Glycogen Synthase Kinase 3β Is a Key Regulator in the Inhibitory Effects of Accumbal Cocaine- and Amphetamine-Regulated Transcript Peptide 55–102 on Amphetamine-Induced Locomotor Activity
Abstract
1. Introduction
2. Results
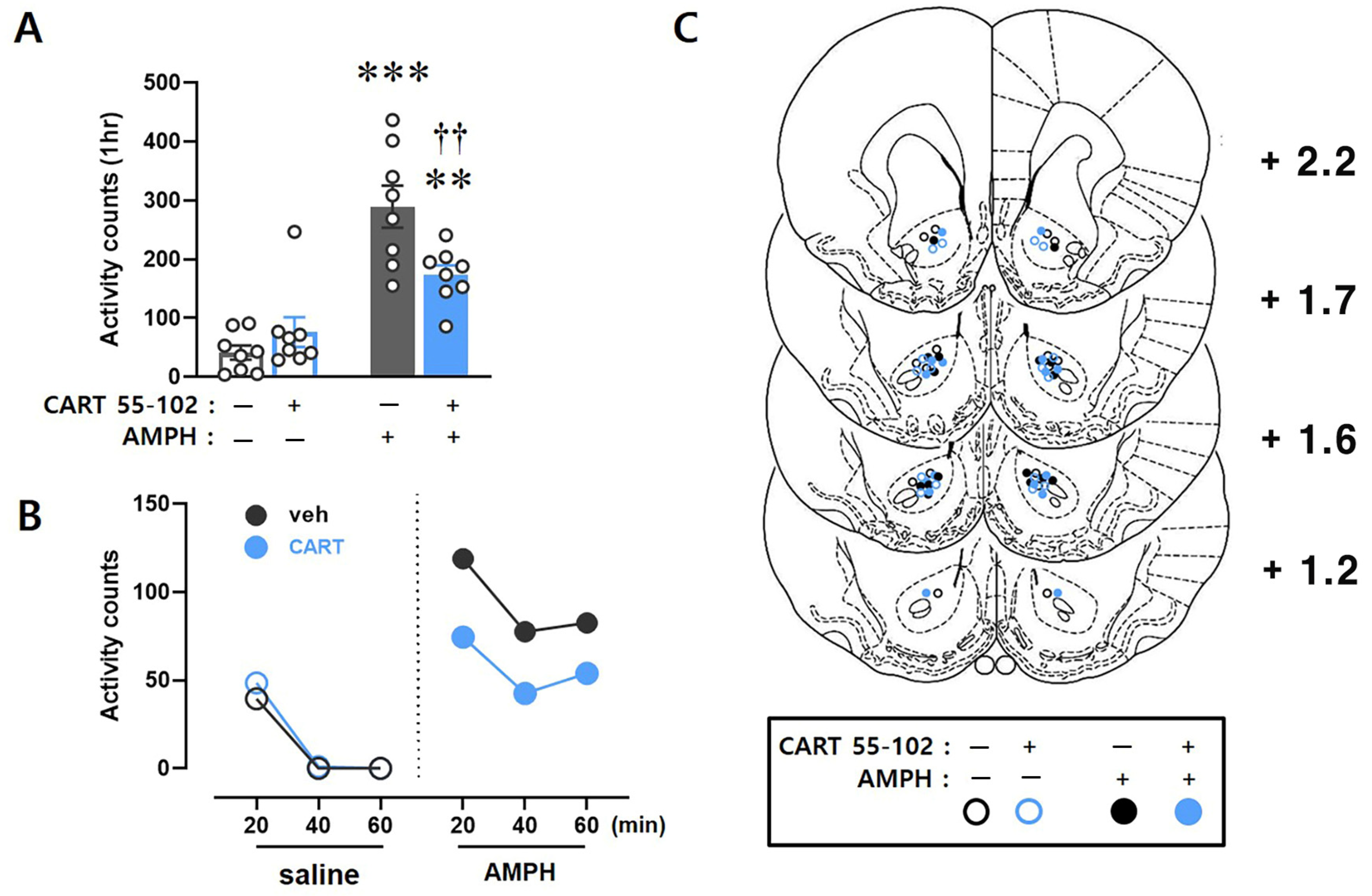
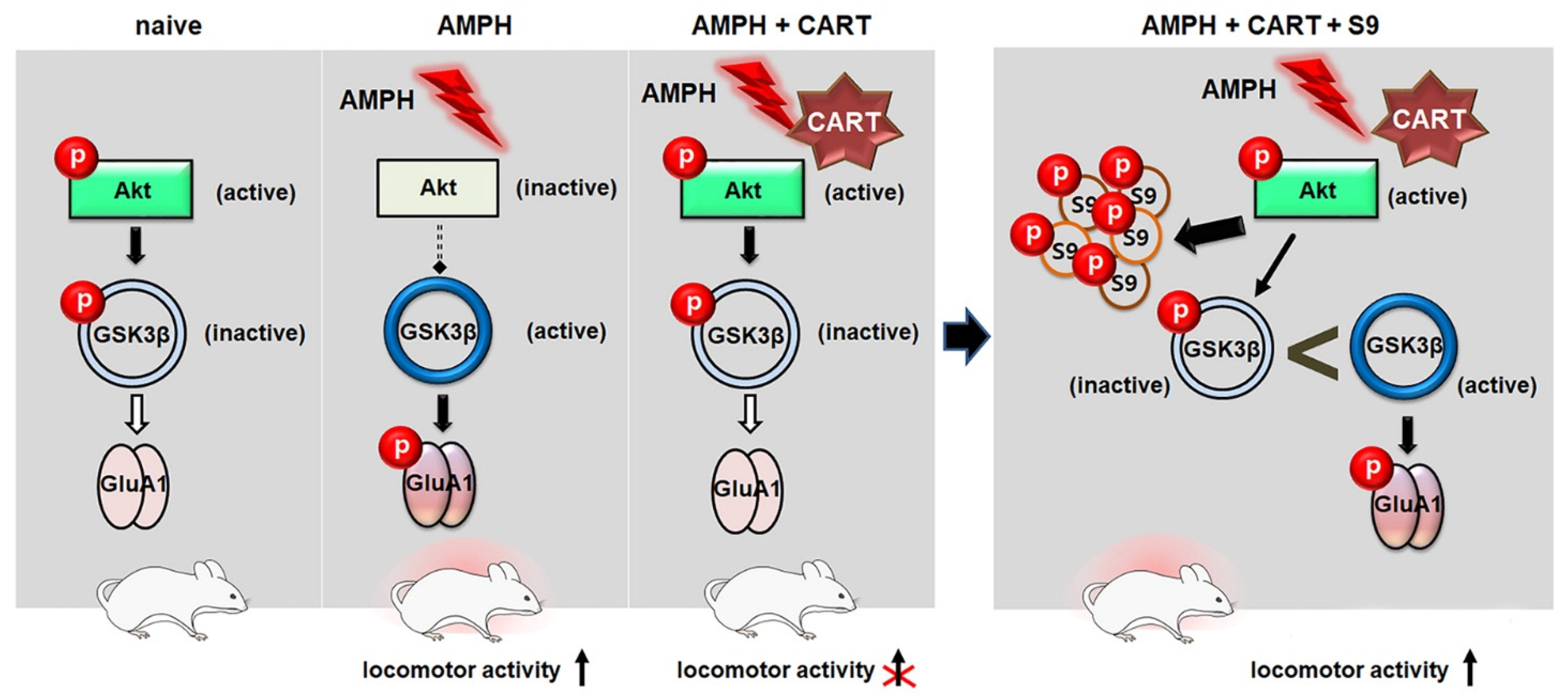
2.1. CART Peptide 55–102 in the NAcc Core Blocks the AMPH-Induced Hyperlocomotor Activity
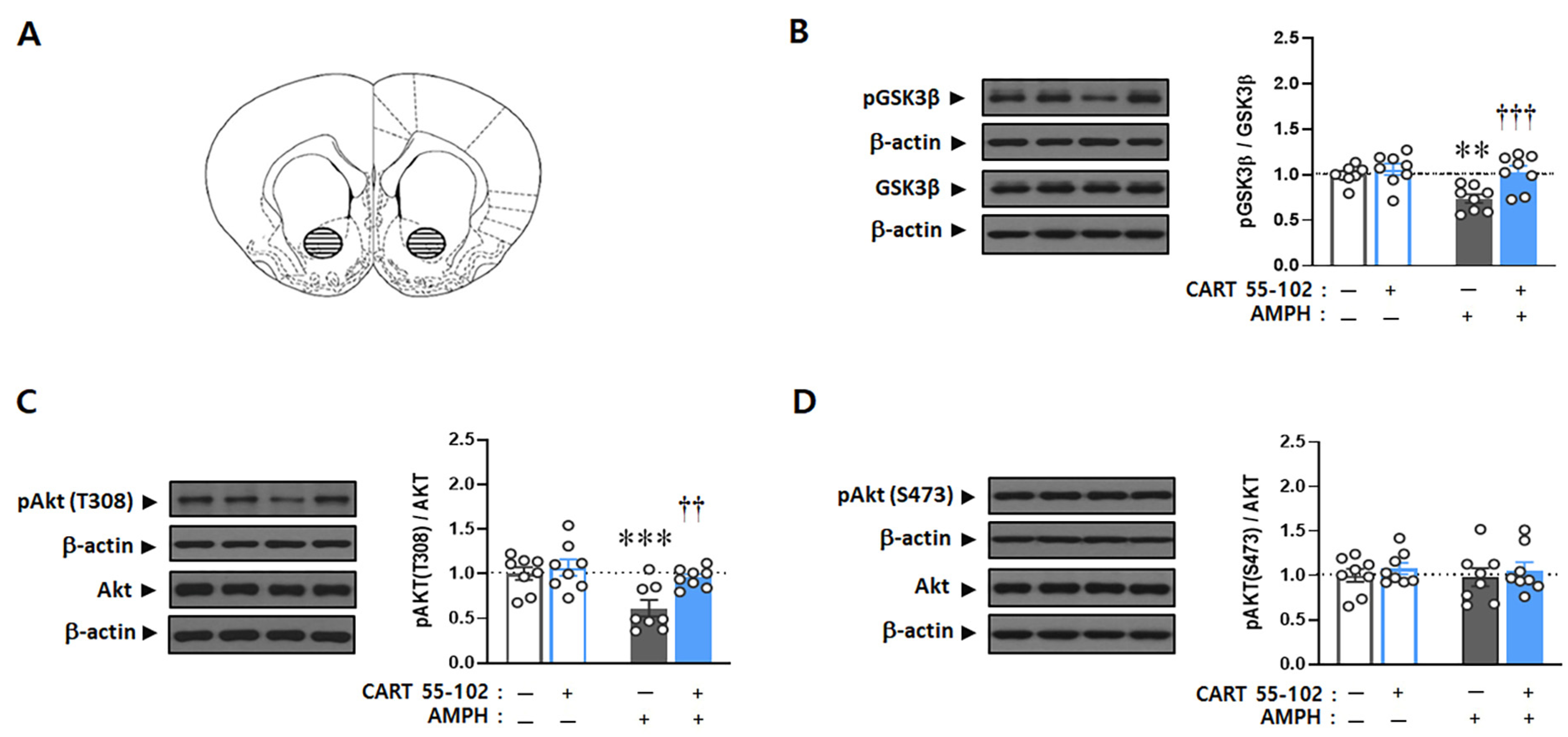
2.2. CART Peptide 55–102 in the NAcc Core Inhibits the AMPH-Induced Decreases in pAkt (T308) and pGSK3β
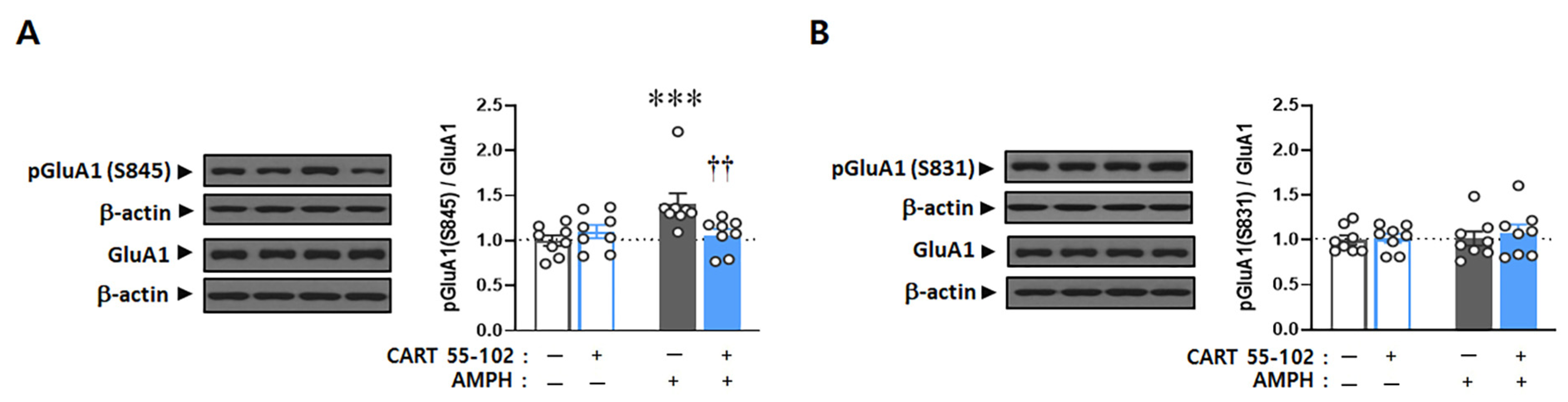
2.3. CART Peptide 55–102 in the NAcc Core Inhibits the AMPH-Induced Increases in pGluA1 (S845) but Not pGluA1 (S831)
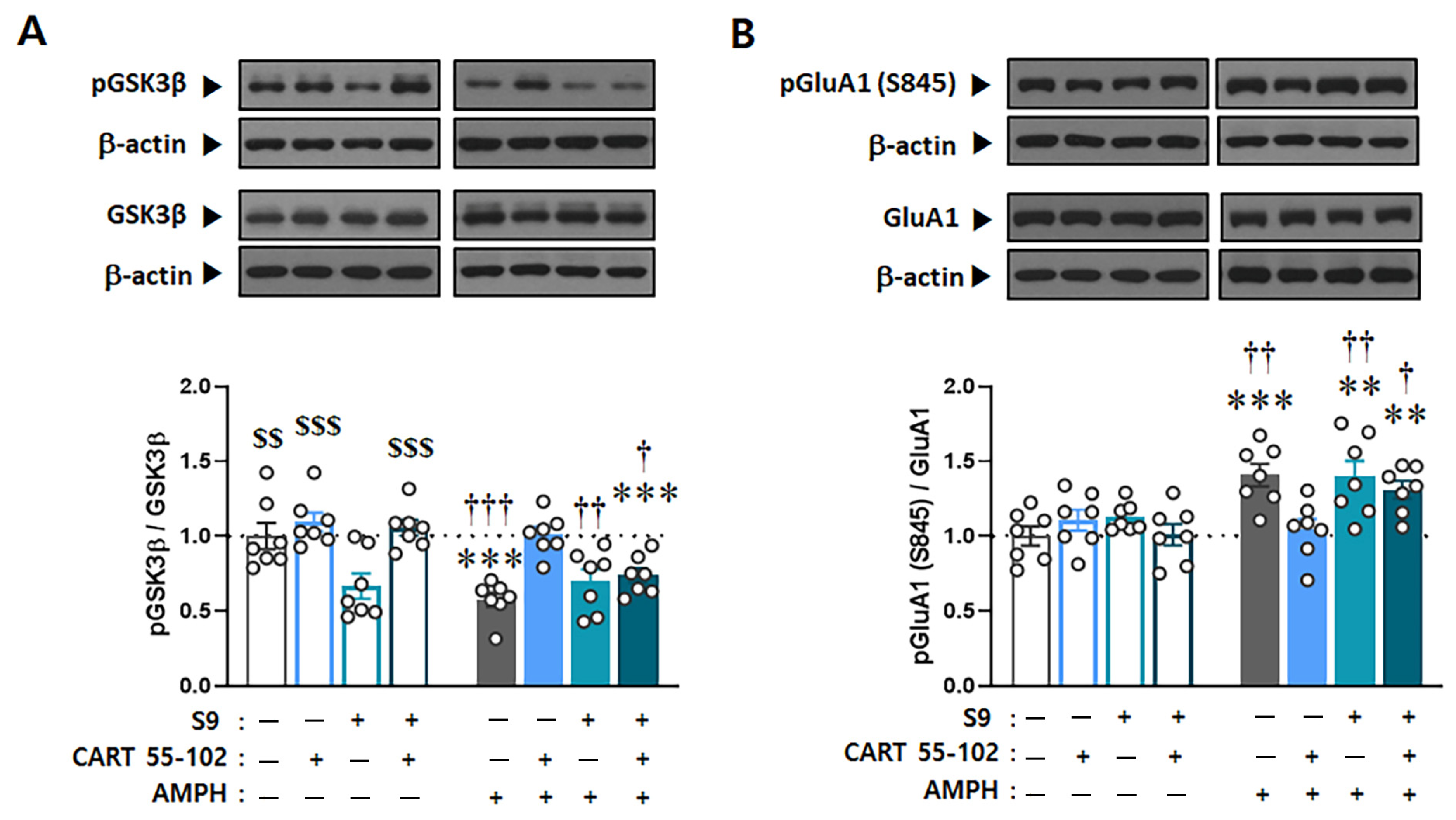
2.4. Interruption to the Akt-GSK3β Signaling in the NAcc Core Abolishes Inhibitory Effects of CART Peptide 55–102 on AMPH
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Drug and Peptides
4.3. Surgery
4.4. Intracranial Microinjection
4.5. Locomotor Activity
4.6. Brain Tissue Preparation
4.7. Western Blot
4.8. Design and Procedures
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Couceyro, P.R.; Koylu, E.O.; Kuhar, M.J. Further studies on the anatomical distribution of CART by in situ hybridization. J. Chem. Neuroanat. 1997, 12, 229–241. [Google Scholar] [CrossRef]
- Koylu, E.O.; Couceyro, P.R.; Lambert, P.D.; Kuhar, M.J. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J. Comp. Neurol. 1998, 391, 115–132. [Google Scholar] [CrossRef]
- Ong, Z.Y.; McNally, G.P. CART in energy balance and drug addiction: Current insight and mechanisms. Brain Res. 2020, 1740, 146852. [Google Scholar] [CrossRef]
