Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Structure and Physiology of Gastrointestinal Tract
3. Interplay between Microbiota and Gastrointestinal Tract
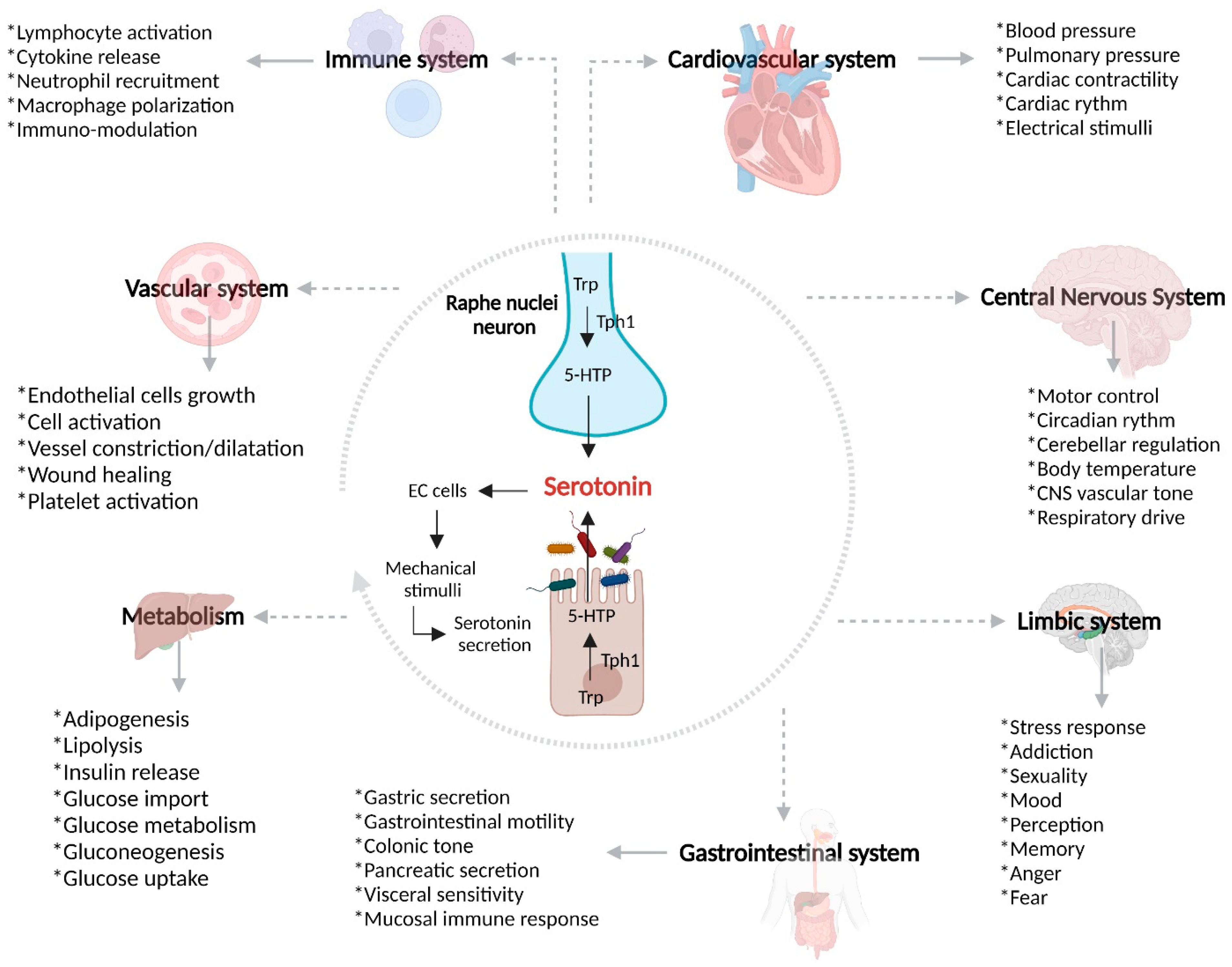
4. Gut–Brain Axis
4.1. Serotonin Receptors and Serotonin Transporter (SERT)
4.1.1. 5-HT1 Receptors
4.1.2. 5-HT2 Receptors
4.1.3. 5-HT3 Receptors
4.1.4. 5-HT4 Receptors
4.1.5. Serotonin Transporter (SERT)
5. Epidemiology and Pathogenesis of Inflammatory Bowel Disease
5.1. Risk Factors and Clinical Manifestations
Genetic Susceptibility and Inflammatory Bowel Disease
5.2. Intestinal Barrier Disruption and Over-Activated Immune Response in Inflammatory Bowel Disease
5.2.1. Immune Over-Activation in IBD
Th1 Response and Crohn’s Disease
Th2 Response and Ulcerative Colitis
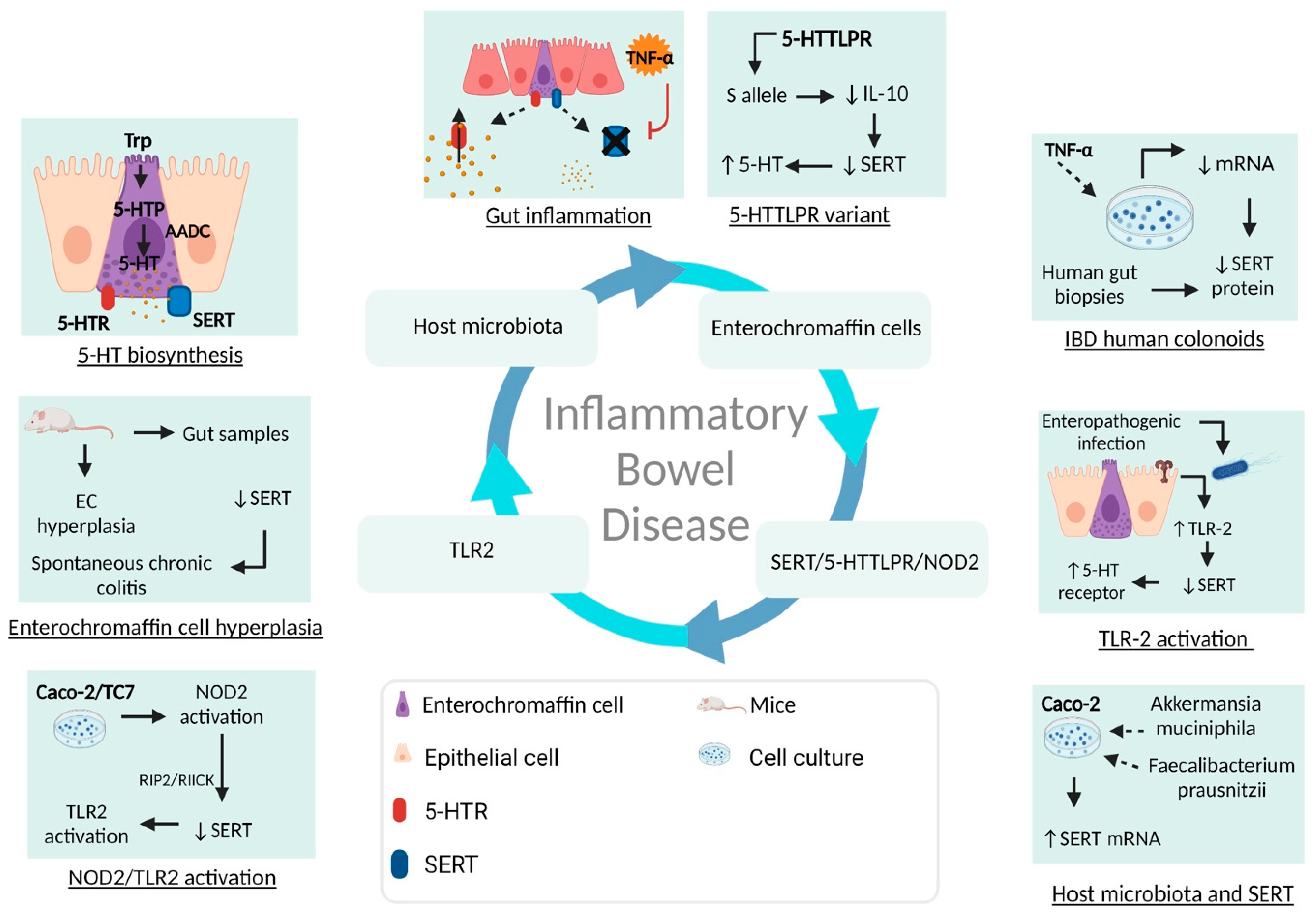
5.3. Serotonin and Gut–Brain Axis Dysfunction in IBD
6. Selective Serotonin Re-uptake Inhibitors in Inflammatory Bowel Disease: Clinical Evidence
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data availability Statement
Conflicts of Interest
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Piovani, D.; Pansieri, C.; Kotha, S.R.R.; Piazza, A.C.; Comberg, C.L.; Peyrin-Biroulet, L.; Danese, S.; Bonovas, S. Ethnic Differences in the Smoking-related Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2021, 15, 1658–1678. [Google Scholar] [CrossRef]
- Gearry, R.B. IBD and Environment: Are There Differences between East and West. Dig. Dis. 2016, 34, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Örtqvist, A.K.; Cao, Y.; Simon, T.G.; Roelstraete, B.; Song, M.; Joshi, A.D.; Staller, K.; Chan, A.T.; Khalili, H.; et al. Antibiotic use and the development of inflammatory bowel disease: A national case-control study in Sweden. Lancet Gastroenterol. Hepatol. 2020, 5, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Higuchi, L.M.; Ananthakrishnan, A.N.; Richter, J.M.; Feskanich, D.; Fuchs, C.S.; Chan, A.T. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013, 62, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e644. [Google Scholar] [CrossRef] [Green Version]
- Dubeau, M.F.; Iacucci, M.; Beck, P.L.; Moran, G.W.; Kaplan, G.G.; Ghosh, S.; Panaccione, R. Drug-induced inflammatory bowel disease and IBD-like conditions. Inflamm. Bowel Dis. 2013, 19, 445–456. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, P.; Lamerz, D.; Hill, P.; Kirchner, M.; Gauss, A. Gene Polymorphisms of NOD2, IL23R, PTPN2 and ATG16L1 in Patients with Crohn’s Disease: On the Way to Personalized Medicine? Genes 2021, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Boukercha, A.; Mesbah-Amroun, H.; Bouzidi, A.; Saoula, H.; Nakkemouche, M.; Roy, M.; Hugot, J.P.; Touil-Boukoffa, C. NOD2/CARD15 gene mutations in North Algerian patients with inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 7786–7794. [Google Scholar] [CrossRef] [PubMed]
- Lacher, M.; Schroepf, S.; Helmbrecht, J.; Von Schweinitz, D.; Ballauff, A.; Koch, I.; Lohse, P.; Osterrieder, S.; Kappler, R.; Koletzko, S. Association of the interleukin-23 receptor gene variant rs11209026 with Crohn’s disease in German children. Acta Paediatr. 2010, 99, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.; Konrad, A.; Schmechel, S.; Dambacher, J.; Seiderer, J.; Schroff, F.; Wetzke, M.; Roeske, D.; Torok, H.P.; Tonenchi, L.; et al. The ATG16L1 gene variants rs2241879 and rs2241880 (T300A) are strongly associated with susceptibility to Crohn’s disease in the German population. Am. J. Gastroenterol. 2008, 103, 682–691. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- NW, H. Hepatocyte nuclear factor- 4alpha regulates expression of the serotonin transporter in intestinal epithelial cells. Physiology 2020, 6, 1294–1304. [Google Scholar] [CrossRef]
- Malik, T.A. Inflammatory Bowel Disease. Surg. Clin. N. Am. 2015, 95, 1105–1122. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Thomas, S.; Przesdzing, I.; Metzke, D.; Bielecki, C.; Lehmann, S.M.; Lehnardt, S.; Dorffel, Y.; Sturm, A.; Scheffold, A.; et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin. Exp. Immunol. 2009, 157, 423–436. [Google Scholar] [CrossRef]
