sweden | Cross-sectional | n = 12 GDM n = 12 controls | hPL sampling across standard breakfast (0 to 120 min), at 29–38 weeks | GDM status Plasma glucose | WHO 1980 | hPL NS diff between GDM and control women at all timepoints. hPL levels NS altered by glucose excursions after standard meal (in either group). | hPL unaltered by meal ingestion, and no different between GDM and control women, in third trimester. | Moderate |
| Ngala et al., 2017 [29] † Ghana | Longitudinal observational | n = 200 preg women in 1st trimester: n = 50 ‘low risk’ for diabetes and n = 150 ‘standard risk’. n = 12 later developed GDM | One-off hPL sample at 24–28 weeks | GDM status GDM risk Fasting glucose, insulin, IR, BMI, total cholesterol, Tg | ADA | When n = 12 GDM compared to all n = 138 non-GDM, NS diff between hPL levels at 24–28 weeks (p = 0.155). When n = 12 GDM compared to subgroup of n = 50 women deemed ‘low risk of diabetes’, hPL sig lower in the GDM women (p < 0.0001). In multiple logistic regression, hPL at 24–28 weeks did not emerge as a sig predictor of GDM risk. All metabolic parameters NS related to 24–28 week hPL in either GDM or non-GDM women. | hPL NS different between GDM and non-GDM women at 24–28 weeks, and did not emerge as a sig predictor of GDM risk. hPL at 24–28 weeks not linked to other metabolic parameters including BMI, lipids, fasting glucose or insulin. | High |
| Persson et al., 1975 [22] Sweden | Cross-sectional | n = 8 GDM n = 5 controls | Five measurements of hPL over one 8 h period at 34–37 weeks | GDM status Blood glucose FFAs, glycerol Ketones Insulin | IVGTT in third trimester in at-risk women, own criteria | Mean hPL over the 8 h sampling period was NS diff between GDM vs. control women. hPL changes over the sampling period bore no apparent relationship to changes in glucose, FFAs, ketones, or insulin over the sampling period. | hPL in over 8 h in third trimester NS diff between GDM and controls. hPL not clearly related to insulin or glucose dynamics over an 8 h period in GDM or controls. | High |
| Rasanen et al., 2013 [28] Finland | Case-control | n = 90 GDM n = 92 controls | GDM cases and controls identified in late preg. hPL at 5–13 weeks then compared between cases and controls | GDM status GDM risk | ADA, but deemed abnormal if only one value exceeded thresholds | Median hPL in first trimester sig higher in women who would go on to get GDM than in controls (GDM vs. controls 0.34 vs. 0.22 µg/mL, p < 0.001). AUC on ROC curve for GDM prediction with threshold 0.80 ng/mL: sensitivity 28%, specificity 90%, AUC 0.63 (0.55–0.71, p < 0.001), i.e., only very marginal classification benefit. | First trimester hPL was sig diff between women who would go on to get GDM and those who would not (i.e., sig association with GDM risk, higher hPL in GDM than controls). However, degree of separation between distributions of hPL in cases and controls was not adequate for use as screening test (low AUC; poor classification performance). | Low |
| Retnakaran et al., 2016 [32] Canada | Cross-sectional | n = 105 GDM n = 290 controls | One-off hPL sample at 29–30 weeks, at time of OGTT | GDM status AUC glucose, Matsuda index, HOMA-IR, fasting insulin, ISSI-2, and IGI/HOMA-IR | NDDG | Median hPL NS diff between GDM women and controls (2.0 vs. 1.9µg/mL, p = 0.1). No variable showed a sig association with hPL in either GDM or control women, before or after adjustment for key covariates. | hPL NS diff between GDM and non-GDM women at time of OGTT. hPL NS associated with AUC glucose in either GDM or non-GDM women. hPL NS associated with other markers of insulin sensitivity or beta cell function in either GDM or non-GDM women. Data suggests that circulating hPL concentrations may not provide direct insights on maternal glucose homeostasis. | Moderate |
