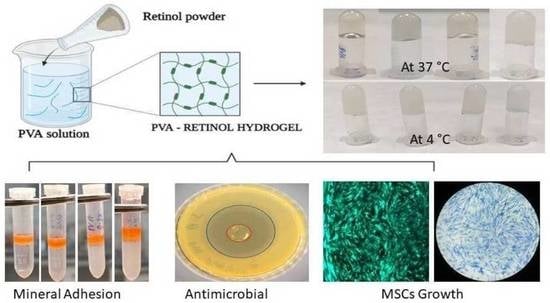
Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
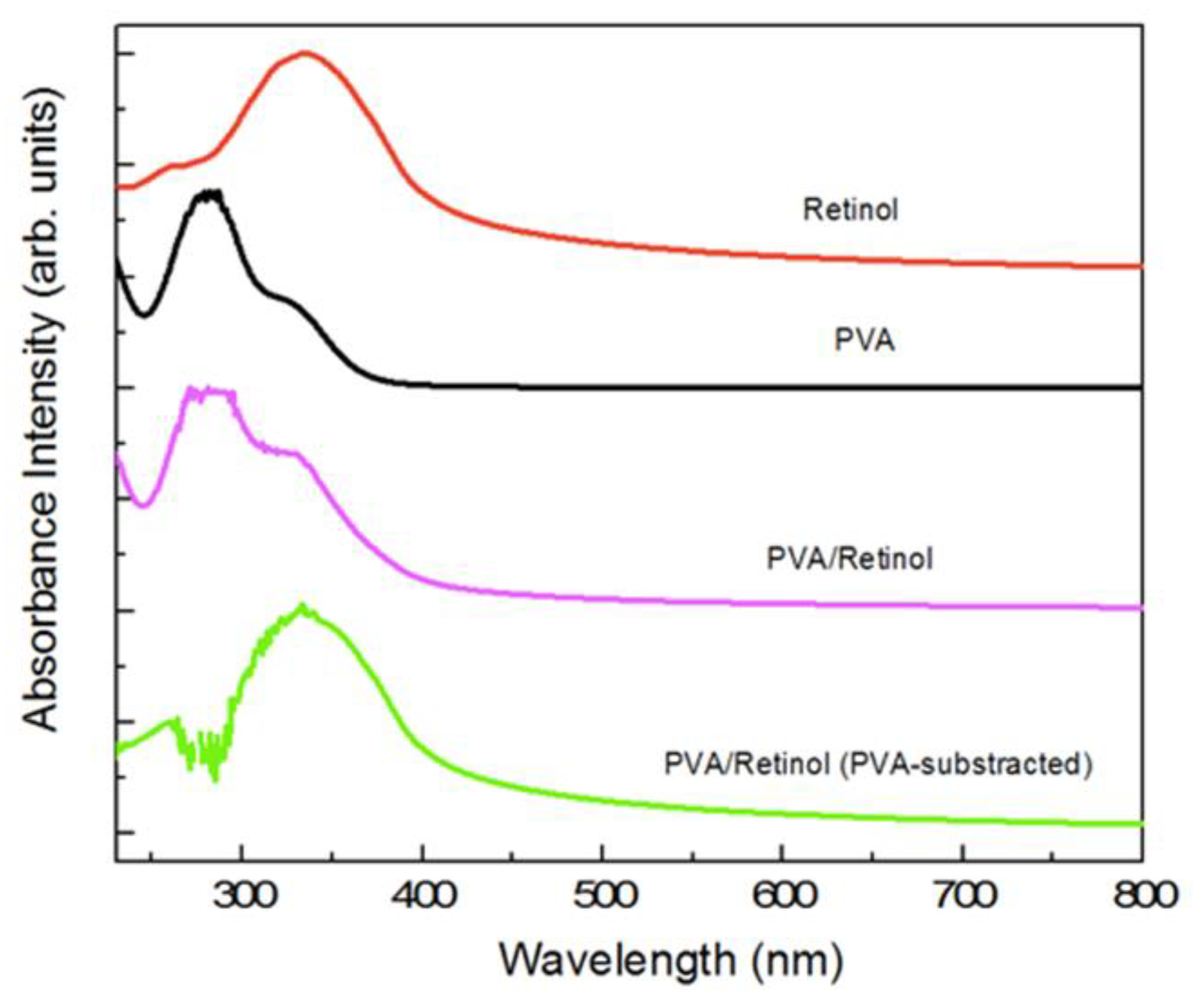
2.1. Maximum Absorbance of Hydrogel
2.2. Retinol Degradation
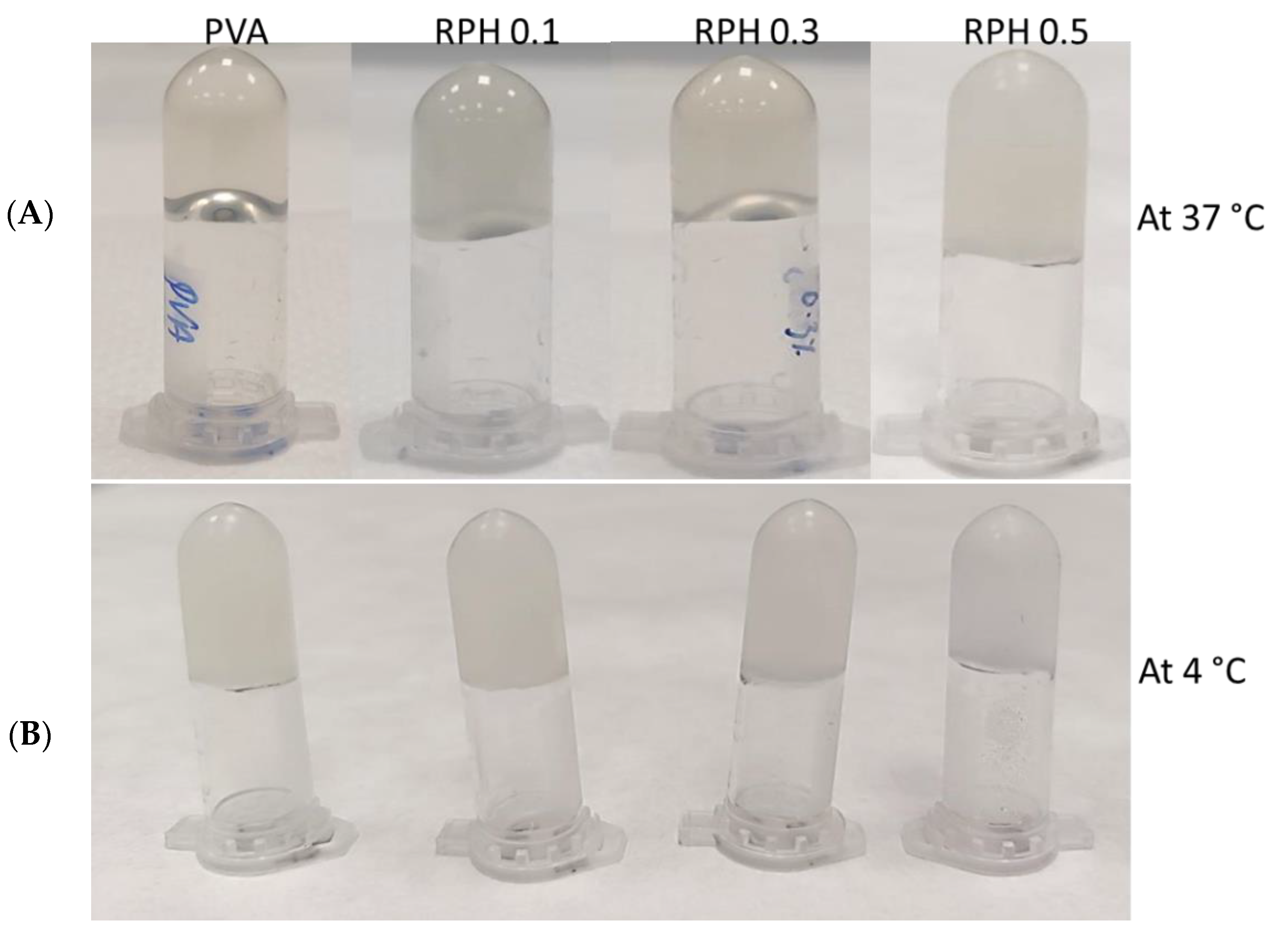
2.3. Textural Analysis
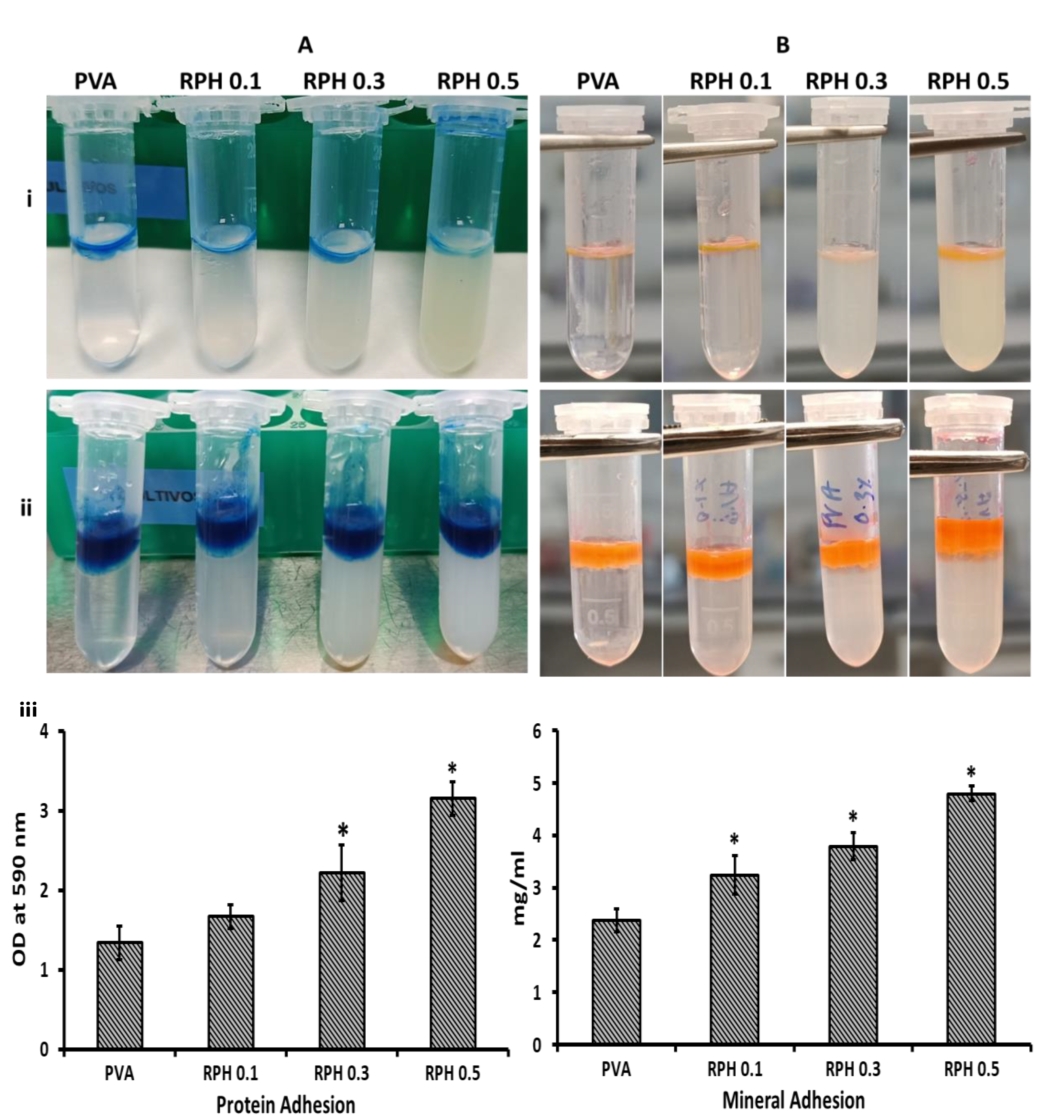
2.4. Protein Adhesion Ability
2.5. Mineral Adhesion Ability
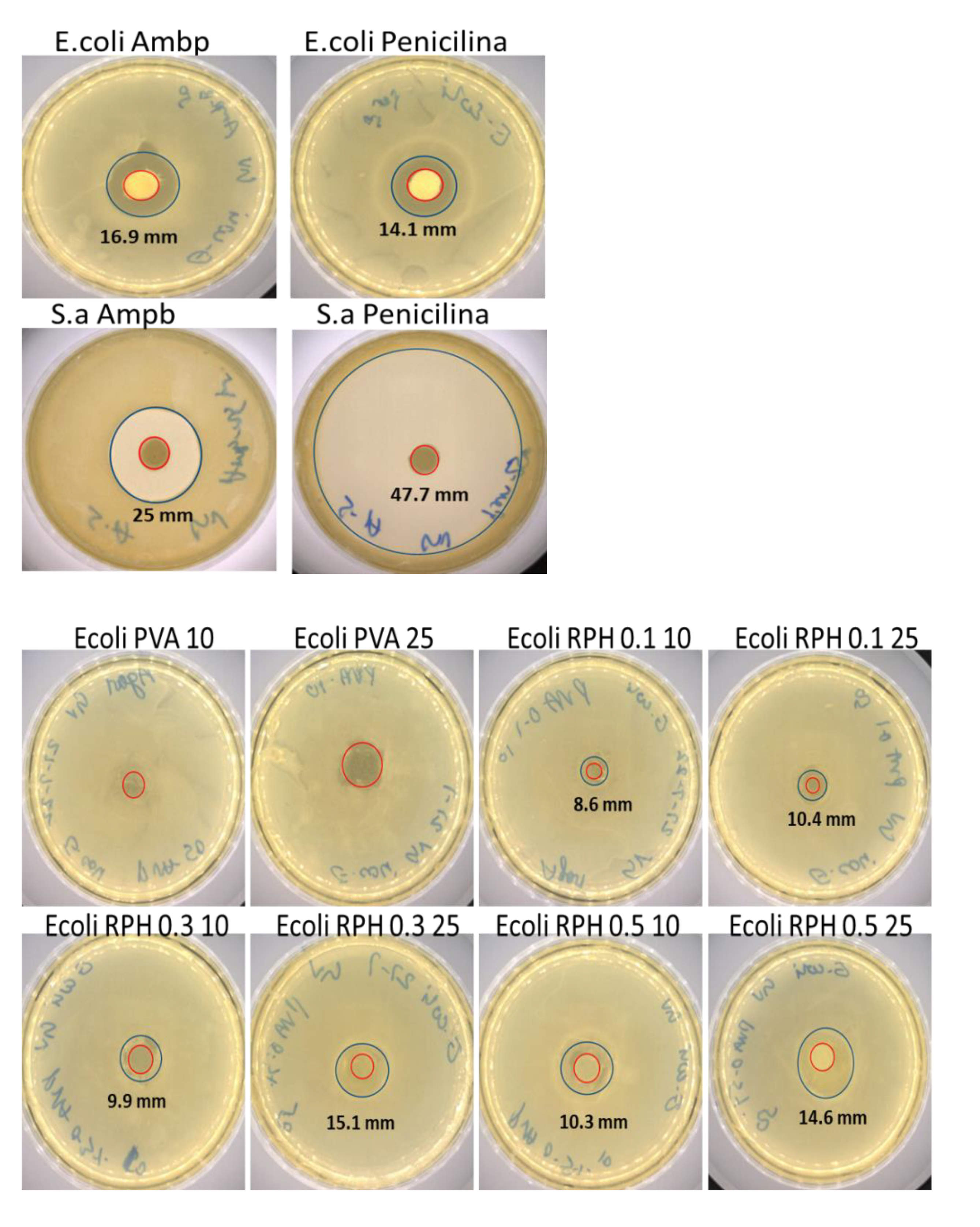
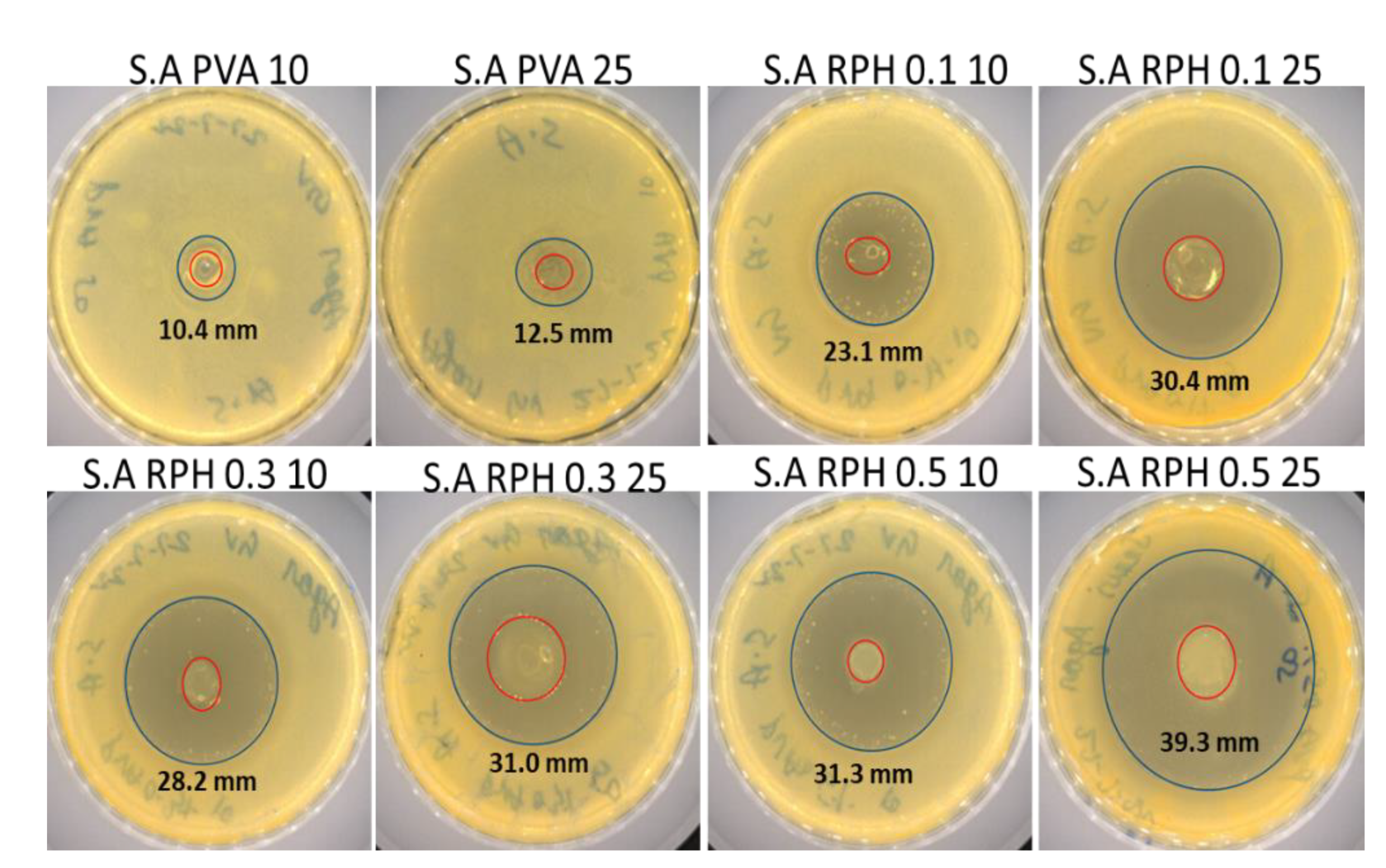
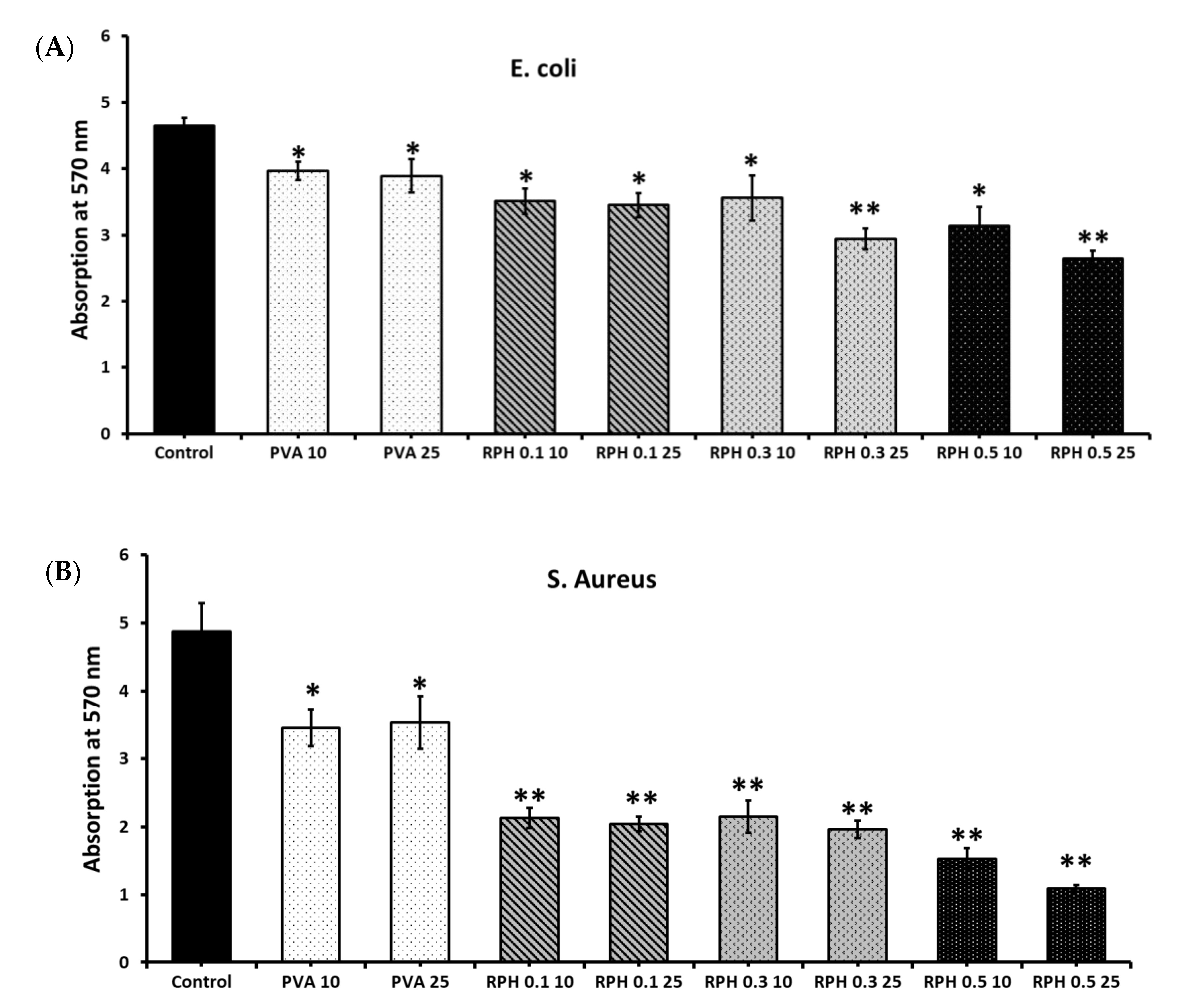
2.6. Antimicrobial Activity of PVA Retinol Hydrogel
2.7. Microbial Attachment
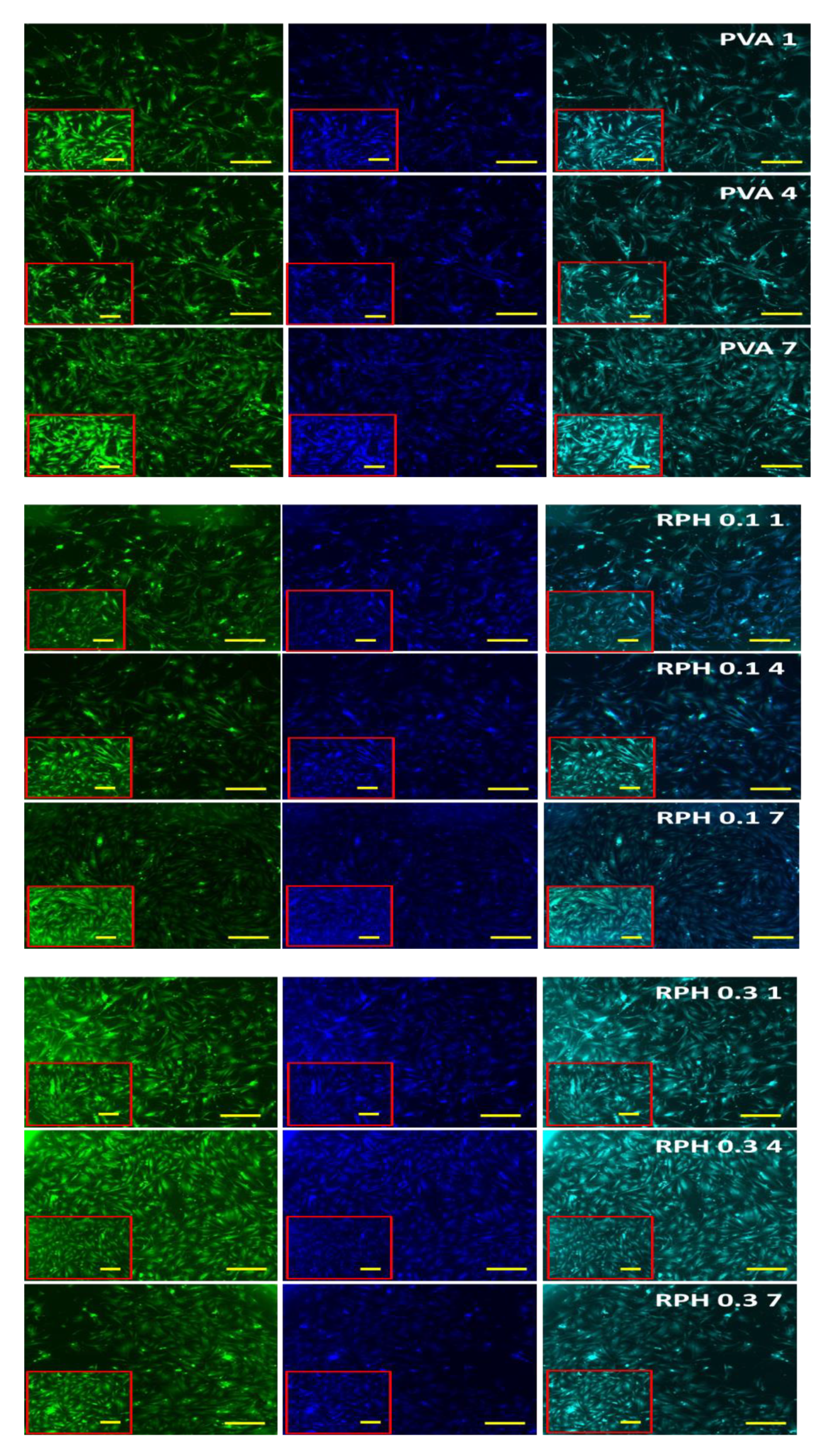
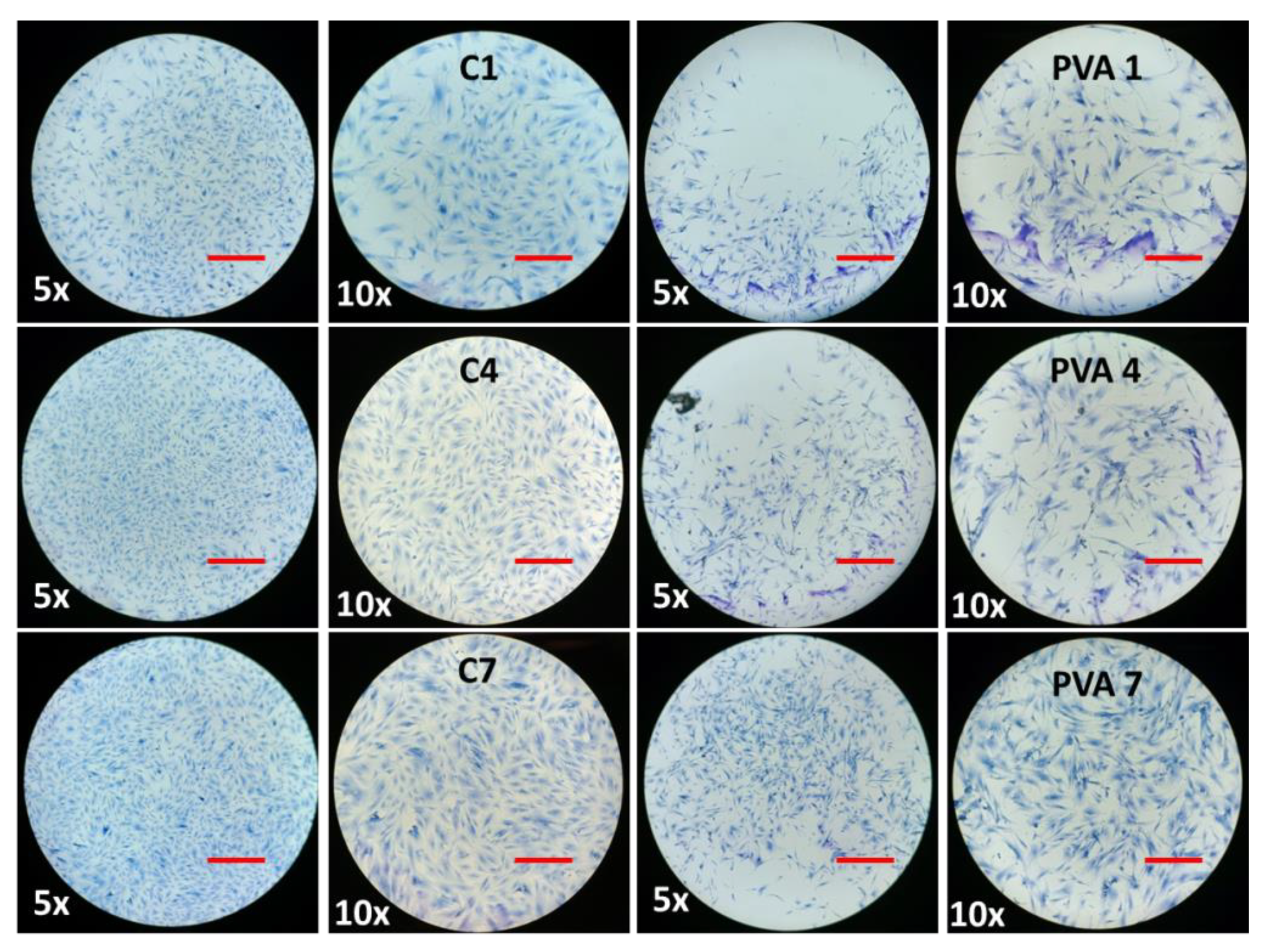
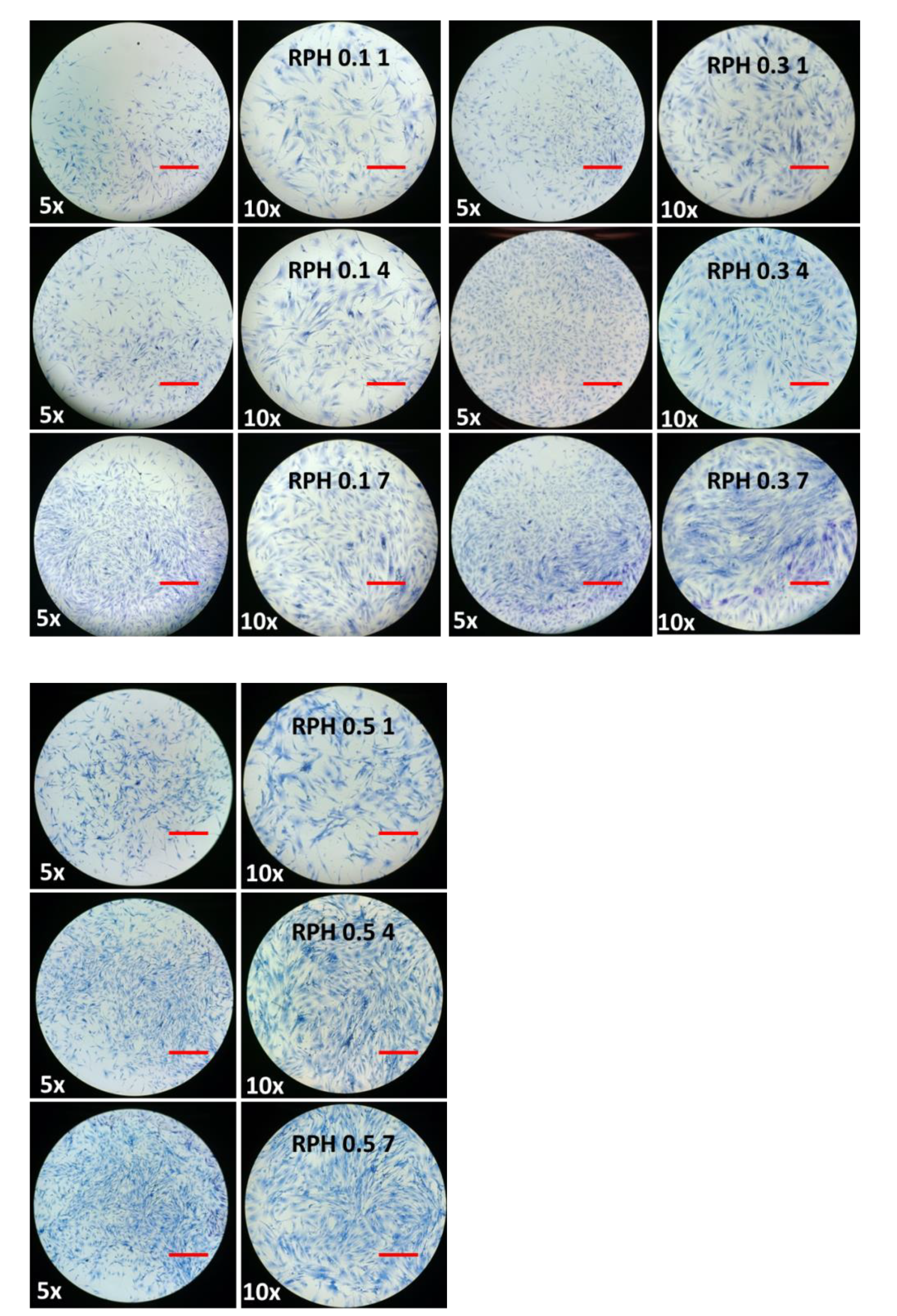
2.8. The Effect of BMMS Cells
2.9. BMMS Cell-Loading Capacity of the Hydrogel
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Aqueous Retinol-Loaded Poly (Vinyl Alcohol) Hydrogels (RPH) Fabrication
4.3. Characterization
4.4. In Vitro Retinol Stability and Degradation
4.5. Textural Properties Analysis
4.6. Protein Adsorption
4.7. Mineral Deposition
4.8. Antimicrobial Activity
4.8.1. Disk Diffusion Method
4.8.2. In Vitro Bacterial Attachment Assay
4.9. In Vitro Cell Proliferation
4.10. Cell-Loading Capacity
4.11. Fluorescence Microscope
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kouser, S.; Sheik, S.; Nagaraja, G.K.; Prabhu, A.; Prashantha, K.; D’souza, J.N.; Navada, K.M.; Manasa, D.J. Functionalization of halloysite nanotube with chitosan reinforced poly (vinyl alcohol) nanocomposites for potential biomedical applications. Int. J. Biol. Macromol. 2020, 15, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2021, 164, 110974. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Maiolo, A.S.; Amado, M.N.; Gonzalez, J.S.; Alvarez, V.A. Development and characterization of poly (vinyl alcohol) based hydrogels for potential use as an articular cartilage replacement. Mater. Sci. Eng. C 2012, 32, 1490–1495. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Liu, L.; Song, P.; Cao, Q.; Xu, Z.; Fang, Z.; Wang, H. Water governs the mechanical properties of poly (vinyl alcohol). Polymer 2021, 213, 123330. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Y.; Xu, M.; Qin, M.; Lan, W.; Huang, D.; Wei, Y.; Chen, W. High strength polyvinyl alcohol/polyacrylic acid (PVA/PAA) hydrogel fabricated by Cold-Drawn method for cartilage tissue substitutes. J. Biomater. Sci. Polym. 2020, 31, 1836–1851. [Google Scholar] [CrossRef]
- Williams, J.K.; Yoo, J.J.; Atala, A. Chapter 59. Regenerative medicine approaches for tissue engineered heart valves. In Principles of Regenerative Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2019; pp. 1041–1058. [Google Scholar]