- Mercado, C.P. Molecular mechanisms of SERT in platelets: Regulation of Plasma Serotonin Levels. Mol. Interv. 2010, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S. Characterization of serotonin signaling components in patients with inflammatory bowel disease. J. Can. Assoc. Gastroenterol. 2018, 20, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.L.; Moya, P.R. Human serotonin transporter gene (SLC6A4) variants: Their contributions to understanding pharmacogenomic and other functional GxG and GxE differences in health and disease. Curr. Opin. Pharmacol. 2011, 11, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Miron, N.; Cristea, V. Enterocytes: Active cells in tolerance to food and microbial antigens in the gut. Clin. Exp. Immunol. 2012, 167, 405–412. [Google Scholar] [CrossRef]
- Krstic Ristivojevic, M.; Apostolovic, D.; Smiljanic, K. Enterocytes in Food Hypersensitivity Reactions. Animals 2021, 11, 2713. [Google Scholar] [CrossRef]
- Fusunyan, R.D. Evidence for an innate immune response in the immature human intestine: Toll-like receptors of fetal enterocytes. Pediatr. Res. 2001, 49, 589–593. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holly, M.K.; Smith, J.G. Paneth Cells during Viral Infection and Pathogenesis. Viruses 2018, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Diwakarla, S.; Fothergill, L.J.; Fakhry, J.; Callaghan, B.; Furness, J.B. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2017, 29, e13101. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Ivanov, I.I. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol. Cell Biol. 2013, 91, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Canny, G. Interactions of Intestinal Epithelial Cells with bacteria and Immune cells. Cell-Cell Interact. Methods Protoc. 2006, 341, 17–35. [Google Scholar] [CrossRef]
- Bekiaris, V. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol. Rev. 2014, 260, 86–101. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Fleissner, D.; Hansen, W.; Buer, J. T cells, dendritic cells and epithelial cells in intestinal homeostasis. Int. J. Med. Microbiol. IJMM 2010, 300, 11–18. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. J. Med. Investig. 2015, 63, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharmacother. 2021, 141, 111817. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Jorg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. CMLS 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Sawaki, E.; Koga, Y.; Benno, Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Front. Syst. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.B.; Witte, A.B. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 2008, 193, 311–323. [Google Scholar] [CrossRef]
- Louwies, T.; Johnson, A.C.; Orock, A.; Yuan, T.; Greenwood-Van Meerveld, B. The microbiota-gut-brain axis: An emerging role for the epigenome. Exp. Biol. Med. 2020, 245, 138–145. [Google Scholar] [CrossRef]
- Weltens, N.; Iven, J.; Van Oudenhove, L.; Kano, M. The gut-brain axis in health neuroscience: Implications for functional gastrointestinal disorders and appetite regulation. Ann. N. Y. Acad. Sci. 2018, 1428, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, J.R. The Brain-Gut Team. Dig. Dis. 2020, 38, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Schatz, R.A.; Moshiree, B. Gastrointestinal and Hepatic Disease in Fibromyalgia. Rheum. Dis. Clin. N. Am. 2018, 44, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Bear, T.; Dalziel, J.; Coad, J.; Roy, N.; Butts, C.; Gopal, P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 2021, 9, 723. [Google Scholar] [CrossRef]
- Ancona, A.; Petito, C.; Iavarone, I.; Petito, V.; Galasso, L.; Leonetti, A.; Turchini, L.; Belella, D.; Ferrarrese, D.; Addolorato, G.; et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 298–305. [Google Scholar] [CrossRef]
- Bhattarai, Y. Irritable Bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 312, G52–G62. [Google Scholar] [CrossRef]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflamm. 2021, 18, 135. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Tack, T. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut 2000, 46, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef] [PubMed]