| Samaan et al., 1985 [35] USA | Cross-sectional | n = 14 GDM n = 17 controls | One-off hPL sample at delivery | GDM status | NR | Mean hPL at time of delivery sig higher in GDM than controls (8.85 ± 1.4 vs. 6.8 ± 2.1 µg/mL, p < 0.001). | hPL at time of delivery sig higher in diet-controlled GDM women than in controls. | High |
| Surmaczynska et al., 1974 [33] USA | Cross-sectional | n = 13 GDM n = 33 controls | One off hPL sample at 30–40 weeks, at time of OGTT | GDM status OGTT | O’Sullivan and Mahan | Mean baseline hPL NS diff between GDM and controls (mean ±SEM 8.8 ± 0.64 vs. 8.4 ± 0.45 µg/mL). Very marginal hPL decrement with 100 g OGTT seen in overall cohort; magnitude NS diff between GDM and controls. | hPL at 30–40 weeks NS diff between GDM and non-GDM women. hPL dipped slightly with OGTT in cohort overall, magnitude no diff between GDM and non-GDM women. | Moderate |
| Author and Year; Country of Origin | Design | Participants and Sample Size | Methodology and hPL Pregnancy timepoints | Metabolic Parameters Analysed | Results | Authors’ Conclusions | Risk of Bias Rating |
|---|---|---|---|---|---|---|---|
| Benny et al., 1980 [40] UK | Cross-sectional | n = 21 women with normal OGTT in preg, none obese (n = 10 Hindi vegetarians, n = 11 Caucasian omnivores) | Eleven serial measures of hPL over one 24 h period at 36–39 weeks | Insulin Glucose | Insulin and glucose sampled serially across 24 h period, as was hPL. hPL peak (at 0500h after overnight fast) coincided with nadir of insulin and glucose. No direct correlation of individuals’ hPL levels with insulin or glucose. However, Hindi women were found to have sig higher mean glucose than Caucasian women. This was unlikely to be mediated by hPL because hPL was sig lower in Hindi than Caucasian women (5.79 ± 0.05 vs. 6.11 ± 0.05 µg/mL; p < 0.01). Lower hPL in the Hindi women likely related to sig lower placental masses. | hPL appeared to peak after overnight fast in pregnancy, temporally coinciding with time of lowest glucose and insulin. Hindi women had higher mean glucose levels in third trimester than Caucasians, but lower hPL levels (maybe related to smaller placentas)–so hPL unlikely to be driving glycaemic differences. | Moderate |
| Enzi et al., 1980 [41] Italy | Longitudinal observational | n = 50 healthy preg women | One-off hPL sample at 34–35 weeks | AUC glucose AUC insulin | hPL at 34/40 positively related to maternal AUC glucose at 34/40, r = 0.62, p < 0.001. hPL at 34/40 positively related to maternal AUC insulin at 34/40, r = 0.31, p < 0.05 | hPL at 34 weeks positively related to maternal AUC insulin and AUC glucose, suggesting diabetogenic effects. | Low |
| Fairweather et al., 1971 [42] UK | Longitudinal observational | n = 33 healthy preg women | hPL sampling: 6–12 weeks 13–19 weeks 20–25 weeks 26–30 weeks 31–32 weeks 33–34 weeks 35–36 weeks 37–38 weeks 39–40 weeks 41–42 weeks | Glucose NEFAs | NS direct relationship between glucose and hPL levels at a given time in a given patient. Positive relationship between hPL and NEFA levels, r = 0.24, p < 0.01; i.e., higher hPL levels at at a given time in a given patient tended to be assoc with higher NEFA levels. | hPL showed NS relationship to glucose levels within a patient at any given time, but higher hPL levels tended to be associated with higher levels of NEFAs. hPL appears to have anti-insulin, diabetogenic effects that promote mobilisation of FFAs and reduce maternal glucose utilisation, sparing glucose to meet fetal demands. | Moderate |