- Wu, Y.; Chee, A.J.Y.; Golzar, H.; Yu, A.C.H.; Tang, X. Embedded 3D Printing of Ultrasound-Compatible Arterial Phantoms with Biomimetic Elasticity. Adv. Funct. Mater. 2022, 32, 2110153. [Google Scholar] [CrossRef]
- Jung, S.; Pant, B.; Climans, M.; Shaw, G.C.; Lee, E.-J.; Kim, N.; Park, M. Transformation of electrospun Keratin/PVA nanofiber membranes into multilayered 3D Scaffolds: Physiochemical studies and corneal implant applications. Int. J. Pharm. 2021, 610, 121228. [Google Scholar] [CrossRef]
- Wen, N.; Jiang, B.; Wang, X.; Shang, Z.; Jiang, D.; Zhang, L.; Sun, C.; Wu, Z.; Yan, H.; Liu, C. Overview of polyvinyl alcohol nanocomposite hydrogels for electro-skin, actuator, supercapacitor and fuel cell. Chem. Rec. 2020, 20, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, P.; Yang, F.; Chen, J.; Wang, C.; Zhou, Y.; Fu, J. Biomimetic epidermal sensors assembled from polydopamine-modified reduced graphene oxide/polyvinyl alcohol hydrogels for the real-time monitoring of human motions. J. Mater. Chem. B 2020, 8, 10549–10558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zilu, L.; Fangli, R.; Lin, D.; Chenyu, L.; Chuanling, S. Green and stable piezoresistive pressure sensor based on lignin-silver hybrid nanoparticles/polyvinyl alcohol hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef]
- Xinxin, S.; Chunhui, L.; Faliang, L. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar] [CrossRef]
- Soleymani, M.; Akbari, A.; Mahdavinia, G.R. Magnetic PVA/laponite RD hydrogel nanocomposites for adsorption of model protein BSA. Polym. Bull. 2019, 76, 2321–2340. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cao, Y. Hydrogels for large-scale expansion of stem cells. Acta Biomater. 2021, 128, 1–20. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, X.; Xu, M.; Zhao, L.; Huang, D.; Wei, X.; Chen, W. Carbon nanotube reinforced polyvinyl alcohol/biphasic calcium phosphate scaffold for bone tissue engineering. RSC Adv. 2019, 9, 38998–39010. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Ren, L.; Wang, Y. Graphene oxide/PVA inorganic/organic interpenetrating hydrogels with excellent mechanical properties and biocompatibility. Carbon 2017, 111, 18–27. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar]