- Fiorica-Howells, E. Serotonin and the 5-HT2B receptor in the Development of enteric neurons. J. Neurosci. 2000, 20, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendig, D.M.; Grider, J.R. Serotonin and colonic motility. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2015, 27, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S. Recent advances in 5-hydroxitryptamine (5-HT) receptor research: How many pathophysiological roles does 5-HT play via its multiiple receptor subtypes? Biol. Pharm. Bull 2013, 36, 1406–1409. [Google Scholar] [CrossRef] [Green Version]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Galligan, J.J. Colonic 5-HT4 receptors are targets for novel prokinetic drugs. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2021, 33, e14125. [Google Scholar] [CrossRef]
- Konen, J.R.; Haag, M.M.; Guseva, D.; Hurd, M.; Linton, A.A.; Lavoie, B.; Kerrigan, C.B.; Joyce, E.; Bischoff, S.C.; Swann, S.; et al. Prokinetic actions of luminally acting 5-HT4 receptor agonists. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2021, 33, e14026. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [Green Version]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Nakamura, M. The human serotonin transporter gene linked polymporphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry 2000, 5, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, T.N.; Christensen, P.M.; Gether, U. Serotonin-induced down-regulation of cell surface serotonin transporter. Neurochem. Int. 2014, 73, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layunta, E.; Latorre, E.; Forcen, R.; Grasa, L.; Castro, M.; Arias, M.A.; Alcalde, A.I.; Mesonero, J.E. NOD2 Modulates Serotonin Transporter and Interacts with TLR2 and TLR4 in Intestinal Epithelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.D. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc. Natl. Acad. Sci. USA 1992, 89, 10993–10997. [Google Scholar] [CrossRef] [Green Version]
- Hagan, C.E.; Schenk, J.O.; Neumaier, J.F. The contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes. Synapse 2011, 65, 1015–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, P.W.; Bosyj, C.; Brenton, L.; Green, L.; Gasser, P.J.; Lowry, C.A.; Pickel, V.M. All the brain’s a stage for serotonin: The forgotten story of serotonin diffusion across cell membranes. Proc. Biol. Sci. 2022, 289, 20221565. [Google Scholar] [CrossRef]
- Grunst, A.S.; Grunst, M.L.; Staes, N.; Thys, B.; Pinxten, R.; Eens, M. Serotonin transporter (SERT) polymorphisms, personality and problem-solving in urban great tits. Sci. Rep. 2021, 11, 24270. [Google Scholar] [CrossRef]
- Schurks, M.; Rist, P.M.; Kurth, T. STin2 VNTR polymorphism in the serotonin transporter gene and migraine: Pooled and meta-analyses. J. Headache Pain 2010, 11, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Pizzo de Castro, M.R.; Vargas Nunes, S.O.; Guembarovski, R.L.; Ariza, C.B.; Oda, J.M.; Vargas, H.O.; Piccoli de Melo, L.G.; Watanabe, M.A.; Berk, M.; Maes, M. STin2 VNTR polymorphism is associated with comorbid tobacco use and mood disorders. J. Affect. Disord. 2015, 172, 347–354. [Google Scholar] [CrossRef]
- Wendland, J.R. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr. Genet. 2006, 18, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hildonen, M.; Levy, A.M.; Dahl, C.; Bjerregaard, V.A.; Birk Moller, L.; Guldberg, P.; Debes, N.M.; Tumer, Z. Elevated Expression of SLC6A4 Encoding the Serotonin Transporter (SERT) in Gilles de la Tourette Syndrome. Genes 2021, 12, 86. [Google Scholar] [CrossRef]
- Ravić, K.G.; Paić, F.; Vucelić, B.; Brinar, M.; Čuković-Čavka, S.; Božina, N.; Krznarić, Ž.; Kalauz, M.; Bešić, D.; Martić, T.N. Association of polymorphic variants in serotonin re-uptake transporter gene with Crohn’s disease: A retrospective case-control study. Croat. Med. J. 2018, 59, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Wang, L.; Yu, B.; Li, Q.; Dong, X. Association between polymorphisms in the serotonin transporter gene-linked polymorphic region and risk for irritable bowel syndrome in China: Evidence based on a meta-analysis. J. Int. Med. Res. 2019, 47, 2810–2818. [Google Scholar] [CrossRef]