| Retnakaran et al., 2016 [44] Canada | Longitudinal observational | n = 301 NGT n = 60 pre-diabetes n = 6 DM (based on OGTT at 3 months postpartum) | hPL sampled at time of OGTT in late second trimester of preg, but then analysed in relation to postpartum metabolic status | Maternal diabetes category at 3 mo postpartum Glycaemic markers at 3 mo postpartum Risk of pre-DM or DM at 3 mo postpartum | hPL in late preg had been no diff between who went on to be NGT at 3 mo postpartum, those with pre-diabetes at 3 mo postpartum, and those with DM at 3 mo postpartum (median hPL in µg/mL = NGT 2.0 vs. pre-DM 2.0 vs. DM 1.5, p = 0.312). On multivariate regression, hPL in late preg not independently related to any glycaemic markers (log Matsuda index, log HOMA-IR, log ISSI-2, log IGI/HOMA-IR, fasting glucose, AUC glucose) at 3 mo post partum. On multivariate regression, hPL in late preg not an independent predictor of the risk of persistent dysglycaemia at 3 mo postpartum. | hPL in late preg had been no diff between those with normal glucose tolerance at 3 mo postpartum and those with postpartum pre-DM or DM. hPL in late preg was not an independent determinant of insulin resistance or beta-cell function at 3 mo postpartum. hPL in late preg was not an independent predictor of the risk of pre-DM or DM at 3 mo postpartum. | Moderate |
| Scott et al., 1992 [43] UK | Cross-sectional | n = 127 healthy preg women (n = 97 European, n = 30 Asian) | One-off hPL sample at 29 weeks, at time of OGTT | 2 h insulin on OGTT 2 h glucose on OGTT | No relationship to hPL in either ethnic group. No relationship to hPL in either ethnic group. | hPL not related to either 2 h OGTT insulin or 2 h OGTT glucose in either race in this study. hPL does not clearly play a role in modifying insulin action. | Low |
| Author and Year; Country of Origin | Design | Participants and Sample Size | Methodology | Metabolic Parameters Analysed | Results | Authors’ Conclusions | Risk of Bias Rating |
|---|---|---|---|---|---|---|---|
| Al-Hussein et al., 2021 [30] † Iraq | Cross-sectional | n = 40 GDM (20 male fetus, 20 female) n = 40 controls (20 male fetus, 20 female) | One-off hPL sampling, presumably > 24–28 weeks after OGTT | Maternal BMI | Maternal BMI NS rel to maternal hPL level in any group. | No sig relationship between maternal BMI and hPL demonstrated in any study subgroup. | High |
| Enzi et al., 1980 [41] Italy | Longitudinal observational | n = 50 healthy preg women | One-off hPL sample at 34–35 weeks | Maternal GWG | Maternal hPL at 34 weeks NS diff between mothers in excessive GWG group (gained >20% IBW, mean 16.5 ± 1.4 kg, n = 23) and those in normal GWG group (gained <20% IBW, mean 8.7 ± 0.5 kg, n = 27). Excessive GWG mean hPL 7.7 ± 1.5 µg/mL vs. normal GWG 6.3 ± 1.1 µg/mL. | hPL at 34 weeks NS diff between mothers who had normal GWG and those with excessive GWG. | Low |
| Lin et al., 1976 [45] USA | Cross-sectional | n = 187 healthy preg women | One-off hPL sample near term (within one week of delivery) | Maternal weight | Maternal weight sig inversely related to maternal hPL at term (r = −0.28, p <0.01). | Maternal weight at term sig inversely related to hPL concentration at term. Authors suggest this might be dilutional effect (?more tissue space in larger women). | Low |
| McCarrick et al., 1979 [46] USA | Longitudinal observational | n = 290 preg women with preg risk factors (eg prev or current GDM, PET, previous losses, IUGR) | Serial hPL sampling across third trimester 33–40 weeks (approx. 4–5 per woman) | Weight category | Obese women (>72.6 kg at 16 weeks) were over-represented in “group 3” of the study, n = 44, all of whom had normal estrogen but low hPL (50% women in that group obese vs. 25% obese in other groups, p < 0.001). These women were at an increased risk of preg complications such as fetal death or SGA (34.1% had complications) compared to those with normal hPL and estrogen (group 1, of whom 4.7% had complications) although not as high as those with both low hPL and low estrogen (group 2, of whom 71.4% had complications.) | Obese women sig proportionally over-represented in the group of women with low hPL but normal estrogen in the study. Authors conclusion was that obesity may impact on hPL regulation and activity. Note that direct impact of the complications themselves on hPL levels was not considered. | High |