- Cador, M.; Bjijou, Y.; Stinus, L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience 1995, 65, 385–395. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Perugini, M.; Vezina, P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J. Pharmacol. Exp. Ther. 1994, 270, 690–696. [Google Scholar]
- Koylu, E.O.; Couceyro, P.R.; Lambert, P.D.; Ling, N.C.; DeSouza, E.B.; Kuhar, M.J. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J. Neuroendocrinol. 1997, 9, 823–833. [Google Scholar] [CrossRef]
- Smith, Y.; Koylu, E.O.; Couceyro, P.; Kuhar, M.J. Ultrastructural localization of CART (cocaine- and amphetamine-regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse 1997, 27, 90–94. [Google Scholar] [CrossRef]
- Thim, L.; Kristensen, P.; Nielsen, P.F.; Wulff, B.S.; Clausen, J.T. Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc. Natl. Acad. Sci. USA 1999, 96, 2722–2727. [Google Scholar] [CrossRef]
- Dylag, T.; Kotlinska, J.; Rafalski, P.; Pachuta, A.; Silberring, J. The activity of CART peptide fragments. Peptides 2006, 27, 1926–1933. [Google Scholar] [CrossRef]
- Kim, J.H.; Creekmore, E.; Vezina, P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides 2003, 37, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.N.; Kozel, M.A.; Philpot, K.B.; Kuhar, M.J. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J. Pharmacol. Exp. Ther. 2003, 307, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.N.; Hansen, S.T.; Kuhar, M.J.; Mark, G.P. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav. Brain Res. 2008, 191, 266–271. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, S.; Park, H.K.; Kim, J.H. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology 2007, 53, 344–351. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, W.Y.; Kim, J.H. Microinjection of CART peptide 55-102 into the nucleus accumbens core inhibits the expression of conditioned hyperactivity in a cocaine-associated environment. Behav. Brain Res. 2010, 207, 169–173. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.S.; Kim, J.H. CART peptide 55-102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul. Peptides 2007, 144, 6–9. [Google Scholar] [CrossRef]
- Xiong, L.; Meng, Q.; Sun, X.; Lu, X.; Fu, Q.; Peng, Q.; Yang, J.; Oh, K.W.; Hu, Z. Cocaine- and amphetamine-regulated transcript peptide in the nucleus accumbens shell inhibits cocaine-induced locomotor sensitization to transient over-expression of -Ca2+/calmodulin-dependent protein kinase II. J. Neurochem. 2018, 146, 289–303. [Google Scholar] [CrossRef]
- Yosten, G.L.C.; Harada, C.M.; Haddock, C.; Giancotti, L.A.; Kolar, G.R.; Patel, R.; Guo, C.; Chen, Z.; Zhang, J.; Doyle, T.M.; et al. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J. Clin. Investig. 2020, 130, 2587–2592. [Google Scholar] [CrossRef]
- Yermolaieva, O.; Chen, J.; Couceyro, P.R.; Hoshi, T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J. Neurosci. 2001, 21, 7474–7480. [Google Scholar] [CrossRef]
- Jones, D.C.; Kuhar, M.J. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse 2008, 62, 122–127. [Google Scholar] [CrossRef]
- Somalwar, A.R.; Ghoudhary, A.G.; Sharma, P.R.; Nagalakshmi, B.; Sagarkar, S.; Sakharkar, A.J.; Subhedar, N.K.; Kokare, D.M. Cocaine- and amphetamine-regulated transcript peptide (CART) induced reward behavior is mediated via Gi/o dependent phosphorylation of PKA/ERK/CREB pathway. Behav. Brain Res. 2018, 348, 9–21. [Google Scholar] [CrossRef]