- Sikander, A.; Sinha, S.K.; Prasad, K.K.; Rana, S.V. Association of Serotonin Transporter Promoter Polymorphism (5-HTTLPR) with Microscopic Colitis and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 887–894. [Google Scholar] [CrossRef]
- Gyawali, S.; Subaran, R.; Weissman, M.M.; Hershkowitz, D.; McKenna, M.C.; Talati, A.; Fyer, A.J.; Wickramaratne, P.; Adams, P.B.; Hodge, S.E.; et al. Association of a polyadenylation polymorphism in the serotonin transporter and panic disorder. Biol. Psychiatry 2010, 67, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Kang, C.; Wang, M.; Wang, Q.; Li, P.; Liu, H.; Hou, Y.; Su, P.; Yang, F.; Wei, Y.; et al. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS ONE 2014, 9, e84414. [Google Scholar] [CrossRef] [PubMed]
- Sangrajrang, S.; Sato, Y.; Sakamoto, H.; Ohnami, S.; Khuhaprema, T.; Yoshida, T. Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: A case–control study in Thai women. Breast Cancer Res. Treat. 2010, 123, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, C.; He, Q.; Chen, C.; Moyzis, R.K.; Xue, G.; Chen, X.; Cao, Z.; Li, J.; Li, H.; et al. Sex determines which section of the SLC6A4 gene is linked to obsessive-compulsive symptoms in normal Chinese college students. J. Psychiatr. Res. 2012, 46, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Hui, P.; Yang, J.; Wang, J.; Zhao, L.; Wang, X.; Su, X.; Wang, J.; Ma, W.; Fan, J.; Chen, W.; et al. Association between 5-hydroxytryptamine gene polymorphism rs140700 and primary insomnia in Chinese population. Intern. Med. J. 2021, 51, 732–738. [Google Scholar] [CrossRef]
- Xu, F.L.; Wang, B.J.; Yao, J. Association between the SLC6A4 gene and schizophrenia: An updated meta-analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 143–155. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Wang, Y.; Wang, X.M.; Qiu, Y.; Long, J.F.; Zhang, S.P. Association of 5-HTT gene polymorphisms with migraine: A systematic review and meta-analysis. J. Neurol. Sci. 2011, 305, 57–66. [Google Scholar] [CrossRef]
- Savas, S. Serotonin Transporter gene (SLC6A4) variations are associated with poor survival in Colorectal Cancer patients. PLoS ONE 2012, 7, e38953. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Ren, D.; Guo, Z.; Ma, G.; Xu, F.; Chen, Z.; An, L.; Zhang, N.; Ji, L.; Yuan, F.; et al. Influence and interaction of genetic, cognitive, neuroendocrine and personalistic markers to antidepressant response in Chinese patients with major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110036. [Google Scholar] [CrossRef]
- Ma, D.Q.; Rabionet, R.; Konidari, I.; Jaworski, J.; Cukier, H.N.; Wright, H.H.; Abramson, R.K.; Gilbert, J.R.; Cuccaro, M.L.; Pericak-Vance, M.A.; et al. Association and gene-gene interaction of SLC6A4 and ITGB3 in autism. Am. J. Med. Genetics. Part B Neuropsychiatr. Genet. 2010, 153B, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, N.N.; Iwayama, Y.; Koshy, L.V.; Natarajan, C.; Nair, C.; Allencherry, P.M.; Yoshikawa, T.; Banerjee, M. Evidence of association of serotonin transporter gene polymorphisms with schizophrenia in a South Indian population. J. Hum. Genet. 2009, 54, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Hasler, W.L. Serotonin and the GI tract. Curretn Gastroenterol. Rep. 2009, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Goldner, D.; Margolis, K.G. Association of Serotonin Transporter Promoter Polymorphism (5HTTLPR) with Microscopic Colitis and Ulcerative Colitis: Time to Be AsSERTive? Dig. Dis. Sci. 2015, 60, 819–821. [Google Scholar] [CrossRef] [Green Version]
- Ikegame, T.; Hidaka, Y.; Nakachi, Y.; Murata, Y.; Watanabe, R.; Sugawara, H.; Asai, T.; Kiyota, E.; Saito, T.; Ikeda, M.; et al. Identification and functional characterization of the extremely long allele of the serotonin transporter-linked polymorphic region. Transl. Psychiatry 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, J.R.; Russo, P.; Sullivan, K.E. Early-Onset Inflammatory Bowel Disease. Immunol. Allergy Clin. N. Am. 2019, 39, 63–79. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio-Med. Atenei Parm. 2018, 89, 60–75. [Google Scholar] [CrossRef]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L. Perianal Crohn’s Disease. Dis. Colon Rectum 2020, 63, 1023–1026. [Google Scholar] [CrossRef]