| Author and Year; Country of Origin | Design | Participants and Sample Size | Methodology | Fetal or Placental Outcomes | Results | Authors’ Conclusions | Risk of Bias Rating |
|---|---|---|---|---|---|---|---|
| Botta et al., 1984 [14] Italy | Longitudinal observational | n = 15 T1DM n = 10 controls | Serial hPL sampling across preg | Placental weight Birthweight | Placental weight positively correlated to week 36 hPL (r = 0.368) across whole cohort, although not sig (p-value NR). Birthweight positively correlated to week 36 hPL (r = 0.319) across whole cohort, although NS (p-value NR). | Late pregnancy hPL positively related to both placental mass and birthweight across combined cohort, although short of sig. | Moderate |
| Lopez-Espinoza et al., 1986 [18] Scotland | Longitudinal observational | n = 15 T1DM n = 8 GDM n = 14 controls | Serial hPL sampling across preg | Placental weight | Positively related to pre-delivery (>37 week) hPL in T1DM (r = 0.8, p < 0.01). Positively related to third trimester (<37 week) hPL in GDM (r = 0.6, p < 0.05). Positively related to pre-delivery (>37 week) hPL in controls (r = 0.6, p < 0.05). | Late preg hPL levels strongly positively correlated with placental weight in control, T1DM and GDM women. | Moderate |
| Luthman et al., 1994 [38] Sweden | Cross-sectional | n = 12 GDM n = 12 controls | One-off hPL sample at 29–38 weeks | Birthweight | Positively correlated to third trimester hPL in GDM cohort (r = 0.59, p < 0.05). No such relationship found in controls or in overall cohort. | Late pregnancy hPL positively correlated with birthweight in the GDM cohort. | Moderate |
| Pedersen et al., 1986 [48] Denmark | Cross-sectional | n = 26 T1DM | One-off hPL sample at 7–16 weeks | Early fetal growth | √hPL in early pregnancy related to menstrual age corrected by 90% of the growth delay (growth delay = diff in days between menstrual age and USS CRL age). √hPL (mg/l) = −0.541 + 0.0142 menstrual age (days) −0.0128 delay (days). | Authors had previously noted that size of T1DM pregnancies (by CRL on USS) may lag by several days behind the age calculated from LMP. Here, hPL could be best mathematically related to menstrual age when it was corrected by this delay. Given hPL reflects functional placental mass, this suggests that the observed growth delay in early T1DM pregnancies is accompanied by a delay in placental development. | High |
| Samaan et al., 1985 [35] USA | Cross-sectional | n = 14 GDM n = 17 controls | One-off hPL sample at time of delivery | Birthweight | Across whole cohort (GDM women, controls; and a third group of women with preterm birth, n = 15); NS correlation between maternal hPL and neonatal weight. Not described for GDM cohort individually. | NS relationship between hPL at time of delivery and birthweight across combined cohort of GDM, preterm birth and control women. | High |
| Small et al., 1987 [47] Scotland | Longitudinal observational | n = 20 T1DM with macrosomia (birthweight >90% for gestation) n = 20 matched T1DM without macrosomia | One-off hPL sample at 34 weeks | Birthweight class | T1DM group with macrosomia (mean birthweight 3.96 kg at 37 weeks) had sig higher hPL at 34 weeks than matched T1DM preg without macrosomia (mean birthweight 3.05 kg at 37 weeks). Macrosomia group mean hPL = 8.3 ± 2.3 µg/mL vs. non-macrosomia group mean hPL = 6.5 ± 2.3 µg/mL; p < 0.005. | hPL at 34 weeks was sig higher in n = 20 T1DM women who gave birth to macrosomic infants than in n = 20 T1DM who gave birth to normal weight infants. Authors suggested that hPL may help with detection of macrosomia early in the third trimester. | Low |