- Hunter, R.G.; Jones, D.; Vicentic, A.; Hue, G.; Rye, D.; Kuhar, M.J. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology 2006, 50, 858–864. [Google Scholar] [CrossRef]
- Jones, D.C.; Kuhar, M.J. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system. J. Pharmacol. Exp. Ther. 2006, 317, 454–461. [Google Scholar] [CrossRef]
- Rogge, G.A.; Jones, D.C.; Green, T.; Nestler, E.; Kuhar, M.J. Regulation of CART peptide expression by CREB in the rat nucleus accumbens in vivo. Brain Res. 2009, 1251, 42–52. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007, 28, 166–172. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK-3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Barr, J.L.; Unterwald, E.M. Glycogen synthase kinase-3 signaling in cellular and behavioral responses to psychostimulant drugs. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118746. [Google Scholar] [CrossRef]
- Perrine, S.A.; Miller, J.S.; Unterwald, E.M. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J. Neurochem. 2008, 107, 570–577. [Google Scholar] [CrossRef]
- Miller, J.S.; Barr, J.L.; Harper, L.J.; Poole, R.L.; Gould, T.J.; Unterwald, E.M. The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PLoS ONE 2014, 9, e88026. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.K.; Lee, J.W.; Jang, H.; Kim, J.H. Decrease of GSK3β phosphorylation in the rat nucleus accumbens core enhances cocaine-induced hyper-locomotor activity. J. Neurochem. 2013, 125, 642–648. [Google Scholar] [CrossRef]