- Gheita, T.A.; El, G., II; El-Fishawy, H.S.; Aboul-Ezz, M.A.; Kenawy, S.A. Involvement of IL-23 in enteropathic arthritis patients with inflammatory bowel disease: Preliminary results. Clin. Rheumatol. 2014, 33, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Colombel, J.F.; Sandborn, W.J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 2011, 17, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef]
- Kuznicki, P.; Kempinski, R.; Neubauer, K. The emerging role of mood disorders in inflammatory bowel diseases. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2020, 29, 1505–1510. [Google Scholar] [CrossRef]
- Hatamnejad, M.R.; Baradaran Ghavami, S.; Shirvani, M.; Asghari Ahmadabad, M.; Shahrokh, S.; Farmani, M.; Sherkat, G.; Asadzadeh Aghdaei, H.; Zali, M.R. Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front. Immunol. 2022, 13, 980189. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pavlidis, P.; Norton, C.; Norton, S.; Pariante, C.; Hayee, B.; Powell, N. Depressive symptoms in inflammatory bowel disease: An extraintestinal manifestation of inflammation? Clin. Exp. Immunol. 2019, 197, 308–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, C.W.P.; Powell, N.; Norton, C.; Dumbrill, J.L.; Hayee, B.; Moulton, C.D. Cognitive Impairment in Adult Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Acad. Consult.-Liaison Psychiatry 2021, 62, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Hugot, J.-P. Association of NOD-2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of inflammatory bowel disease: Beyond NOD2. Lancet. Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.L.; Shao, B.Z.; Zhao, S.B.; Chang, X.; Wang, P.; Miao, C.Y.; Li, Z.S.; Bai, Y. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis. 2019, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Festen, E.A.; Goyette, P.; Green, T.; Boucher, G.; Beauchamp, C.; Trynka, G.; Dubois, P.C.; Lagace, C.; Stokkers, P.C.; Hommes, D.W.; et al. A meta-analysis of genome-wide association scans identifies IL18RAP, PTPN2, TAGAP, and PUS10 as shared risk loci for Crohn’s disease and celiac disease. PLoS Genet. 2011, 7, e1001283. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Hegarty, J.P.; Berg, A.; Kelly, A.A.; Wang, Y.; Poritz, L.S.; Franke, A.; Schreiber, S.; Koltun, W.A.; Lin, Z. PTPN2 is associated with Crohn’s disease and its expression is regulated by NKX2-3. Dis. Mrk. 2012, 32, 83–91. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Parmentier-Decrucq, E.; Branche, J.; Desreumaux, P. IL-23R, a novel susceptibility gene for inflammatory bowel disease. Med. Sci. M/S 2007, 23, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Castro-Santos, P.; Suarez, A.; Lopez-Rivas, L.; Mozo, L.; Gutierrez, C. TNFalpha and IL-10 gene polymorphisms in inflammatory bowel disease. Association of -1082 AA low producer IL-10 genotype with steroid dependency. Am. J. Gastroenterol. 2006, 101, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meddens, C.A.; Harakalova, M.; van den Dungen, N.A.; Foroughi Asl, H.; Hijma, H.J.; Cuppen, E.P.; Bjorkegren, J.L.; Asselbergs, F.W.; Nieuwenhuis, E.E.; Mokry, M. Systematic analysis of chromatin interactions at disease associated loci links novel candidate genes to inflammatory bowel disease. Genome Biol. 2016, 17, 247. [Google Scholar] [CrossRef] [Green Version]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Peters, C.P.; Mjosberg, J.M.; Bernink, J.H.; Spits, H. Innate lymphoid cells in inflammatory bowel diseases. Immunol. Lett. 2016, 172, 124–131. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharm. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermudez-Humaran, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Glover, S.C. Innate Lymphoid Cells in Inflammatory Bowel Disease. Arch. Immunol. Ther. Exp. 2018, 66, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic infections modulate intestinal serotonin transporter (SERT) function by activating Toll-like receptor 2 (TLR-2) in Crohn’s disease. Sci. Rep. 2021, 11, 22624. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Manzella, C.; Soni, V.; Alrefai, W.A.; Saksena, S.; Hecht, G.A.; Dudeja, P.K.; Gill, R.K. Role of SHP2 protein tyrosine phosphatase in SERT inhibition by enteropathogenic E. coli (EPEC). Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G443–G449. [Google Scholar] [CrossRef] [Green Version]