| Stewart et al., 1989 [20] UK | Longitudinal observational | n = 40 T1DM n = 69 controls | Serial hPL sampling across preg | Placental weight Birthweight | hPL at 32 or 36 weeks NS related to placental weight in T1DM group as a whole, using sig value of p < 0.01. hPL at 32 or 36 weeks NS related to birthweight in T1DM group as a whole, using sig value of p < 0.01. However, was a positive correlation between birthweight and 32 week hPL if p < 0.02 accepted (r = 0.48, p < 0.02). Both birth and placental weight corrected for maternal parity, maternal stature, infant sex and length of gestation. | NS relationship seen between hPL and placental mass, or hPL and birthweight, in T1DM in this cohort (using stringent sig threshold of p < 0.01). Did see a positive relationship between hPL and birthweight in T1DM group when sig level of p < 0.02 accepted. | Low |
References
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Sibiak, R.; Gutaj, P.; Wender-Ozegowska, E.; Jankowski, M.; Mozdziak, P.; Kempisty, B. Placental lactogen as a marker of maternal obesity, diabetes, and fetal growth abnormalities: Current knowledge and clinical perspectives. J. Clin. Med. 2020, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Roman, M.A. Prolactin and lactation as modifiers of diabetes risk in gestational diabetes. Horm. Metab. Res. 2011, 43, 593–600. [Google Scholar] [CrossRef]
- Handwerger, S. Clinical counterpoint: The physiology of placental lactogen in human pregnancy. Endocr. Rev. 1991, 12, 329–336. [Google Scholar] [CrossRef]
- Brelje, T.C.; Scharp, D.W.; Lacy, P.E.; Ogren, L.; Talamantes, F.; Robertson, M.; Friesen, H.G.; Sorenson, R.L. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: Implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993, 132, 879–887. [Google Scholar] [CrossRef]
- Spellacy, W.N. The use of human placental lactogen in the antepartum monitoring of pregnancy. Clin. Obstet. Gynaecol. 1979, 6, 245–258. [Google Scholar] [CrossRef]
- Hobbins, J.C.; Berkowitz, R.L. Current status of human placental lactogen. Clin. Obstet. Gynecol. 1978, 21, 363–373. [Google Scholar] [CrossRef]
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef]
- Rassie, K.L.; Giri, R.; Melder, A.; Joham, A.; Mousa, A.; Teede, H.J. Lactogenic hormones in relation to maternal metabolic health in pregnancy and postpartum: Protocol for a systematic review. BMJ Open 2022, 12, e055257. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- MCHRI. Evidence Synthesis Program Templates for Critical Appraisal and Risk of Bias (Adapted from Critical Appraisal Templates, Centre for Clinical Effectiveness, Southern Health, Melbourne 2010); Monash University and Monash Health: Melbourne, Australia, 2013. [Google Scholar]
- Deeks, J.; Dinnes, J.; D’Amico, R.; Sowden, A.; Sakarovitch, C. Evaluating non-randomised intervention studies. Health Technol. Assess 2003, 7. [Google Scholar] [CrossRef] [PubMed]
- Botta, R.M.; Donatelli, M.; Bucalo, M.L.; Bellomonte, M.L.; Bompiani, G.D. Placental lactogen, progesterone, total estriol and prolactin plasma levels in pregnant women with insulin-dependent diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D.; Mills, J.L.; Reed, G.F.; Jovanovic, L.G.; Holmes, L.B.; Aarons, J.; Simpson, J.L. Comparison of serum placental protein hormone levels in diabetic and normal pregnancy. J. Clin. Endocrinol. Metab. 1989, 68, 3–8. [Google Scholar] [CrossRef]
- De Hertogh, R.; Thomas, K.; Hoet, J.J.; Vanderheyden, I. Plasma concentrations of unconjugated estrone, estradiol-17beta and estriol, and HCS throughout pregnancy in diabetics and gestational diabetics. Diabetologia 1976, 12, 455–461. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Sorensen, S.; Molsted-Pedersen, L. Serum levels of human placental lactogen, pregnancy-associated plasma protein A and endometrial secretory protein PP14 in first trimester of diabetic pregnancy. Acta Obstet. Et Gynecol. Scand. 1998, 77, 155–158. [Google Scholar]
- Lopez-Espinoza, I.; Smith, R.F.; Gillmer, M.; Schidlmeir, A.; Hockaday, T.D. High levels of growth hormone and human placental lactogen in pregnancy complicated by diabetes. Diabetes Res. 1986, 3, 119–125. [Google Scholar]