- Nešović, K.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Silver/poly (vinyl alcohol)/chitosan/graphene hydrogels–synthesis, biological and physicochemical properties and silver release kinetics. Compos. Part B Eng. 2018, 154, 175–185. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 2013, 103, 318–325. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A novel high-strength poly (ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Sainz-Díaz, C.I.; Perioli, L.; Viseras, C. Theoretical Study of Retinol, Niacinamide and Glycolic Acid with Halloysite Clay Mineral as Active Ingredients for Topical Skin Care Formulations. Molecules 2021, 26, 4392. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology: Thematic review series: Fat-soluble vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef]
- Khillan, J.S. Vitamin A/retinol and maintenance of pluripotency of stem cells. Nutrients 2014, 6, 1209–1222. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutri. Burn. Inj. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Shimo, T.; Takebe, H.; Okui, T.; Kunisada, Y.; Ibaragi, S.; Obata, K.; Kurio, N.; Shamsoon, K.; Fujii, S.; Hosoya, A.; et al. Expression and Role of IL-1β Signaling in Chondrocytes Associated with Retinoid Signaling during Fracture Healing. Int. J. Mol. Sci. 2020, 21, 2365. [Google Scholar] [CrossRef]
- Grafanaki, K.; Skeparnias, I.; Kontos, C.K.; Anastasakis, D.; Korfiati, A.; Kyriakopoulos, G.; Theofilatos, K.; Mavroudi, S.; Magoulas, G.; Papaioannou, D.; et al. Pharmacoepigenomics circuits induced by a novel retinoid-polyamine conjugate in human immortalized keratinocytes. Pharmacogenom. J. 2021, 21, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.A.; Kushram, P.; Bose, S. Effects of Vitamin A (Retinol) Release from Calcium Phosphate Matrices and Porous 3D Printed Scaffolds on Bone Cell Proliferation and Maturation. ACS Appl. Biomater. 2022, 5, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly (vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Škufca, P.; Kristl, A.; Roškar, R. Retinoid stability and degradation kinetics in commercial cosmetic products. J. Cosmet. Dermatol. 2021, 20, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kamboj, M. Assay of Retinol and α-tocopherol involving Ce(IV)-Ce(III) Redox Couple Spectrophotometrically. Vietnam. J. Chem. 2020, 58, 815–825. [Google Scholar]
- Allwood, M.; Martin, H. The photodegradation of vitamins A and E in parenteral nutrition mixtures during infusion. Clin. Nutr. 2000, 19, 339–342. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Babaladimath, G.; Badalamoole, V.; H Mallaiah, S. Gamma irradiation synthesis and in vitro drug release studies of ZnO/PVA hydrogel nanocomposites. Adv. Mater. Lett. 2017, 8, 546–552. [Google Scholar] [CrossRef]