- Enman, N.M.; Unterwald, E.M. Inhibition of GSK3 attenuates amphetamine-induced hyperactivity and sensitization in the mouse. Behav. Brain Res. 2012, 231, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.R.; Li, X.; Wang, X.; Reimers, J.M.; Uejima, J.L.; Wolf, M.E. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology 2010, 35, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Kalivas, P.W. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology 1996, 127, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.C.; Bell, K.; Duffy, P.; Kalivas, P.W. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996, 16, 1550–1560. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.B.; Permenter, L.K.; Lake, R.; Worley, P.F.; Kalivas, P.W. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur. J. Neurosci. 2003, 18, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Peineau, S.; Taghibiglou, C.; Bradley, C.; Wong, T.P.; Liu, L.; Lu, J.; Lo, E.; Wu, D.; Saule, E.; Bouschet, T.; et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 2007, 53, 703–717. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, Y.C.; Snyder, M.A.; Wang, H.; Li, F.; Gao, W.J. Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons. PLoS ONE 2013, 8, e61787. [Google Scholar] [CrossRef]
- Li, Y.Z.; Tang, X.H.; Wang, C.Y.; Hu, N.; Xie, K.L.; Wang, H.Y.; Yu, Y.H.; Wang, G.L. Glycogen synthase kinase-3beta inhibition prevents remifentanil-induced postoperative hyperalgesia via regulating the expression and function of AMPA receptors. Anesth. Analg. 2014, 119, 978–987. [Google Scholar] [CrossRef]