- Larinkari, J.; Laatikainen, L.; Ranta, T. Metabolic control and serum hormone levels in relation to retinopathy in diabetic pregnancy. Diabetologia 1982, 22, 327–332. [Google Scholar] [CrossRef]
- Stewart, M.O.; Whittaker, P.G.; Persson, B.; Hanson, U.; Lind, T. A longitudinal study of circulating progesterone, oestradiol, hCG and hPL during pregnancy in type 1 diabetic mothers. Br. J. Obstet. Gynaecol. 1989, 96, 415–423. [Google Scholar] [CrossRef]
- Gillmer, M.D.G.; Beard, R.W.; Oakley, N.W. Plasma human placental lactogen profiles over 24 hours in normal and diabetic pregnancy. Br. J. Obstet. Gynaecol. 1977, 84, 197–204. [Google Scholar] [CrossRef]
- Persson, B.; Lunell, N.O. Metabolic control in diabetic pregnancy. Variations in plasma concentrations of glucose, free fatty acids, glycerol, ketone bodies, insulin, and human chorionic somatomammotropin during the last trimester. Am. J. Obstet. Gynecol. 1975, 122, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.; Ditzel, J. Correlation of serum unconjugated oestriol to red cell 2,3-diphosphoglycerate levels in diabetic pregnancy. Diabetologia 1983, 24, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Spellacy, W.N.; Cohn, J.E. Human placental lactogen levels and daily insulin requirements in patients with diabetes mellitus complicating pregnancy. Obstet. Gynecol. 1973, 42, 330–333. [Google Scholar] [PubMed]
- Schmitz, O.; Klebe, J.; Moller, J.; Arnfred, J.; Hermansen, K.; Orskov, H.; Beck-Nielsen, H. In vivo insulin action in type 1 (insulin-dependent) diabetic pregnant women as assessed by the insulin clamp technique. J. Clin. Endocrinol. Metab. 1985, 61, 877–881. [Google Scholar] [CrossRef]
- Catalano, P.M.; Nizielski, S.E.; Shao, J.; Preston, L.; Qiao, L.; Friedman, J.E. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: Relationship to FFA during pregnancy. Am. J. Physiol. 2002, 282, E522–E533. [Google Scholar]
- Al Busaidi, F.; Al Wahaibi, A.; Krolikowski, A. Elevated levels of beta-human chorionic gonadotropin and human placental lactogen between 11–13 weeks’ gestation and subsequent pregnancy complications in Omani women. Saudi Med. J. 2004, 25, 382–384. [Google Scholar]
- Rasanen, J.P.; Snyder, C.K.; Rao, P.V.; Mihalache, R.; Heinonen, S.; Gravett, M.G.; Roberts, C.T., Jr.; Nagalla, S.R. Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet. Gynecol. 2013, 122, 586–594. [Google Scholar] [CrossRef]
- Ngala, R.A.; Fondjo, L.A.; Gmagna, P.; Awe, M.A.; Ghartey, F.N. Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. PLoS ONE 2017, 12, e0181613. [Google Scholar] [CrossRef]
- Al-Hussein, R.K.A.; Jawad, S.M.A. Effect of human placental lactogen hormone and some physiological parameter changes association with fetal sex in women with gestational diabetes. Biochem. Cell. Arch. 2021, 21, 4251–4257. [Google Scholar]
- Grigorakis, S.I.; Beis, C.; Anastasiou, E.; Alevizaki, C.C.; Souvatzoglou, A.; Alevizaki, M. Hormonal parameters in gestational diabetes mellitus during the third trimester: High glucagon levels. Gynecol. Obstet. Investig. 2000, 49, 106–109. [Google Scholar] [CrossRef]
- Retnakaran, R.; Ye, C.; Kramer, C.K.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B. Evaluation of Circulating Determinants of Beta-Cell Function in Women with and without Gestational Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Surmaczynska, B.Z.; Nitzan, M.; Metzger, B.E.; Freinkel, N. Carbohydrate metabolism in pregnancy. XII. The effect of oral glucose on plasma concentrations of human placental lactogen and chorionic gonadotropin during late pregnancy in normal subjects and gestational diabetics. Isr. J. Med. Sci. 1974, 10, 1481–1486. [Google Scholar] [PubMed]
- Henderson, C.E.; Divon, M.Y. Combining human placental lactogen with routine glucose challenge tests. Prim. Care Update Ob Gyns 1998, 5, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Samaan, N.A.; Vassilopoulou-Sellin, R.; Schulz, P.N. Nonsuppressible insulin-like activity and somatomedin C levels in normal pregnant women, in pregnant women with gestational diabetes, and in umbilical cord blood of mature and premature infants. Am. J. Obstet. Gynecol. 1985, 153, 457–461. [Google Scholar] [CrossRef]