- Esmaili, A.; Nazir, S.F.; Borthakur, A.; Yu, D.; Turner, J.R.; Saksena, S.; Singla, A.; Hecht, G.A.; Alrefai, W.A.; Gill, R.K. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 2009, 137, 2074–2083. [Google Scholar] [CrossRef] [Green Version]
- Latorre, E.; Pradilla, A.; Chueca, B.; Pagan, R.; Layunta, E.; Alcalde, A.I.; Mesonero, J.E. Listeria monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells. Microb. Ecol. 2016, 72, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef]
- Cao, Y.N.; Feng, L.J.; Wang, B.M.; Jiang, K.; Li, S.; Xu, X.; Wang, W.Q.; Zhao, J.W.; Wang, Y.M. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2018, 24, 59–66. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatcroft, J.; Wakelin, D.; Smith, A.; Mahoney, C.R.; Mawe, G.; Spiller, R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2005, 17, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, C. Lypopolysaccharide inducces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun. 2009, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef]
- Luo, W.; Tian, L.; Tan, B.; Shen, Z.; Xiao, M.; Wu, S.; Meng, X.; Wu, X.; Wang, X. Update: Innate Lymphoid Cells in Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 67, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Che, X.; Kwak, M.S.; Kim, S.; Kim, J.H.; Ma, H.W.; Kim, D.H.; Kim, T.I.; Kim, W.H.; Kim, S.W.; et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci. Rep. 2017, 7, 851. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, A. Escherichia Coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 8751. [Google Scholar] [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Zhou, G.X. Potential roles of neutrophils in regulating intestinal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Lukas, D.; Clausen, B.E.; Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2017, 39, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Chaparro, M.; Gisbert, J.P. Human Intestinal Dendritic Cells in Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2018, 62, e1700931. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A. Intestinal dendritic cells and epithelial barrier dysfunction in Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Ballester Ferre, M.P.; Bosca-Watts, M.M.; Minguez Perez, M. Crohn’s disease. Med. Clin. 2018, 151, 26–33. [Google Scholar] [CrossRef]
- Okazawa, A. Th1-mediated intestinal inflammation in Crohn’s disease may be induced by activation of lamina propria lymphocytes through synergistic stimulation of interleukin-12 and interleukin-18 without T cel receptor engagement. Am. J. Gastroenterol. 2002, 97, 3108–3117. [Google Scholar] [CrossRef]
- Sedda, S.; Bevivino, G.; Monteleone, G. Targeting IL-23 in Crohn’s disease. Expert Rev. Clin. Immunol. 2018, 14, 907–913. [Google Scholar] [CrossRef]
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 Pathway in Crohn’s Disease. Front. Immunol. 2021, 12, 622934. [Google Scholar] [CrossRef]
- Ogino, H.; Fukaura, K.; Iboshi, Y.; Nagamatsu, Y.; Okuno, H.; Nishioka, K.; Nishihara, Y.; Tanaka, Y.; Chinen, T.; Ihara, E.; et al. Role of the IL-23-T-bet/GATA3 Axis for the Pathogenesis of Ulcerative Colitis. Inflammation 2021, 44, 592–603. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Burgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Mannon, P.; Reinisch, W. Interleukin 13 and its role in gut defence and inflammation. Gut 2012, 61, 1765–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzi, A.; Frohlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurother. J. Am. Soc. Exp. NeuroTher. 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filaretova, L. The realization of the brain-gut interactions with corticotropin-releasing factor and glucocorticoids. Curr. Neuropharmacol. 2016, 14, 876–881. [Google Scholar] [CrossRef] [Green Version]