- Boran, F.; Karakaya, Ç. Polyvinyl alcohol/CuO nanocomposite hydrogels: Facile synthesis and long-term stability. Balıkesir Üniversitesi Fen Bilimleri Enstitüsü Derg. 2019, 21, 512–530. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Antimicrobial chitosan–PVA hydrogel as a nanoreactor and immobilizing matrix for silver nanoparticles. Appl. Nanosci. 2012, 2, 179–188. [Google Scholar] [CrossRef]
- Failloux, N.; Bonnet, I.; Baron, M.-H.; Perrier, E. Quantitative analysis of vitamin A degradation by Raman spectroscopy. Appl. Spectrosc. 2003, 57, 1117–1122. [Google Scholar] [CrossRef]
- Park, H.; Mun, S.; Kim, Y.-R. UV and storage stability of retinol contained in oil-in-water nanoemulsions. Food Chem. 2019, 272, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Kim, H.; Lee, H.; Song, J.E.; Park, S.G.; Kang, N.-G. Synthesis of retinol-loaded lipid nanocarrier via vacuum emulsification to improve topical skin delivery. Polymers 2021, 13, 826. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, F.; Alami-Milani, M.; Salatin, S.; Hadavi, A.; Jelvehgari, M. Freeze-thaw-induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: The experimental design and optimization. Res. Pharm. Sci. 2019, 14, 175. [Google Scholar] [PubMed]
- Bai, C.; Li, X.; Cui, X.; Yang, X.; Zhang, X.; Yang, K.; Wang, T.; Zhang, H. Transparent Stretchable Thermogalvanic PVA/Gelation Hydrogel Electrolyte for Harnessing Solar Energy Enabled by a Binary Solvent Strategy. Nano Energy 2022, 100, 107449. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, Y.; Sun, X.; Hu, B. Developing high strength, antiseptic and swelling-resistant polyvinyl alcohol/chitosan hydrogels for tissue engineering material. Mater. Lett. 2020, 280, 128499. [Google Scholar] [CrossRef]
- Farzinfar, E.; Paydayesh, A. Investigation of polyvinyl alcohol nanocomposite hydrogels containing chitosan nanoparticles as wound dressing. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 628–638. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Zhao, Y.-Q.; Huang, J.-F. Retinoid-binding proteins: Similar protein architectures bind similar ligands via completely different ways. PLoS ONE 2012, 7, e36772. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, C.; Peng, X.; Liu, T.; Shi, Y.; Liang, M.; Wang, H. Biomimetic anisotropic poly(vinyl alcohol) hydrogels with significantly enhanced mechanical properties by freezing–thawing under drawing. J. Mater. Chem. B 2019, 7, 3243–3249. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Diaz-Suntaxi, D.F.; Carrión-Matamoros, L.M.; Guerrero, V.H.; Almeida-Naranjo, C.E.; Morales-Flórez, V.; Debut, A.; Vizuete, K.; Mowbray, D.J.; Zamora-Ledezma, C. Impact of the solvent composition on the structural and mechanical properties of customizable electrospun poly (vinylpyrrolidone) fiber mats. Phys. Chem. Chem. Phys. 2021, 23, 22923–22935. [Google Scholar] [CrossRef]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.A.; Loutfy, S.A.; El-Fakharany, E.M.; Taha, T.H.; Hussien, Y.; Kamoun, E.A. Influence of chitosan and hydroxyapatite incorporation on properties of electrospun PVA/HA nanofibrous mats for bone tissue regeneration: Nanofibers optimization and in vitro assessment. J. Drug Deliv. Sci. Technol. 2021, 62, 102417. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.; Lyoo, W.S.; Han, S.S. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly (vinyl alcohol)-hyaluronic acid membranes for wound dressing applications: Synthesis and in vitro bio-evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Qi, R.; Cao, X.; Shen, M.; Guo, R.; Yu, J.; Shi, X. Biocompatibility of electrospun halloysite nanotube-doped poly (lactic-co-glycolic acid) composite nanofibers. J. Biomater. Sci. Polym. Ed. 2012, 23, 299–313. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Chicaiza-Zambrano, A.; Santiago Vispo, N.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Almeida, C.E.; Alexis, F. Frequency Based Control of Antifouling Properties Using Graphene Nanoplatelet/Poly (Lactic-co-Glycolic Acid) Composite Films. Compos. Interfaces 2021, 28, 1137–1153. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Tian, Y.; Ren, L. Hydroxyapatite gradient on poly (vinyl alcohol) hydrogels surface to mimic calcified cartilage zone for cartilage repair. J. Biomater. Appl. 2022, 36, 1579–1587. [Google Scholar] [CrossRef]
- Ye, M.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C 2014, 44, 310–316. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2011, 2, 55. [Google Scholar] [CrossRef]
- Hezma, A.; Rajeh, A.; Mannaa, M.A. An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Ghanem, S. Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J. Polym. Res. 2009, 16, 1–10. [Google Scholar] [CrossRef]
- Imtiaz, N.; Niazi, M.B.K.; Fasim, F.; Khan, B.A.; Bano, S.A.; Shah, G.M.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an original transparent PVA/gelatin hydrogel: In vitro antimicrobial activity against skin pathogens. Int. J. Polym. Sci. 2019, 2019, 7651810. [Google Scholar] [CrossRef]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Jang, Y.-M.; Kim, Y.-M.; Park, W.S.; Choi, I.-W.; Jung, W.-K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018, 198, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Mussatto, S.I.; Jha, H. Synthesis and characterization of silver nanoparticles loaded poly (vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 120, 763–767. [Google Scholar] [CrossRef]