- Kuhl, C.; Gaede, P.; Klebe, J.G.; Pedersen, J. Human placental lactogen concentration during physiological fluctuations of serum glucose in normal pregnant and gestational diabetic women. Acta Endocrinol. 1975, 80, 365–373. [Google Scholar] [CrossRef]
- Catalano, P.M.; Avallone, D.A.; Drago, N.M.; Amini, S.B. Reproducibility of the oral glucose tolerance test in pregnant women. Am. J. Obstet. Gynecol. 1993, 169, 874–881. [Google Scholar] [CrossRef]
- Luthman, M.; Stock, S.; Werner, S.; Bremme, K. Growth hormone-binding protein in plasma is inversely correlated to placental lactogen and augmented with increasing body mass index in healthy pregnant women and women with gestational diabetes mellitus. Gynecol. Obstet. Investig. 1994, 38, 145–150. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef]
- Benny, P.S.; MacVicar, J.; Parkin, E.N.; Montague, W. Carbohydrate profiles in two groups of mothers with differing perinatal mortality. J. Obstet. Gynaecol. 1980, 1, 20–23. [Google Scholar] [CrossRef]
- Enzi, G.; Inelmen, E.M.; Caretta, F.; Rubaltelli, F.; Grella, P.; Baritussio, A. Adipose tissue development ‘in utero’. Relationships between some nutritional and hormonal factors and body fat mass enlargement in newborns. Diabetologia 1980, 18, 135–140. [Google Scholar] [CrossRef]
- Fairweather, D.V.I. Changes in levels of serum non esterified fatty acid and blood glucose in pregnancy. J. Obstet. Gynaecol. Br. Commonw. 1971, 78, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.H.; Malhotra, A.; Scott, J.; Douse, T.; Pogmore, J.R.; Wharton, B.A. Glucose tolerance, insulin resistance and birth size in Asian and European mothers. J. Obstet. Gynaecol. 1992, 12, 87–93. [Google Scholar] [CrossRef]
- Retnakaran, R.; Chang, Y.; Kramer, C.K.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B.; Ye, C. Maternal Serum Prolactin and Prediction of Postpartum β-Cell Function and Risk of Prediabetes/Diabetes. Diabetes Care 2016, 39, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.M.; Halbert, S.P.; Spellacy, W.N. Relation of obstetric parameters to the concentrations of four pregnancy-associated plasma proteins at term in normal gestation. Am. J. Obstet. Gynecol. 1976, 125, 17–24. [Google Scholar] [CrossRef]
- McCarrick, J.; Moshirpur, J.; Levy, E.; Allerhand, J. Correlation of HPL and total estrogen in complicated pregnancies and the influence of maternal weight on HPL result. Mt. Sinai J. Med. 1979, 46, 75–78. [Google Scholar]
- Small, M.; Cameron, A.; Lunan, C.B.; MacCuish, A.C. Macrosomia in pregnancy complicated by insulin-dependent diabetes mellitus. Diabetes Care 1987, 10, 594–599. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Molsted-Pedersen, L.; Lebech, P.E. Is the early growth delay in the diabetic pregnancy accompanied by a delay in placental development? Acta Obstet. Et Gynecol. Scand. 1986, 65, 675–677. [Google Scholar] [CrossRef]
- Gaspard, U.; Sandront, H.; Luyckx, A. Glucose-insulin interaction and the modulation of human placental lactogen (hPL) secretion during pregnancy. BJOG Int. J. Obstet. Gynaecol. 1974, 81, 201–209. [Google Scholar] [CrossRef]
- Jovanovic, L.; Peterson, C.M.; Saxena, B.B.; Dawood, M.Y.; Saudek, C.D. Feasibility of maintaining normal glucose profiles in insulin-dependent pregnant diabetic women. Am. J. Med. 1980, 68, 105–112. [Google Scholar] [CrossRef]
- Ursell, W.; Brudenell, M.; Chard, T. Placental Lactogen Levels in Diabetic Pregnancy. Br. Med. J. 1973, 2, 80–82. [Google Scholar] [CrossRef][Green Version]
- Varner, M.W.; Hauser, K.S. Current Status of Human placental lactogen. Semin. Perinatol. 1981, 5, 123–130. [Google Scholar] [PubMed]