- Nelson, C.D.; Kim, M.J.; Hsin, H.; Chen, Y.; Sheng, M. Phosphorylation of threonine-19 of PSD-95 by GSK3 is required for PSD-95 mobilization and long-term depression. J. Neurosci. 2013, 33, 12122–12135. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Beaulieu, J.M.; Sotnikova, T.D.; Yao, W.D.; Kockeritz, L.; Woodgett, J.R.; Gainetdinov, R.R.; Caron, M.G. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA 2004, 101, 5099–5104. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Sotnikova, T.D.; Marion, S.; Lefkowitz, R.J.; Gainetdinov, R.R.; Caron, M.G. An Akt/-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122, 261–273. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Caron, M.G. Looking at lithium: Molecular moods and complex behavior. Mol. Interv. 2008, 8, 230–241. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Dalia, A.; Uretsky, N.J.; Wallace, L.J. Dopaminergic agonists administered into the nucleus accumbens: Effects on extracellular glutamate and on locomotor activity. Brain Res. 1998, 788, 111–117. [Google Scholar] [CrossRef]
- Reid, M.S.; Hsu, K., Jr.; Berger, S.P. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: Studies on the involvement of dopamine. Synapse 1997, 27, 95–105. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Dopamine regulation of extracellular glutamate in the nucleus accumbens. Brain Res. 1997, 761, 173–177. [Google Scholar] [CrossRef]
- Mao, L.M.; Diaz, J.A.; Fibuch, E.E.; Wang, J.Q. Regulation of phosphorylation of synaptic and extrasynaptic GluA1 AMPA receptors in the rat forebrain by amphetamine. Eur. J. Pharmacol. 2013, 715, 164–171. [Google Scholar] [CrossRef]