- de Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Haub, S.; Ritze, Y.; Bergheim, I.; Pabst, O.; Gershon, M.D.; Bischoff, S.C. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 826–834.e229. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.H. Osteoporosis and Osteoporotic Fractures in Gastrointestinal Disease. J. Bone Metab. 2018, 25, 213–217. [Google Scholar] [CrossRef]
- Lavoie, B.; Roberts, J.A.; Haag, M.M.; Spohn, S.N.; Margolis, K.G.; Sharkey, K.A.; Lian, J.B.; Mawe, G.M. Gut-derived serotonin contributes to bone deficits in colitis. Pharmacol. Res. 2019, 140, 75–84. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Thorkelson, G.; Bielefeldt, K.; Szigethy, E. Empirically Supported Use of Psychiatric Medications in Adolescents and Adults with IBD. Inflamm. Bowel Dis. 2016, 22, 1509–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Gardier, A.M. The pharmacological basis of the serotonin system: Application to antidepressant response. L’Encephale 2016, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2018, 68, 1606–1612. [Google Scholar] [CrossRef]
- Hall, B.J.; Hamlin, P.J.; Gracie, D.J.; Ford, A.C. The Effect of Antidepressants on the Course of Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2047242. [Google Scholar] [CrossRef] [Green Version]
- Goodhand, J.R.; Greig, F.I.; Koodun, Y.; McDermott, A.; Wahed, M.; Langmead, L.; Rampton, D.S. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm. Bowel Dis. 2012, 18, 1232–1239. [Google Scholar] [CrossRef]
- Blackwell, J.; Alexakis, C.; Saxena, S.; Creese, H.; Bottle, A.; Petersen, I.; Hotopf, M.; Pollok, R.C.G. Association between antidepressant medication use and steroid dependency in patients with ulcerative colitis: A population-based study. BMJ Open Gastroenterol. 2021, 8, e000588. [Google Scholar] [CrossRef]
- Coates, M.D.; Johnson, A.C.; Greenwood-Van Meerveld, B.; Mawe, G.M. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2006, 18, 464–471. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Prady, S.L.; Pollok, J.; Esterman, A.J.; Gordon, A.L.; Knowles, S.; Andrews, J.M. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 4, CD012680. [Google Scholar] [CrossRef]
- Koh, S.-J. Fluoxetine inhibits NF-kB signaling in intestinal epithelial cells and ameliorates colitis and colitis-associated colon cancer in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 301, G9–G19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Chen, P.; Tang, Y.; Zhang, C.; Lei, N.; Luo, Y.; Duan, S.; Zhang, Y. Venlafaxine as an Adjuvant Therapy for Inflammatory Bowel Disease Patients With Anxious and Depressive Symptoms: A Randomized Controlled Trial. Front. Psychiatry 2022, 13, 880058. [Google Scholar] [CrossRef] [PubMed]
- Daghaghzadeh, H.; Naji, F.; Afshar, H.; Sharbafchi, M.R.; Feizi, A.; Maroufi, M.; Tabatabaeeyan, M.; Adibi, P.; Tavakoli, H. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: A double-blind controlled study. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Kristensen, M.S.; Kjærulff, T.M.; Ersbøll, A.K.; Green, A.; Hallas, J.; Thygesen, L.C. The influence of antidepressants on the disease course among patients with Crohn’s disease and ulcerative colitis—A Danish nationwide register–based Cohort Study. Inflamm. Bowel Dis. 2019, 25, 886–893. [Google Scholar] [CrossRef] [PubMed]




| Genetic Variant | Location | Disease | Effect | References |
|---|---|---|---|---|
| STin2 VNTR | Intron 2 | Migraine Tobacco use disorder | Risk (OR = 1.34; 95% CI: 1.09–1.64) Risk (OR = 3.07; 95% CI: 1.41–6.68) | [88,89] |
| I425V | Transmembrane region 8 | Obsessive–compulsive disorder | Risk (OR = 6.54; 95% CI: 1.7–24.8) | [90] |
| 5-HTTLPR | Promoter | Irritable bowel syndrome GTS Crohn’s disease MC and Ulcerative Colitis | Risk (Allele S; OR = 1.36) Risk (S/S; OR = 1.5; 95% CI: 0.8–2.98) Risk (L; OR = 0.9; 95% CI: 0.4–2.0) Higher levels of serotonin (p < 0.01) * | [91,92,93,94] |
| rs3813034 | 3′ UTR | Panic disorder | Risk (OR = 1.44; 95% CI: 1.13–1.85) | [95] |
| rs3794808 | Intron | Irritable bowel syndrome | No significant association * | [96] |
| rs140701 | Intron | Breast cancer | Risk (OR = 1.56; 95% CI: 1.01–2.41) | [97] |
| rs4583306 | Intron | Obsessive–compulsive symptoms | Relation with cleanliness dimension (p = 0.004) * | [98] |