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host–commensal–pathogen interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef]
- Chen, G.; Du, Y.; Liu, J.; Li, Y.; Zheng, L.; Qin, X.; Cao, Y. Modulation of anti-malaria immunity by vitamin A in C57BL/6J mice infected with heterogenic plasmodium. Int. Immunopharmacol. 2019, 76, 105882. [Google Scholar] [CrossRef]
- Pechere, M.; Germanier, L.; Siegenthaler, G.; Pechère, J.-C.; Saurat, J.-H. The antibacterial activity of topical retinoids: The case of retinaldehyde. Dermatology 2002, 205, 153–158. [Google Scholar] [CrossRef]
- VanHook, A.M. How vitamin A protects the skin. Sci. Signal. 2019, 12, eaay6954. [Google Scholar] [CrossRef]
- Shahzad, S.; Ashraf, M.A.; Sajid, M.; Shahzad, A.; Rafique, A.; Mahmood, M.S. Evaluation of synergistic antimicrobial effect of vitamins (A, B1, B2, B6, B12, C, D, E and K) with antibiotics against resistant bacterial strains. J. Glob. Antimicrob. Resist. 2018, 13, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ates-Alagöz, Z.; Alp, M.; Kus, C.; Yildiz, S.; Buyukbingöl, E.; Göker, H. Synthesis and potent antimicrobial activities of some novel retinoidal monocationic benzimidazoles. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2006, 339, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wheelwright, M.; Kim, E.W.; Inkeles, M.S.; De Leon, A.; Pellegrini, M.; Krutzik, S.R.; Liu, P.T. All-trans retinoic acid–triggered antimicrobial activity against Mycobacterium tuberculosis Is Dependent on NPC2. J. Immunol. 2014, 192, 2280–2290. [Google Scholar] [CrossRef]
- Kim, T.H.; An, D.B.; Oh, S.H.; Kang, M.K.; Song, H.H.; Lee, J.H. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing–thawing method to investigate stem cell differentiation behaviors. Biomaterials 2015, 40, 51–60. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J.L.; Melzig, M.F.; Chen, W.; Haag, R. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 77, 28–37. [Google Scholar] [CrossRef]
- Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A.A.; Masoud, M.S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021, 16, 035014. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef]
- Zhang, H.; Cong, Y.; Osi, A.R.; Zhou, Y.; Huang, F.; Zaccaria, R.P.; Chen, J.; Wang, R.; Fu, J. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv. Funct. Mater. 2020, 30, 1910573. [Google Scholar] [CrossRef]