- Xue, B.; Edwards, M.C.; Mao, L.M.; Guo, M.L.; Jin, D.Z.; Fibuch, E.E.; Wang, J.Q. Rapid and sustained GluA1 S845 phosphorylation in synaptic and extrasynaptic locations in the rat forebrain following amphetamine administration. Neurochem. Int. 2014, 64, 48–54. [Google Scholar] [CrossRef][Green Version]
- Santos, S.D.; Carvalho, A.L.; Mao, A.L.; Caldeira, M.V.; Duarte, C.B. Regulation of AMPA receptors and synaptic plasticity. Neuroscience 2009, 158, 105–125. [Google Scholar] [CrossRef]
- Dajani, R.; Fraser, E.; Roe, S.M.; Young, N.; Good, V.; Dale, T.C.; Pearl, L.H. Crystal structure of glycogen synthase kinase 3 beta: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 2001, 105, 7217–7232. [Google Scholar]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Zahm, D.S. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000, 24, 85–105. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Parkinson, J.A.; Hall, J.; Everitt, B.J. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002, 26, 321–352. [Google Scholar] [CrossRef]
- Meredith, G.E.; Baldo, B.A.; Andrezjewski, M.E.; Kelley, A.E. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct. 2008, 213, 17–27. [Google Scholar] [CrossRef]
- Pascoli, V.; Cahill, E.; Bellivierl, F.; Caboche, J.; Vanhoutte, P. Extracellular signal-regulated protein kinase 1 and 2 activation by addictive drugs: A signal toward pathological adaptation. Biol. Psychol. 2014, 76, 917–926. [Google Scholar] [CrossRef]
- Choi, J.M.; Ahn, M.H.; Chae, W.J.; Jung, Y.G.; Park, J.C.; Song, H.M.; Kim, Y.E.; Shin, J.A.; Park, C.S.; Park, J.W.; et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat. Med. 2006, 12, 574–579. [Google Scholar] [CrossRef]