| rs140700 | Intron | Primary insomnia Schizophrenia | Not risk factor (OR = 1.32; 95% CI: 0.49–3.55) No association * | [99,100] |
| rs2020942 | Intron | Migraine | No significant association (OR = 1.09; 95% CI: 0.82–1.44) | [101] |
| rs12150214 | Intron | Colorectal cancer Antidepressant response | Shorter overall survival (OR = 1.57; 95% CI: 1.14–2.16) Poorer response to fluoxetine (OR = 4.24; 95% CI: 1.39–12.98) | [102,103] |
| rs2066713 | Intron | Autism Schizophrenia | No significant association * Significant association (p < 0.001)* | [104,105] |
| Gene | Locus | Effect | Reference |
|---|---|---|---|
| NOD2 | 16q12.1 | IBD increased risk | [13,14,123,126] |
| ATG16L1 | 2q37.1 | Impaired intracellular bacteria clearance in IBD, intestinal autophagy | [13,16,127,128] |
| PTPN2 | 18p11.21 | IBD increased risk | [13,129,130] |
| IL-23R | 1p31.3 | IBD susceptibility, Crohn’s disease risk | [13,15,131] |
| IL-10 | 1q32.1 | IBD steroid dependency, early onset IBD | [13,132,133] |
| HNF4α | 20q13.12 | IBD susceptibility | [13,18,134] |
| Bacteria | Mechanism | Model | References |
|---|---|---|---|
| Enteropathogenic E. coli | Activation of protein tyrosine phosphatase, a process that leads to SERT inhibition | Caco-2 cells infected with E. coli | [146,147] |
| Listeria monocytogenes | Reduced SERT expression related to a transcriptional change in TLR10 and TLR2 | Caco-2/TC7 cells infected with Listeria monocytogenes | [148] |
| Akkermansia muciniphila | Interaction between activated TLR2 and SERT causes reduced SERT expression | Caco-2 cells infected with Akkermansia muciniphila | [149] |
| Lactobacillus acidophilus | Up-regulation of SERT mRNA | Lactobacillus acidophilus and B. longum interaction with HT-29 and Caco-2 cells | [150] |
| Lactobacillus rhamnosus | SERT Gene and protein up-regulation | Wistar rats implemented with probiotics and prebiotics | [151] |
| Campylobacter jejuni | EC hyperplasia and reduced SERT expression | C57BL/6 mice infected with T. Spiralis and C. jejuni | [152] |
| Salmonella typhimurium | Inhibition of SERT by TLR4 activation | Mice and Caco-2 cells infected with S. typhimurium | [153,154] |
| Type of study | Results | Reference |
|---|---|---|
| Review | Antidepressants are highly used for depression and anxiety problems in IBD, even though gut side-effects are questionable | [182] |
| Retrospective | Antidepressants showed a protective role over IBD | [185] |
| Review | Useful effects: anti-inflammatory properties, immune regulation | [120] |
| Retrospective | Increased risk of corticosteroid dependency after long-term SSRI intake | [188] |
| In vivo | Decreased stool output, delayed transit, and attenuated colonic sensitivity related with paroxetine intake | [189] |
| Longitudinal | Antidepressants predispose lower medical therapy escalation | [186] |
| Review | The results for the outcomes are uncertain | [190] |
| In vitro | Fluoxetine inhibited NF-κβ and up-regulated expression of IL-8 in COLO 205 colon epithelial cells stimulated with TNF-α | [191] |
| Prospective, randomized, double-blind, and placebo-controlled clinical trial | Venlafaxine reduced TNF-α levels in patients with IBD | [192] |
| Double-blind | Duloxetine can be used as a therapy for reducing depression, anxiety, and severity of physical symptoms | [193] |
| Population-based cohort study. Prospectively collected data | Patients with IBD and a 180-day antidepressant therapy showed lower relapse rates, hospitalization, and less risk of initiating anti-TNF therapy | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Delgado, S.; Garza-Veloz, I.; Trejo-Vazquez, F.; Martinez-Fierro, M.L. Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 15632. https://doi.org/10.3390/ijms232415632
González Delgado S, Garza-Veloz I, Trejo-Vazquez F, Martinez-Fierro ML. Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2022; 23(24):15632. https://doi.org/10.3390/ijms232415632
Chicago/Turabian StyleGonzález Delgado, Samantha, Idalia Garza-Veloz, Fabiola Trejo-Vazquez, and Margarita L Martinez-Fierro. 2022. "Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease" International Journal of Molecular Sciences 23, no. 24: 15632. https://doi.org/10.3390/ijms232415632