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef]
- Oda, H.; Konno, T.; Ishihara, K. Efficient differentiation of stem cells encapsulated in a cytocompatible phospholipid polymer hydrogel with tunable physical properties. Biomaterials 2015, 56, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, Y.; Han, J.; Zhu, Y.; Zhang, H.; Zhang, L.; Li, L.; Xu, K.; Fu, G.; Mou, X. Structure and properties of PVA/silk fibroin hydrogels and their effects on growth behavior of various cell types. Mater. Res. Express 2020, 7, 015413. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; Moghadam, Z.M. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromole 2018, 14, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Carlos Godoy-Parejo, C.; Deng, C.; Zhang, Y.; Liu, W. Roles of vitamins in stem cells. Cell. Mol. Life Sci. 2020, 77, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Hore, T.A.; von Meyenn, F.; Ravichandran, M.; Bachman, M.; Ficz, G.; Oxley, D.; Santos, F.; Balasubramanian, S.; Jurkowski, T.P.; Reik, W. Retinol and ascorbate drive erasure of epigenetic memory and enhance reprogramming to naive pluripotency by complementary mechanisms. Proc. Natl. Acad. Sci. USA 2016, 113, 12202–12207. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Bittner, A.; Schlicht, K.; Mekhemar, M.; Enthammer, K.; Höppner, M.; Es-Souni, M.; Schulz, J.; Laudes, M.; Graetz, C.; et al. Ascorbic Acid/Retinol and/or Inflammatory Stimuli’s Effect on Proliferation/Differentiation Properties and Transcriptomics of Gingival Stem/Progenitor Cells. Cells 2021, 10, 3310. [Google Scholar] [CrossRef]
- Lia, X.; Long, X.; Xie, Y.; Zeng, X.; Chen, X.; Mo, Z. The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. Clin. Chim. Acta 2019, 497, 54–60. [Google Scholar] [CrossRef]
- Haghighat, M.; Iranbakhsh, A.; Baharara, J.; Ebadi, M.; Sotoodehnejadnematalahia, F. Evaluation of the Potential Effects of Retinol and Alginate/Gelatin-Based Scaffolds on Differentiation Capacity of Mouse Mesenchymal Stem Cells (MSCs) into Retinal Cells. Int. J. Stem Cells 2022, 15, 183. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Hein, D.; Dörfer, C.E. Retinol/inflammation affect stemness and differentiation potential of gingival stem/progenitor cells via Wnt/β-catenin. J. Periodontal Res. 2019, 54, 413–423. [Google Scholar] [CrossRef]