- Pellegrino, L.J.; Pellegrino, A.S.; Cushman, A.J. A Stereotaxic Atlas of the Rat Brain, 2nd ed.; Plenum Press: New York, NY, USA, 1979. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.R.; Kim, W.Y.; Jang, J.K.; Lee, J.W.; Kim, J.-H. Glycogen Synthase Kinase 3β Is a Key Regulator in the Inhibitory Effects of Accumbal Cocaine- and Amphetamine-Regulated Transcript Peptide 55–102 on Amphetamine-Induced Locomotor Activity. Int. J. Mol. Sci. 2022, 23, 15633. https://doi.org/10.3390/ijms232415633
Cho BR, Kim WY, Jang JK, Lee JW, Kim J-H. Glycogen Synthase Kinase 3β Is a Key Regulator in the Inhibitory Effects of Accumbal Cocaine- and Amphetamine-Regulated Transcript Peptide 55–102 on Amphetamine-Induced Locomotor Activity. International Journal of Molecular Sciences. 2022; 23(24):15633. https://doi.org/10.3390/ijms232415633
Chicago/Turabian StyleCho, Bo Ram, Wha Young Kim, Ju Kyong Jang, Jung Won Lee, and Jeong-Hoon Kim. 2022. "Glycogen Synthase Kinase 3β Is a Key Regulator in the Inhibitory Effects of Accumbal Cocaine- and Amphetamine-Regulated Transcript Peptide 55–102 on Amphetamine-Induced Locomotor Activity" International Journal of Molecular Sciences 23, no. 24: 15633. https://doi.org/10.3390/ijms232415633
APA StyleCho, B. R., Kim, W. Y., Jang, J. K., Lee, J. W., & Kim, J.-H. (2022). Glycogen Synthase Kinase 3β Is a Key Regulator in the Inhibitory Effects of Accumbal Cocaine- and Amphetamine-Regulated Transcript Peptide 55–102 on Amphetamine-Induced Locomotor Activity. International Journal of Molecular Sciences, 23(24), 15633. https://doi.org/10.3390/ijms232415633