- Palomba, S.; Daolio, J. Pregnancy Endocrinology. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, MS, USA, 2018; pp. 408–417. [Google Scholar]
- Parsons, J.A.; Brelje, T.C.; Sorenson, R.L. Adaptation of islets of Langerhans to pregnancy: Increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992, 130, 1459–1466. [Google Scholar] [PubMed]
- Sorenson, R.L.; Brelje, T.C. Adaptation of islets of Langerhans to pregnancy: Beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Cao-Minh, L.; Galasso, R.; Rizza, R.A.; Corradin, A.; Cobelli, C.; Butler, P.C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010, 53, 2167–2176. [Google Scholar] [CrossRef]
- Le, T.N.; Francis, G.L.; Elsea, S.H.; Romero, R.; Chaiworapongsa, T. Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet. Test. Mol. Biomark. 2013, 17, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Saxena, B.N.; Emerson, K., Jr.; Selenkow, H.A. Serum placental lactogen (HPL) levels as an index of placental function. New Engl. J. Med. 1969, 281, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, J.J.; Sherwood, L.M.; Varma, A.A.; Lundberg, W.B. Human placental lactogen (HPL) and placental weight. Am. J. Obstet. Gynecol. 1968, 101, 413–416. [Google Scholar] [CrossRef]
- Houghton, D.J.; Shackleton, P.; Obiekwe, B.C.; Chard, T. Relationship of maternal and fetal levels of human placental lactogen to the weight and sex of the fetus. Placenta 1984, 5, 455–458. [Google Scholar] [CrossRef]
- Knopp, R.H.; Bergelin, R.O.; Wahl, P.W.; Walden, C.E. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes 1985, 34 (Suppl. 2), 71–77. [Google Scholar] [CrossRef]
- Markestad, T. Prediction of fetal growth based on maternal serum concentrations of human chorionic gonadotropin, human placental lactogen and estriol. Acta Obstet. Et Gynecol. Scand. Suppl. 1997, 76, 50–55. [Google Scholar]
- Nahavandi, S.; Seah, J.-m.; Shub, A.; Houlihan, C.; Ekinci, E.I. Biomarkers for Macrosomia Prediction in Pregnancies Affected by Diabetes. Front. Endocrinol. 2018, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Goetzmann, L.N.; Cantlon, J.D.; Jeckel, K.M.; Winger, Q.A.; Anthony, R.V. Development of ovine chorionic somatomammotropin hormone-deficient pregnancies. Am. J. Physiol Regul. Integr. Comp. Physiol. 2016, 310, R837–R846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeckel, K.M.; Boyarko, A.C.; Bouma, G.J.; Winger, Q.A.; Anthony, R.V. Chorionic somatomammotropin impacts early fetal growth and placental gene expression. J. Endocrinol. 2018, 237, 301–310. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Serek, R.; Crane, D.I.; Veveris-Lowe, T.; Parry, A.; Johnson, S.; Leung, K.C.; Ho, K.K.; Bougoussa, M.; Hennen, G.; et al. Placental growth hormone (GH), GH-binding protein, and insulin-like growth factor axis in normal, growth-retarded, and diabetic pregnancies: Correlations with fetal growth. J. Clin. Endocrinol. Metab. 2000, 85, 1143–1150. [Google Scholar] [PubMed]
- Mannik, J.; Vaas, P.; Rull, K.; Teesalu, P.; Rebane, T.; Laan, M. Differential expression profile of growth hormone/chorionic somatomammotropin genes in placenta of small- and large-for-gestational-age newborns. J. Clin. Endocrinol. Metab. 2010, 95, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rassie, K.; Giri, R.; Joham, A.E.; Teede, H.; Mousa, A. Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15621. https://doi.org/10.3390/ijms232415621
Rassie K, Giri R, Joham AE, Teede H, Mousa A. Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(24):15621. https://doi.org/10.3390/ijms232415621
Chicago/Turabian StyleRassie, Kate, Rinky Giri, Anju E. Joham, Helena Teede, and Aya Mousa. 2022. "Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 23, no. 24: 15621. https://doi.org/10.3390/ijms232415621
APA StyleRassie, K., Giri, R., Joham, A. E., Teede, H., & Mousa, A. (2022). Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 23(24), 15621. https://doi.org/10.3390/ijms232415621