- Heydari, P.; Zargar Kharazi, A.; Asgary, S.; Parham, S. Comparing the wound healing effect of a controlled release wound dressing containing curcumin/ciprofloxacin and simvastatin/ciprofloxacin in a rat model: A preclinical study. J. Biomed. Mater. Res. Part A 2022, 110, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Rahman, M.M.; Hossain, M.S.; Jahan, M.S.; Jahan, N.A.; Bari, L.M. A Simple and Alternative UV Spectrometric Method for the Estimation of Vitamin D3. Microb. Bioact. 2019, 2, E098–E105. [Google Scholar]
- Elango, J.; Bushin, R.; Lijnev, A.; Aza, P.N.D.; Martínez, C.P.A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. [Google Scholar] [CrossRef]
- López-González, I.; Zamora-Ledezma, C.; Sanchez-Lorencio, M.I.; Tristante Barrenechea, E.; Gabaldón-Hernández, J.A.; Meseguer-Olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar] [PubMed]






| Sample | Gel Strength (N) | Consistency (mJ) | |
|---|---|---|---|
| At 4 °C | PVA | 0.29 ± 0.02 | 90 ± 20 |
| RPH0.1 | 0.25 ± 0.02 | 50 ± 20 | |
| RPH0.3 | 0.25 ± 0.02 | 50 ± 20 | |
| RPH0.5 | 0.25 ± 0.02 | 60 ± 20 | |
| At 37 °C | PVA | ND | ND |
| RPH0.1 | ND | ND | |
| RPH0.3 | ND | ND | |
| RPH0.5 | ND | ND |
| Hydrogels | E. coli (mm) | S. aureus (mm) |
|---|---|---|
| Penicillin 20 µL | 14.1 ± 0.27 | 47.7 ± 1.38 |
| Amphotericin B 20 µL | 16.9 ± 0.15 | 25.0 ± 2.56 |
| PVA 10 µL | - | 10.4 ± 0.59 |
| PVA 25 µL | - | 12.5 ± 0.24 |
| RPH 0.1 10 µL | 8.6 ± 0.22 * | 23.1 ± 1.88 * |
| RPH 0.1 25 µL | 10.4 ± 0.27 * | 30.4 ± 3.42 * |
| RPH 0.3 10 µL | 9.9 ± 0.16 * | 28.2 ± 2.46 * |
| RPH 0.3 25 µL | 15.1 ± 0.31 * | 31.0 ± 4.55 * |
| RPH 0.5 10 µL | 10.3 ± 0.48 * | 31.3 ± 3.98 * |
| RPH 0.5 25 µL | 14.6 ± 0.39 * | 39.3 ± 6.72 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 15623. https://doi.org/10.3390/ijms232415623
Elango J, Zamora-Ledezma C, Negrete-Bolagay D, Aza PND, Gómez-López VM, López-González I, Belén Hernández A, De Val JEMS, Wu W. Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2022; 23(24):15623. https://doi.org/10.3390/ijms232415623
Chicago/Turabian StyleElango, Jeevithan, Camilo Zamora-Ledezma, Daniela Negrete-Bolagay, Piedad N. De Aza, Vicente M. Gómez-López, Ivan López-González, Ana Belén Hernández, José Eduardo Maté Sánchez De Val, and Wenhui Wu. 2022. "Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells" International Journal of Molecular Sciences 23, no. 24: 15623. https://doi.org/10.3390/ijms232415623
APA StyleElango, J., Zamora-Ledezma, C., Negrete-Bolagay, D., Aza, P. N. D., Gómez-López, V. M., López-González, I., Belén Hernández, A., De Val, J. E. M. S., & Wu, W. (2022). Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. International Journal of Molecular Sciences, 23(24), 15623. https://doi.org/10.3390/ijms232415623